The role of exosomes in therapeutic resistance of hepatocellular carcinoma
Abstract
Hepatocellular carcinoma (HCC) stands as one of the most prevalent malignant tumors globally. Despite considerable advancements in HCC therapies, therapeutic resistance remains a significant challenge that compromises patient prognosis. Increasing evidence indicates that exosomes, which are secreted by cells in the tumor microenvironment (TME), are pivotal players in the development of therapeutic resistance in HCC. These nano-sized vesicles mediate intercellular communication in TME through the transfer of bioactive molecules such as nucleic acids, lipids, and proteins. A comprehensive understanding of the role of exosomes in therapeutic resistance could provide promising strategies for both the diagnosis and treatment of HCC. This review mainly summarizes the involvement of exosomal cargos and elucidates their underlying mechanisms in resistance to therapeutic treatments for HCC, and further discusses the potential clinical applications of exosomes as diagnostic biomarkers and therapeutic targets to overcome drug resistance in HCC.
Keywords
INTRODUCTION
HCC, accounting for approximately 75% to 85% of primary liver cancers, consistently ranks as one of the most lethal malignancies worldwide[1]. In clinical, HCC patients are frequently diagnosed at intermediate or advanced stages, largely due to the lack of noticeable symptoms and effective diagnostic markers during the early stages[2,3]. Moreover, the availability of effective treatment strategies for advanced HCC is still limited, especially in the case of traditional chemotherapy involving cytotoxic drugs, which have shown unsatisfactory results for the treatment of advanced HCC[4]. Since the U.S. Food and Drug Administration (FDA) approved Sorafenib as the first-line molecularly targeted therapy for advanced HCC in 2008, there has been rapid development in this field, offering new therapeutic possibilities. Numerous such drugs have now entered different stages of clinical trials[5]. Notably, Lenvatinib was approved by the FDA as another first-line targeted drug in 2018 for treating advanced HCC (NCT01761266). In recent years, immunotherapies that target immune checkpoints have shown potential in improving therapeutic outcomes for advanced HCC. Several inhibitors (ICIs) against immune checkpoints, such as PD-1, PD-L1, and CTLA-4, are currently under clinical trials[6]. Interestingly, recent studies found the potential of combining molecular targeted therapies with immune inhibitors to produce promising therapeutic effects on advanced HCC[7]. For example, the combination of Atezolizumab (a PD-L1 monoclonal antibody) and Bevacizumab (a VEGF antagonist) demonstrated good tolerability and effectiveness in advanced HCC, thus becoming the first FDA-approved combined therapy involving a molecular targeted drug and an immune inhibitor[8]. At present, systemic treatments for advanced HCC primarily include molecular targeted therapies, immune checkpoint inhibitors, or a combination of both. However, clinical studies have shown that tumor cells frequently develop resistance during treatment, which significantly reduces the therapeutic efficacy, thereby greatly limiting the impact of these strategies[9]. As a result, overcoming therapeutic resistance remains a significant challenge in HCC treatment. Investigating the underlying molecular mechanisms that induce therapeutic resistance and identifying reliable biomarkers for predicting and monitoring therapeutic responses, are of paramount importance in addressing the issue of treatment resistance and enhancing therapeutic efficacy across various tumors, including HCC.
The development of therapeutic resistance in HCC is generally associated with several key factors, including tumor burden, tumor heterogeneity, tumor stem cells, undruggable cancer drivers, and TME, among others[10]. Exosomes, serving as critical mediators of intercellular communication within the TME, have been shown to markedly impact a range of biological functions and pathological processes, including proliferation, metastasis, stemness, and immune response in HCC[11,12]. Exosomes are capable of modulating drug sensitivity through several mechanisms, primarily by transmitting bioactive molecules such as functional proteins and non-coding RNA molecules among heterogeneous tumor cells, immune cells, and other stromal cells within the TME[13]. These communications may further result in therapeutic resistance by altering the biological regulation process such as promotion of tumor cell survival, inhibition of programmed tumor cell death, remodeling of the tumor environment, facilitation of epithelial-mesenchymal transition (EMT) and tumor cell stemness, as well as deactivation or excretion of drug molecules to decrease intracellular drug concentrations. Therefore, exploring the role of exosomes and their contents in the emergence of HCC therapeutic resistance has attracted extensive attention. In this review, we examine the roles of exosomes in contributing to therapeutic resistance in HCC. Specifically, we summarized the latest research progress on the exosomal cargos and mechanisms by which exosomes contribute to therapeutic resistance in HCC. Furthermore, we provide an overview of current advancements in the application of engineered exosomes loaded with ani-tumor cargos such as nuclear acids, proteins, or small molecules to overcome drug resistance and improve the treatment outcome of HCC.
EXOSOMES-MEDIATED THERAPEUTIC RESISTANCE IN HCC
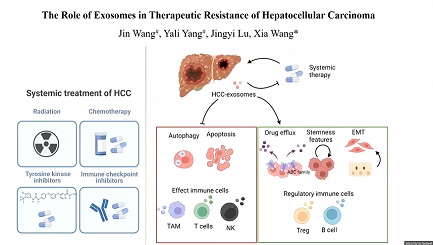
Exosomes, derived from tumor, stromal, and immune cells, contribute to the multiple stages of cancer progression. Previously, we have summarized the functions of exosomes originating from different sources in tumor development[12]. Here, given the proposed critical roles of exosomes in promoting therapeutic resistance in HCC, we conducted a detailed review of research exploring the roles of exosomes in resistance to systemic treatments including molecular targeted therapy, immunotherapy, chemotherapy, and radiotherapy for HCC. Exosomal contents and mechanisms identified as promoters of therapeutic resistance in HCC are summarized in Table 1. The representative mechanisms of exosome-mediated therapeutic resistance are encapsulated in Figure 1.
Figure 1. Representative mechanisms of exosome-mediated drug resistance in HCC. Exosomes derived from cells in TME serve as mediators to transport functional cargos that subsequently influence the signaling pathways and characteristics of target cells. HCC: hepatocellular carcinoma; CMA: Chaperone-mediated autophagy; BMMSCs: bone marrow-derived mesenchymal stem cells; EMT: Epithelial-Mesenchymal Transition; TAM, tumor-associated macrophage.
Exosomal contents involved in therapeutic resistance of HCC
| Contents classification | Origin of exosome | Exosomal contents | Functions | Mechanisms | Study type | Year | References |
| circRNAs | HCC cells | circUHRF1 | Drive anti-PD1 resistance | Inhibit NK cell function by regulating miR-449c-5p/TIM-3 axis | In vitro and in vivo | 2020 | [14] |
| HCC cells | circRNA-SORE | Sorafenib resistance | Block PRP19-mediated YBX1 degradation | In vitro and in vivo | 2020 | [15] | |
| HCC cells | cricTMEM181 | Contribute to immunosuppression and anti-PD1 resistance | Regulate miR-488-3p/CD39/eATP-adenosine pathway | In vitro and in vivo | 2021 | [16] | |
| Cancer-associated fibroblast | circZFR | Enhance cisplatin (DDP) resistance | Promote HCC development and cisplatin-resistance by targeting STAT3/ NF-κB signaling pathway | In vitro and in vivo | 2022 | [17] | |
| HCC cells | circPAK1 | Drive resistance to lenvatinib | CircPAK1 competitively binds 14-3-3 ζ with YAP, promoting YAP nucleus localization, leading to the inactivation of a Hippo signaling pathway | In vitro and in vivo | 2022 | [18] | |
| HCC cells | circCCAR1 | Promote CD8 + T-cell dysfunction and anti-PD1 resistance | CircCCAR1 induces the upregulation of PD-L1 expression by binding β-catenin and triggers the dysfunction of CD8+ T cells by stabilization of PD-1 | In vitro and in vivo | 2023 | [19] | |
| miRNAs | HCC cells | miR-21 | Lead to sorafenib resistance | Inhibit autophagy activity by the PTEN/AKT pathway | In vitro and in vivo | 2015 | [20] |
| Adipose tissue-derived mesenchymal stem cells | miR-122 | Chemoresistance (Sorafenib and 5-fluorouracil) | Communicate between adipose tissue-derived mesenchymal stem cells (AMSCs) and HCC cells, rendering cancer cells sensitive to chemotherapeutic agents | In vitro and in vivo | 2015 | [21] | |
| HCC cells | miR-1247-3p | Enhance sorafenib resistance | MiR-1247-3p converts fibroblasts to CAFs via downregulating B4GALT3, to activate the β1-integrin–NF-κB signaling pathway, and the activated fibroblasts further promote stemness, EMT by IL-6 and IL-8 secretion | In vitro | 2018 | [22] | |
| M2 macrophage | miR-27a-3p | Drug resistance | MiR-27a-3p downregulates TXNIP to induce stemness of HCC cells | In vitro | 2021 | [23] | |
| HepG2 cells | miR-135a-5p | Promote chemoresistance | Inhibit Dox-induced apoptosis via the miR-135a-5p/VAMP2 axis | In vitro | 2021 | [24] | |
| HCC cells | miR-494-3p | Promote sorafenib resistance | Inhibit PTEN expression by directly targeting the 3` UTR of PTEN mRNA | In vitro | 2022 | [25] | |
| HCC cells | miR-4669 | Lead to acquired sorafenib resistance | Induct M2 macrophage polarization to promote the immunosuppressive TME | In vitro | 2023 | [26] | |
| lncRNAs | HCC cells | linc-VLDLR | Induce chemotherapy resistance | Increase the expression of multidrug efflux transporter ABCG2 | In vitro | 2014 | [27] |
| HCC cells | linc-ROR | Mediate TGFβ-dependent chemoresistance | TGFβ increased the expression of CD133+ cells and colony growth | In vitro | 2014 | [28] | |
| Protein | HCC patient sera and HCC cells | plgR | Promote sorafenib resistance | Activate the PDK1/Akt/GSK3β/β-catenin signaling axis | In vitro and in vivo | 2022 | [29] |
| HCC cells | S100A10 | Enhance chemoresistance including sorafenib, 5-FU, and cisplatin | Upregulated EGFR, AKT and ERK signaling to increase the stemness and EMT features of HCC cells | In vitro and in vivo | 2023 | [30] | |
| No classificati on | HCC cells | exosomes | Induce sorafenib resistance | Activate the HGF/c-Met/Akt signaling pathway | In vitro and in vivo | 2016 | [31] |
| Liver cancer stem cells | exosomes | Induce regorafenib resistance | Induce Nanog expression to increase stemness features of HCC cells | In vitro and in vivo | 2021 | [32] | |
| HepG2 cells | exosomes | Transfer cisplatin resistance to cisplatin-sensitive cells | Upregulate the expression of drug efflux transporters P-glycoprotein P-gp | In vitro and in vivo | 2021 | [33] | |
| HCC cells | exosomes | Induce everolimus (EVE) resistance in EVE-sensitive HCC cells | Induce mesenchymal phenotype and deregulation of the mTOR pathway | In vitro and in vivo | 2022 | [34] |
Exosomes induce molecular targeted therapy resistance in HCC
Over the past few years, molecular targeted therapies have shown promising breakthroughs for patients with advanced HCC[35]. However, targeted therapy still faces challenges including adverse events, low response rates, and the presence of drug resistance[36,37]. Currently, molecular targeted therapy mainly involves the use of tyrosine kinase inhibitors (TKIs) and/or monoclonal antibodies. Sorafenib, the first FDA-approved multiple-target TKI for advanced HCC, effectively inhibits tumor angiogenesis and cell proliferation[38,39]. However, despite its efficacy, patients with HCC usually develop resistance to sorafenib[10,40,41].
Exosomes have emerged as key players in the development of resistance to sorafenib in HCC. Qu et al. indicated that exosomes derived from HCC cells mediated sorafenib resistance by activating the HGF/c-Met/Akt signaling pathway and inhibiting sorafenib-induced apoptosis[31]. Subsequent investigations revealed that the cargo components of exosomes are closely associated with sorafenib resistance. In 2014, Takahashi et al. identified the enrichment of long intergenic non-protein coding RNA, regulator of reprogramming (lincRNA-ROR), within exosomes derived from HCC cells in response to TGFβ[28]. After sorafenib treatment, the expression of lincRNA-ROR increased both in HCC cells and exosomes, leading to a reduction in sorafenib-induced cell death. In 2019, Zhi et al. discovered that LincRNA-ROR could function as a molecular sponge of miR-876-5p to upregulate FOXM1, a potent transcription factor with oncogenic functions, resulting in the attenuated sensitivity to sorafenib[42]. Linc-VLDLR, an exosome-enriched lncRNA, was identified to be upregulated in HCC cell-derived exosomes upon exposure to sorafenib. The transfer of linc-VLDLR through exosomes was correlated with elevated expression of multidrug efflux transporter ABCG2, resulting in reduced sensitivity of HCC cells to sorafenib, doxorubicin, and camptothecin[27]. Circular RNAs (circRNAs) and micro RNAs (miRNAs) have also been found to play important roles in the development of sorafenib resistance in HCC cells. CircRNA-SORE was discovered to be highly expressed in sorafenib-resistant HCC cells and could interact with YBX1 to inhibit its ubiquitination and degradation, thereby maintaining sorafenib resistance in HCC cells. Furthermore, circRNA-SORE can be transferred between tumor cells via exosomes, leading to the acquisition of sorafenib resistance in previously sensitive liver cancer cells. Therefore, exosomal circRNA-SORE plays a crucial role in the maintenance and transmission of sorafenib resistance in HCC[15]. In addition, exosomal miR-494-3p, upregulated by the oncoprotein Golgi phosphoprotein 3 (GOLPH3), has been found to be transferred to recipient human umbilical vein endothelial cells (HUVECs) and HCC cells, suppressing PTEN protein expression by directly targeting the 3' UTR of PTEN mRNA, and subsequently promoting angiogenesis and enhancing sorafenib resistance[25].
Besides sorafenib, studies have also reported the involvement of exosomes in HCC resistance to other TKIs. Lenvatinib is another first-line molecular targeted drug approved by the FDA in 2018 for the treatment of advanced HCC. Hao et al. reported that exosomes derived from lenvatinib-resistant HCC cells facilitate lenvatinib resistance by transferring circular RNA PAK1 (circPAK1) to neighboring sensitive cells[18]. In HCC cells, circPAK1 interacts with YAP, a downstream regulator of the Hippo signaling pathway, leading to the disruption of the recruitment and cytoplasmic binding of 14-3-3 ζ to YAP, thus promoting the nuclear localization of YAP and modulating the expression of genes associated with the malignant progression of HCC.
In summary, exosomes facilitate the transfer of drug resistance information among heterogeneous HCC cells through their cargo components, such as lncRNAs, circRNAs, miRNAs and proteins, thereby modulating the sensitivity of HCC cells to molecular targeted drugs.
Exosomes induce immunotherapy resistance in HCC
The advent of checkpoint inhibitors in cancer immunotherapy has dramatically promoted the management of advanced malignancies, including HCC. The immune checkpoint system, including inhibitory and stimulatory molecules, represents a set of ligand-receptor pairs that play important roles in modulating immune responses. The majority of immune checkpoint molecules, expressed on adaptive immune cells (especially T cells) and innate immune cells, serve to maintain self-tolerance and regulate the duration and intensity of immune responses in various tissues. Increasing evidence indicates that immune checkpoint molecules are expressed in a substantial proportion of tumors, thereby facilitating immune evasion[43]. Immune checkpoint inhibitors (ICIs) can block the interactions between checkpoint molecules and their corresponding ligands, thereby activating T cell-mediated antitumor immune responses.
Currently, a wide range of immune checkpoint inhibitors targeting PD-1, PD-L1, and CTLA-4 have been developed and are being evaluated at different stages of clinical trials for the treatment of various malignancies. Notably, Nivolumab and Pembrolizumab, which specifically target PD-L1, have been approved by the FDA as conditional second-line therapies for HCC[44,45]. However, despite the positive response observed in certain HCC patients treated with ICIs, only a subset of HCC patients derive benefits from ICIs, and even those who initially favorably respond to ICIs may develop resistance during treatment[46].
Recent evidence indicates the crucial involvement of exosomes in the remodeling of the tumor immune microenvironment. Notably, studies demonstrated that PD-L1 could be packaged into exosomes by tumor cells and transferred to other cells in the tumor microenvironment, thereby facilitating the evasion of antitumor immunity by suppressing effector T cell activation[47]. Moreover, exosomal PD-L1 was observed to compromise the effectiveness of antibody-mediated blockade of the PD-1 and PD-L1 interaction[48,49]. Additionally, the presence of other specific molecules within exosomes is crucial for modulating the tumor immunosuppressive microenvironment.
Lu et al. reported that exosomal circTMEM181 functions as a miR-488-3p sponge, thereby upregulating CD39 expression in macrophages[16]. The increased CD39 levels in macrophages and CD73 levels in HCC cells synergistically activate the eATP-adenosine pathway, resulting in enhanced adenosine production, which further impairs CD8+ T cell function, leading to immunosuppression and resistance to anti-PD1 therapy[16]. Exosomal HMGB1 was demonstrated to induce the expansion of TIM-1+ regulatory B (TIM-1+Breg) cells through the Toll-like receptor (TLR) 2/4 and mitogen-activated protein kinase (MAPK) signaling pathways. These TIM-1+ Breg cells exhibited increasing expression of the immunosuppressive cytokine IL-10 and displayed potent suppressive activity against CD8+ T cells, thereby facilitating the immune evasion of HCC cells[50]. Zhang et al. demonstrated that HCC-derived exosomal circUHRF1(circular ubiquitin-like with PHD and ring finger domain 1 RNA) inhibits the activity of NK cells by upregulating the expression of TIM-3 via degradation of miR-449c-5p, thereby suppressing the secretion of NK cell-derived IFN-γ and TNF-α[14]. CircUHRF1 was also identified to promote resistance to anti-PD1 immunotherapy in HCC patients, but the potential mechanisms remain to be further clarified[14]. Recently, Hu et al. revealed a marked overexpression of circCCAR1 in both tumor tissues and the plasma exosomes of HCC patients[19]. In HCC cells, circCCAR1 could induce the upregulation of PD-L1 expression by binding β-catenin. Moreover, upon being internalized by CD8+ T cells, exosomal circCCAR1 could trigger the dysfunction of CD8+ T cells by stabilization of PD-1, collectively inducing the resistance to anti-PD1 immunotherapy[19]. In addition, Wang et al. uncovered that the 14-3-3ζ protein, transmitted by exosomes derived from HCC cells, has the potential to compromise the antitumor functionality of tumor-infiltrating T lymphocytes[51]. Pu et al. demonstrated that exosomes derived from M2 macrophage could induce CD8+ T cell exhaustion through the miR-21-5p/YOD1/YAP/β-catenin signaling pathway, resulting in an immunosuppressive microenvironment[52]. All these findings hold substantial significance for elucidating the mechanisms driving the tumor immunosuppression microenvironment of HCC.
In summary, exosomes mediate the transmission of immunosuppressive molecules in the TME, particularly facilitating the communication between HCC cells and various immune cells, thereby impacting the function of the immune cells through a variety of mechanisms. Currently, the research on exosome-mediated immunosuppression remains in its infancy. The identification of novel, effective exosomal components originating from various cells in the TME, including HCC cells, immune cells, adipocytes, or other stromal cells, and understanding underlying mechanisms necessitates further exploration. The potential application of these exosomal components as predictive biomarkers presents a promising non-invasive strategy for monitoring responses to immunotherapy.
Exosomes promote chemotherapy resistance in HCC
Currently, chemotherapy remains one of the principal therapeutic approaches for HCC. Nonetheless, the frequent development of chemoresistance following long-term chemotherapy for HCC significantly impedes effective treatment outcomes. Resistance to clinically used therapeutic drugs such as oxaliplatin, cisplatin, gemcitabine, doxorubicin, and 5-fluorouracil (5-FU) is a major cause of the failure of chemotherapy in patients with advanced HCC. Studies demonstrated that exosomes could counteract the therapeutic effects of chemotherapeutic drugs by suppressing apoptosis and promoting the survival of HCC cells during chemotherapy[53]. For example, Wei et al. identified that exosomal miR-135a-5p derived from HCC with HBV infection promotes anti-apoptosis, cell proliferation, and chemical resistance to doxorubicin hydrochloride (DOX) through miR-135a-5p/VAMP2 (vesicle-associated membrane protein 2) axis[24]. In 2022, Zhou et al. discovered that exosomes derived from cancer-associated fibroblasts could transfer circZFR to HCC cells, fostering HCC progression and cisplatin resistance by targeting the STAT3/NF-κB signaling pathway[17].
Moreover, studies also have revealed that exosomes play important roles in HCC chemoresistance by mediating the efflux of chemotherapeutic drugs. Multidrug resistance (MDR) is a critical obstacle in cancer pharmacotherapy, representing a defensive mechanism employed by cancer cells that significantly limits the effectiveness of treatment. One of the common mechanisms underlying MDR involves the overexpression of ATP-binding cassette (ABC) efflux transporters in cancer cells[54]. The ABC family transporters, including P-glycoprotein (P-gp/ABCB1), multidrug resistance-associated protein 2 (MRP2/ABCC2), and breast cancer resistance protein (BCRP/ABCG2), utilize ATP to efflux various xenobiotics, thereby compromising the efficacy of chemotherapeutic agents[55].
Exosomes were demonstrated to regulate drug efflux via modulation of ABC transporter family members, which have been implicated in the development of chemoresistance. For example, in several studies, exosomes were identified to mediate the transfer of drug efflux pumps such as the permeability glycoprotein (P-gp), ABCG2 or MRP1 from donor drug-resistant cells to recipient-sensitive cancer cells, thereby fostering the acquisition of drug resistance in the heterogeneous tumor cells[56-58]. Undoubtedly, exosomes can confer chemoresistance to tumor cell populations via the aforementioned pathway. However, it should be noted that direct in vitro and in vivo experimental evidence substantiating the role of exosome-mediated transfer of drug efflux pumps among HCC cells, which leads to acquired drug resistance, remains elusive. Therefore, additional experimental investigations are imperative to validate this mechanism, specifically in the context of HCC. Beyond the above mechanism, exosomes can influence the chemosensitivity of HCC cells by regulating the expression of ABC transporters through the intercellular transfer of their functional cargo molecules. As alluded to earlier, exosomal linc-VLDLR has the capacity to elevate the expression of ABCG2, consequently engendering therapeutic resistance to drug molecules such as sorafenib, camptothecin, and doxorubicin in HCC cells[27]. Similarly, Tang et al. found exosomes derived from cisplatin-resistant HepG2 cells enhance the protein expression of P-gp in HepG2 cells, inducing these sensitive cells to develop a drug-resistant phenotype[33].
In summary, through the intercellular transmission of bioactive molecules in TME, exosomes not only modulate the pro-survival and anti-apoptotic abilities of HCC cells, but also regulate the expression of multidrug resistance proteins, which consequently leads to the development of drug resistance against various therapeutic agents.
Exosomes mediate radioresistance in radiotherapy of HCC
Radiotherapy, including radiofrequency ablation and stereotactic body radiation therapy, remains the standard locoregional therapeutic option for unresectable or medically inoperable HCC[59,60]. However, the resistance against radiotherapy poses a significant challenge to the successful treatment of HCC. The mechanisms underlying radioresistance are complex, involving various factors such as radiation-induced DNA damage repair, apoptosis escape, cell cycle arrest, abnormal autophagy, abundance of cancer stem cells, and other dysregulated biological processes[61]. Radioresistance is also highly associated with TME, a critical factor influencing tumor progression and therapeutic response, which can be modulated through intercellular communications mediated by exosomes[62,63].
Exosomes represent a significant environmental stressor for cells, with radiation treatment known to intensify their release and modify their components. Exosomes derived from a variety of irradiated cancer cells, including those of lung, breast, and glioblastoma, have been implicated in mediating bystander effects[64]. These effects facilitate the transmission of multiple factors in TME, thereby promoting radiotherapy resistance by regulating pro-survival and anti-apoptotic pathways[65]. Moreover, alterations noted in exosomes and their contents post-radiotherapy could potentially serve as prognostic and predictive biomarkers for monitoring radiation response[66]. However, based on our literature review, there is a noticeable scarcity of research focusing on the significant changes in exosomal components and their associated mechanisms contributing to the development of radioresistance in HCC following radiotherapy. Consequently, exploring the alterations of exosomes in the TME post-radiotherapy is crucial for assessing treatment efficacy and avoiding radioresistance.
Additionally, CSCs inherently exhibit greater resistance to radiation than typical cancer cells and are more likely to survive post-radiotherapy[67-69]. The surviving CSCs may transfer resistant or refractory signals to recipient cells via exosomes, consequently reducing treatment efficacy. Therefore, exosomes associated with liver CSCs represent potential contributors to radioresistance, emphasizing the need for more comprehensive investigations in the future.
Exosomes promote stemness-related characteristics and EMT in drug resistance of HCC
Cancer stem cells (CSCs) are considered key determinants of drug sensitivity. Liver CSCs, a minority subset of HCC cells, display distinctive stem cell-like characteristics such as potent self-renewal, differentiation potential, and tumorigenicity, which significantly contribute to the recurrence, metastasis, and therapeutic resistance in HCC[70,71]. CD133+ HCC cells have been identified as a CSC subset demonstrating substantial resistance to chemotherapeutic agents such as doxorubicin and fluorouracil[72].
Exosomes have been demonstrated to modulate the stemness of HCC cells and mediate drug resistance through their cargos. Regorafenib, a potent multikinase inhibitor for second-line targeted therapy against HCC, was shown to induce regorafenib resistance through RAB27A-dependent exosomes released by liver CSCs[32]. These exosomes could induce Nanog expression in their differentiated progenies, thereby conferring regorafenib resistance in HCC. In 2021, Li et al. identified that exosomal miR-27a-3p, derived from M2 macrophage, promotes both cancer stemness and drug resistance in HCC cells by facilitating the downregulating TXNIP[23]. Recently, Tey et al. found the expression of polymeric immunoglobulin receptor (pIgR) was significantly elevated in circulating exosomes from patients with late-stage HCC[29]. pIgR-enriched exosomes activate PDK1/Akt/GSK3β/β-catenin signaling cascades, promoting the cancer stemness and suppressing the sensitivity of HCC cells to sorafenib[29]. Our previous study showed that S100 calcium-binding protein A10 (S100A10) is enriched in exosomes derived from HCC cells. S100A10-enriched exosomes promote the stemness and epithelial-mesenchymal transition (EMT) of HCC cells, thereby inducing resistance against sorafenib, cisplatin, and 5-flurouracil[30].
EMT is a biological process characterized by the loss of epithelial features and the acquisition of mesenchymal cell phenotypes. Cells undergoing EMT experience a range of biochemical changes, such as a decrease in tight cell-cell adhesion and an increase in invasive, migratory, and anti-apoptotic capabilities[73]. Emerging evidence indicates that exosomes can mediate the transmission of pro-EMT factors in the TME, thereby promoting the progression of HCC[74]. Exosomes derived from highly metastatic HCC cells were identified to induce EMT in low metastatic HCC cells by activating the MAPK/ERK signaling pathway, leading to HCC progression and recurrence[75]. Similarly, exosomes derived from HCC cells resistant to everolimus (an mTOR inhibitor currently under phase III trials) were shown to transmit the aggressive phenotype by promoting the EMT phenotype and inducing the deregulation of the mTOR pathway[34]. Karaosmanoğlu et al. discovered that HCC cells transfected with Slug, a key transcription factor of EMT, demonstrated significant chemoresistance and stem-like characteristics[76]. Intriguingly, exosomes from these HCC cells contained pro-EMT proteins such as fibronectin 1 (FN1), collagen type II alpha 1 (COL2A1), and native fibrinogen gamma chain (FGG). These findings suggest a potential role of these exosomes in promoting the malignant progression and therapeutic resistance of HCC cells in the TME. In 2018, Fang et al. demonstrated that exosomal miR-1247-3p, derived from HCC cells, induces the transformation of fibroblasts into cancer-associated fibroblasts (CAFs) through the downregulation of B4GALT3[22]. Importantly, these activated fibroblasts further stimulate stemness and EMT via the secretion of IL-6 and IL-8, thereby enhancing resistance to sorafenib[22]. Recently, Shi et al. revealed that upregulation of Linc-ROR promotes the EMT program and drives Adriamycin resistance by targeting AP-2α/Wnt/β-catenin axis in HCC[77]. Together with the aforementioned studies, Linc-ROR can be transferred via exosomes to mediate resistance to sorafenib. These findings suggest that Linc-ROR can exert a potent drug resistance effect by inducing EMT and CSC features through various signaling pathways. As a result, Linc-ROR represents a promising target for drug design to overcome the therapeutic resistance of HCC. Despite the identification of numerous exosomal components that contributed to EMT in HCC cells[78-80], experimental evidence substantiating their role as key contributors to drug resistance in HCC remains scarce. Consequently, comprehensive studies elucidating how these exosomal cargos influence drug resistance in HCC cells through the regulation of EMT are imperative. Such insights are pivotal for the development of combined treatment strategies targeting multiple key biological processes.
In summary, highly malignant HCC cells in tumor cell populations can induce stem-like or mesenchymal-like phenotypes in less malignant HCC cells through the secretion of exosomes, thereby promoting acquired therapeutic resistance. Therefore, these exosomes, by modulating CSCs or EMT characteristics in tumor cell subpopulations, represent a common mechanism employed by HCC to develop drug resistance and can serve as crucial potential targets for drug design strategies.
Exosomes induce autophagy in drug resistance
Autophagy, an evolutionarily conserved process responsible for the degradation of intracellular components, plays a dual role in both suppressing and promoting tumor survival according to the circumstance. Studies indicate that autophagy is intricately associated with the development and maintenance of drug resistance in HCC[81-83]. As early as 2011, Ying-Hong Shi and colleagues reported that inhibiting autophagy, either through pharmacological inhibitors or the knockdown of essential autophagy-related genes, enhanced cell death in sorafenib-treated HCC cells. A combination therapy involving sorafenib and the autophagy inhibitor chloroquine yielded more substantial suppression of HCC[84]. In 2018, Zhang et al. identified that miR-142-3p could potentiate the sensitivity of HCC cells to sorafenib by inhibiting autophagy via targeting autophagy-related 5 (ATG5) and autophagy-related 16-like 1 (ATG16L1)[85]. Similarly, autophagy inhibition has also been shown to increase the anticancer effect of bevacizumab in HCC[86].
Recent studies indicate that exosome-induced autophagy may participate in the drug resistance of HCC cells. Liu et al. found that exosomes derived from HBV-associated liver cancer affected cell survival by upregulating the chaperone-mediated autophagy pathway, eventually facilitating chemoresistance in patients with HBV-associated HCC[87]. Additionally, miR-21 was found to be upregulated in sorafenib-resistant HCC cells in 2015. Exposure of HCC cells to sorafenib resulted in increased expression of miR-21. MiR-21 contributes to the acquired resistance to sorafenib by suppressing autophagy through the Akt/PTEN pathway in HCC[20]. Furthermore, in 2019, Tian et al. discovered that the acidic microenvironment in HCC induces the release of miR-21-enriched exosomes to promote cancer cell proliferation and metastasis[88]. Based on these findings, we hypothesize that the transmission of miR-21 through exosomes in response to an acidic microenvironment among HCC cells could suppress autophagy by modulating the Akt/PTEN pathway, which plays a crucial role in the acquisition of sorafenib resistance in HCC.
In summary, most anticancer drugs primarily exert their therapeutic effects by inducing apoptosis in cancer cells. However, under the stress of treatment, some cancer cells may adapt by evolving the mechanisms that regulate cell death, thereby achieving evasion from programmed cell death. In HCC, autophagy emerges as a key mechanism regulating cell survival or death, acting as a double-edged sword contingent on specific circumstances, conferring an enhanced adaptive capacity to cancer cells. Exosomes play a crucial role in propagating these death-resistant signals among tumor cells, which consequently fosters cancer cell survival and engenders therapeutic resistance through the modulation of autophagy. Although our understanding of how exosome-derived cargos regulate autophagy and impact drug resistance is gradually improving, further elucidation is necessary. Therefore, investigating the role and mechanism of exosomes in overcoming drug-induced cell death modalities holds significant promise for the advancement of innovative anticancer drugs and therapeutic strategies.
POTENTIAL CLINICAL APPLICATIONS OF EXOSOMES IN HCC THERAPEUTIC RESISTANCE
With an increased understanding of the characteristics of exosomes and their association with therapeutic resistance, as well as the different cargos identified under various disease conditions, exosomes have emerged as one of the most promising research fields for improving treatment outcomes of HCC[89]. Given their roles in facilitating therapeutic resistance in HCC, exosomes not only represent potential targets for cancer therapy but also prospective biomarkers for cancer diagnosis, therapeutic response evaluation, and prognosis prediction[14,90].
Exosomes as predictive biomarkers for treatment efficacy in HCC
Biomarkers, which are molecules or substances detectable in blood, tissues, or other biological samples, have the capacity to indicate the presence, progression, or prognosis of a disease. They hold important roles in the detection and monitoring of disease status during cancer diagnosis and treatment. The number of studies have been conducted to explore the potential use of exosomes as diagnostic or prognostic biomarkers for HCC. Remarkably, non-coding RNAs and other components present in exosomes have been identified as promising biomarker candidates for HCC[12,91]. For instance, overexpression of exosomal miR-4669 was identified to promote the immunosuppressive TME, potentially predicting therapeutic response and recurrence probability of HCC[26]. Serum exosomal lnc-FAM72D-3 and lnc-EPC1-4 were demonstrated as potential diagnostic biomarkers of HCC[92]. In a study by Ji et al., it was elucidated that exosomes carrying ZFPM2-AS1 could promote HCC progression via the miR-18b-5p/PKM axis, thereby highlighting the diagnostic and therapeutic potential of exosomal ZFPM2-AS1 in HCC patients[93]. Circulating exosomal PD-L1 was found to enhance the adaptive response of tumor cells to T cell reinvigoration and may serve as a potential predictor of anti-PD-1 therapy efficacy in melanoma[47]. However, the potential role of exosomal PD-L1 in the context of immune therapy for HCC remains to be further elucidated. Moreover, Hoshino et al. revealed a set of tumor-type-specific exosomal proteins in plasma and tissue explants, which facilitate the classification of cancers of unknown primary origin and may serve as reliable biomarkers for cancer detection and cancer type determination[94].
In summary, exosomes present a promising avenue for the identification of predictive biomarkers in HCC. The analysis of exosomal cargos, especially differentially enriched non-coding RNAs or proteins that are enriched in disease status, holds the potential for improving treatment selection and monitoring response in HCC patients. Additional research efforts are required to validate the clinical utility of these exosomal biomarkers and to develop standardized protocols for their detection and quantification.
Exosomes as therapeutic interventions in HCC therapeutic resistance
As discussed above, exosomes serve as important mediators to promote therapeutic resistance of HCC by transferring specific components intercellularly in TME. Targeting exosomes emerges as a promising strategy to counteract therapy resistance. An effective approach to mitigate exosome-mediated drug resistance involves impeding the production of exosomes or obstructing the secretion of exosomes that are enriched with drug resistance-related contents originating from specific cell types. For example, the knocking-down of the expression of RAB27A or RAB27B, regulator of exosome secretion, significantly improved the therapeutic effects of drugs in HCC[32,95]. Therefore, a series of inhibitors targeting different stages in exosome biogenesis and release were developed to interfere with the function of exosomes in the progression of cancers[96,97]. Additionally, targeted elimination of circulating exosomes is another viable strategy for tumor inhibition. Neutralizing antibodies that target exosomal S100A10 or pIgR have been shown to effectively overcome drug resistance in HCC cells[29,30]. Hence, strategies that interfere with the biogenesis, release, or transmission of exosomes present substantial potential for the development of effective therapeutic approaches in treating various types of cancers. Particularly, the combination of exosome inhibitors and anticancer drugs may offer a promising solution to overcoming tumor cell drug resistance, thereby significantly improving clinical therapeutic outcomes. Nevertheless, there are still numerous challenges in these therapeutic strategies, such as the lack of specific inhibitors. Moreover, the paucity of relevant experimental research, especially those focusing on drug resistance in HCC, highlights the need for further validation through extensive basic and clinical investigations.
In addition, exosomes, increasingly recognized as one of the most effective delivery vehicles for cancer therapy, have the capability to transport antitumor drugs or therapeutic biomolecules with remarkable histocompatibility, less immunogenicity, low cytotoxicity, and high specificity in targeting recipient cells[98-100]. These nano-sized vesicles can be readily internalized by recipient cells, facilitating the effective delivery of therapeutic cargos such as nucleic acids, proteins, and anticancer drugs to the target site[101]. Lou et al. revealed that miR-122 could be effectively packaged into exosomes released from AMSCs (adipose mesenchymal stem cells) post-transfection[21]. These miR-122-enriched exosomes mediate miR-122 crosstalk between AMSCs and HCC cells, consequently enhancing HCC cell sensitivity to sorafenib both in vitro and in vivo. Further research from the same group discovered that miR-199a-loaded exosomes from AMSCs could increase HCC chemosensitivity to doxorubicin by targeting the mTOR pathway[102]. Similarly, exosomes derived from siGRP78-modified bone marrow-derived mesenchymal stem cells (BMMSCs) were reported to be able to sensitize sorafenib-resistant HCC cells and reverse the drug resistance[103]. CD38 siRNA-loaded exosomes from BMMSCs were identified to reverse HCC resistance to PD-1/PD-L1 inhibitors in vivo[104]. Recently, engineered exosomes carrying sgRNAs/Cas9 targeting IQGAP1 and FOXM1 were developed to effectively reverse sorafenib resistance in HCC by suppressing CD133+ HCC cells[105]. Moreover, exosomes are also being developed as prospective carriers for therapeutic drugs such as curcumin, doxorubicin, and paclitaxel to increase drug efficacy, reduce side effects, and overcome drug resistance in cancer treatment[13,106]. In HCC, exosomes encapsulating asiatic acid or sorafenib have shown effective antitumor effects[105,107]. In brief, the development of innovative exosome-based strategies holds great potential in improving the therapeutic outcomes for HCC patients.
CONCLUSIONS AND PERSPECTIVES
The resistance and low response rates remain significant challenges in the treatment of HCC. Exosomes, emerging as important mediators of intercellular communication, exert critical roles in various biological processes, including cancer progression and therapeutic resistance. In TME, exosomes act as a crucial regulator of drug resistance by modulating mechanisms such as promoting drug efflux, inducing EMT and CSC properties, regulating autophagic phenotypes, and driving immunosuppression. For clinical applications, exosomes may serve as potential biomarkers for predicting and monitoring therapeutic efficacy in HCC patients, especially for those undergoing radiotherapy. Moreover, exosomes serve as promising targets to reverse exosome-mediated drug resistance. Additionally, utilizing exosomes for the delivery of therapeutic anticancer drugs or other therapeutic agents provides a new approach to enhance the efficacy of therapeutic drugs in HCC patients.
Similar to the antibiotic resistance developed by bacteria, tumor cells inevitably acquire drug resistance under the prolonged influence of anticancer drugs. As anomalous tissues composed of eukaryotic multicellular entities, tumors harbor complex intracellular and intercellular signaling networks that sustain their survival, rendering the mechanisms of developing drug resistance more intricate. Therapies targeting a single site often demonstrate effectiveness in the short term but are prone to induce therapeutic resistance in tumor cells upon long-term use. Hence, developing multi-target anticancer drugs has greater therapeutic potential. For example, the first-line molecular targeted drugs such as sorafenib and lenvatinib, which are currently used successfully for advanced HCC treatment, typically inhibit the activity of a series of kinases. Additionally, another effective strategy to prevent drug resistance involves the combination of drugs targeting different specific cellular processes, such as the combination of molecular targeted drug bevacizumab and immune checkpoint inhibitor atezolizumab, which brings new hope for cancer patients. More importantly, since exosomes serve as the hub carriers of therapeutic resistant signals in TME, by blocking the functionality of these exosomes, therapeutic resistance can be effectively counteracted. Consequently, a combination strategy involving targeted inhibitors to suppress exosomal functions in the TME along with other treatment modalities could emerge as a potent method to effectively inhibit tumor progression and drug resistance. This approach may present one of the most promising therapeutic strategies for cancer treatment in the foreseeable future.
Despite the exciting potential applications, research on exosomes in HCC therapeutic resistance remains nascent, with several pivotal challenges that need to be addressed in future investigations. (1) Currently, most studies have primarily focused on exosomes derived from HCC cells, resulting in a significant knowledge gap regarding the contributions of exosomes from other cellular sources such as immune and stromal cells in TME. Deciphering the influences of these exosomes on HCC drug resistance and pathological processes is critical for effectively exploiting the therapeutic potential of exosomes; (2) Although RNAs in exosomes, including miRNA, lncRNA, and circRNA, have been extensively studied, other cargos such as proteins, lipids, and other metabolic substrates remain inadequately investigated. Thus, a more holistic understanding of the underlying mechanisms of exosome-mediated drug resistance is crucial; (3) So far, although emerging studies investigate the role of exosomes in HCC therapeutic resistance, the underlying mechanisms are still vague. Systematic and in-depth studies about the mechanisms of exosome-mediated drug resistance are in urgent need and will further facilitate the development of targeting exosomes to reverse the therapeutic resistance in HCC; (4) The utilization of exosomes as drug delivery vehicles for antitumor therapies offers a promising avenue for enhancing the efficacy of therapeutic agents in HCC patients. Despite recent advances in exosome-based carrier platforms, these techniques often fall short in terms of yield, specificity, sensitivity, and heterogeneity. There is an urgent need for simplified, reproducible methodologies for the purification and large-scale production of quality-controlled exosomes and drug/gene-encapsulating exosomes. Additionally, the rigorous characterization of exosomal RNA, lipids, and proteins also remains a pressing requirement. By addressing these challenges, the therapeutic outcomes for HCC patients will be significantly improved with the use of exosome-based strategies in the future.
DECLARATIONS
AcknowledgmentsAll figures were created with BioRender.com
Authors’ contributionsWrote the manuscript: Wang J, Yang Y
Assistance: Lu J
Initiated the study and finalized the manuscript: Wang X
Availability of data and materialsNot applicable.
Financial support and sponsorshipTalent Introduction Research Startup Funding of Liaoning University (a280001122).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209-49.
3. Jiang HY, Chen J, Xia CC, Cao LK, Duan T, Song B. Noninvasive imaging of hepatocellular carcinoma: From diagnosis to prognosis. World J Gastroenterol 2018;24:2348-62.
4. Rizzo A, Ricci AD, Brandi G. Systemic adjuvant treatment in hepatocellular carcinoma: tempted to do something rather than nothing. Future Oncol 2020;16:2587-9.
5. Zhang H, Zhang W, Jiang L, Chen Y. Recent advances in systemic therapy for hepatocellular carcinoma. Biomark Res 2022;10:3.
6. Yang C, Zhang H, Zhang L, et al. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2023;20:203-22.
7. Vafaei S, Zekiy AO, Khanamir RA, et al. Combination therapy with immune checkpoint inhibitors (ICIs); a new frontier. Cancer Cell Int 2022;22:2.
8. Lee MS, Ryoo BY, Hsu CH, et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol 2020;21:808-20.
10. Ladd AD, Duarte S, Sahin I, Zarrinpar A. Mechanisms of drug resistance in HCC. Hepatology 2023:online ahead of print.
11. Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells 2019;8:307.
12. Wang X, Tian L, Lu JY, Ng IO. Exosomes and cancer-diagnostic and prognostic biomarkers and therapeutic vehicle. Oncogenesis 2022;11:54.
13. Dai J, Su YZ, Zhong SY, et al. Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther 2020;5:145.
14. Zhang PF, Gao C, Huang XY, et al. Cancer cell-derived exosomal circUHRF1 induces natural killer cell exhaustion and may cause resistance to anti-PD1 therapy in hepatocellular carcinoma. Mol Cancer 2020;19:110.
15. Xu J, Ji L, Liang Y, et al. CircRNA-SORE mediates sorafenib resistance in hepatocellular carcinoma by stabilizing YBX1. Signal Transduct Target Ther 2020;5:298.
16. Lu JC, Zhang PF, Huang XY, et al. Amplification of spatially isolated adenosine pathway by tumor-macrophage interaction induces anti-PD1 resistance in hepatocellular carcinoma. J Hematol Oncol 2021;14:200.
17. Zhou Y, Tang W, Zhuo H, et al. Cancer-associated fibroblast exosomes promote chemoresistance to cisplatin in hepatocellular carcinoma through circZFR targeting signal transducers and activators of transcription (STAT3)/nuclear factor -kappa B (NF-κB) pathway. Bioengineered 2022;13:4786-97.
18. Hao X, Zhang Y, Shi X, et al. CircPAK1 promotes the progression of hepatocellular carcinoma via modulation of YAP nucleus localization by interacting with 14-3-3ζ. J Exp Clin Cancer Res 2022;41:281.
19. Hu Z, Chen G, Zhao Y, et al. Exosome-derived circCCAR1 promotes CD8 + T-cell dysfunction and anti-PD1 resistance in hepatocellular carcinoma. Mol Cancer 2023;22:55.
20. He C, Dong X, Zhai B, et al. MiR-21 mediates sorafenib resistance of hepatocellular carcinoma cells by inhibiting autophagy via the PTEN/Akt pathway. Oncotarget 2015;6:28867-81.
21. Lou G, Song X, Yang F, et al. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol 2015;8:122.
22. Fang T, Lv H, Lv G, et al. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat Commun 2018;9:191.
23. Li W, Xin X, Li X, Geng J, Sun Y. Exosomes secreted by M2 macrophages promote cancer stemness of hepatocellular carcinoma via the miR-27a-3p/TXNIP pathways. Int Immunopharmacol 2021;101:107585.
24. Wei XC, Xia YR, Zhou P, et al. Hepatitis B core antigen modulates exosomal miR-135a to target vesicle-associated membrane protein 2 promoting chemoresistance in hepatocellular carcinoma. World J Gastroenterol 2021;27:8302-22.
25. Gao Y, Yin Z, Qi Y, et al. Golgi phosphoprotein 3 promotes angiogenesis and sorafenib resistance in hepatocellular carcinoma via upregulating exosomal miR-494-3p. Cancer Cell Int 2022;22:35.
26. Nakano T, Chen CL, Chen IH, et al. Overexpression of miR-4669 enhances tumor aggressiveness and generates an immunosuppressive tumor microenvironment in hepatocellular carcinoma: its clinical value as a predictive biomarker. Int J Mol Sci 2023;24:7908.
27. Takahashi K, Yan IK, Wood J, Haga H, Patel T. Involvement of extracellular vesicle long noncoding RNA (linc-VLDLR) in tumor cell responses to chemotherapy. Mol Cancer Res 2014;12:1377-87.
28. Takahashi K, Yan IK, Kogure T, Haga H, Patel T. Extracellular vesicle-mediated transfer of long non-coding RNA ROR modulates chemosensitivity in human hepatocellular cancer. FEBS Open Bio 2014;4:458-67.
29. Tey SK, Wong SWK, Chan JYT, et al. Patient pIgR-enriched extracellular vesicles drive cancer stemness, tumorigenesis and metastasis in hepatocellular carcinoma. J Hepatol 2022;76:883-95.
30. Wang X, Huang H, Sze KM, et al. S100A10 promotes HCC development and progression via transfer in extracellular vesicles and regulating their protein cargos. Gut 2023;72:1370-84.
31. Qu Z, Wu J, Wu J, Luo D, Jiang C, Ding Y. Exosomes derived from HCC cells induce sorafenib resistance in hepatocellular carcinoma both in vivo and in vitro. J Exp Clin Cancer Res 2016;35:159.
32. Huang H, Hou J, Liu K, et al. RAB27A-dependent release of exosomes by liver cancer stem cells induces Nanog expression in their differentiated progenies and confers regorafenib resistance. J Gastroenterol Hepatol 2021;36:3429-37.
33. Tang Z, He J, Zou J, Yu S, Sun X, Qin L. Cisplatin-resistant HepG2 cell-derived exosomes transfer cisplatin resistance to cisplatin-sensitive cells in HCC. PeerJ 2021;9:e11200.
34. Negri M, Amatrudo F, Gentile A, et al. Vitamin D Reverts the exosome-mediated transfer of cancer resistance to the mTOR inhibitor everolimus in hepatocellular carcinoma. Front Oncol 2022;12:874091.
35. Huang A, Yang XR, Chung WY, Dennison AR, Zhou J. Targeted therapy for hepatocellular carcinoma. Signal Transduct Target Ther 2020;5:146.
36. Chen J, Jin R, Zhao J, et al. Potential molecular, cellular and microenvironmental mechanism of sorafenib resistance in hepatocellular carcinoma. Cancer Lett 2015;367:1-11.
37. Kudo M, Ikeda M, Takayama T, et al. Safety and efficacy of sorafenib in Japanese patients with hepatocellular carcinoma in clinical practice: a subgroup analysis of GIDEON. J Gastroenterol 2016;51:1150-60.
38. Galle PR. Sorafenib in advanced hepatocellular carcinoma - we have won a battle not the war. J Hepatol 2008;49:871-3.
39. Keating GM, Santoro A. Sorafenib: a review of its use in advanced hepatocellular carcinoma. Drugs 2009;69:223-40.
40. Ping L. Sorafenib plus capecitabine for patients with advanced hepatocellular carcinoma. Available from: https://www.semanticscholar.org/paper/Sorafenib-plus-Capecitabine-for-Patients-with-Ping/7ac9d458ad5c48b9f13dbff60eb7b2f45084854c?utm_source=direct_link [Last accessed on 27 Oct 2023].
41. Bruix J, Qin S, Merle P, et al. RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56-66.
42. Zhi Y, Abudoureyimu M, Zhou H, et al. FOXM1-mediated LINC-ROR regulates the proliferation and sensitivity to sorafenib in hepatocellular carcinoma. Mol Ther Nucleic Acids 2019;16:576-88.
43. Zhang Y, Zheng J. Functions of immune checkpoint molecules beyond immune evasion. In: Xu J, editor. Regulation of cancer immune checkpoints. Singapore: Springer; 2020. pp. 201-26.
44. Llovet JM, Castet F, Heikenwalder M, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol 2022;19:151-72.
45. Sangro B, Sarobe P, Hervás-stubbs S, Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2021;18:525-43.
46. Wang Z, Wang Y, Gao P, Ding J. Immune checkpoint inhibitor resistance in hepatocellular carcinoma. Cancer Lett 2023;555:216038.
47. Chen G, Huang AC, Zhang W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018;560:382-6.
48. Poggio M, Hu T, Pai CC, et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell 2019;177:414-27.e13.
49. Yang Y, Li CW, Chan LC, et al. Exosomal PD-L1 harbors active defense function to suppress T cell killing of breast cancer cells and promote tumor growth. Cell Res 2018;28:862-4.
50. Ye L, Zhang Q, Cheng Y, et al. Tumor-derived exosomal HMGB1 fosters hepatocellular carcinoma immune evasion by promoting TIM-1+ regulatory B cell expansion. J Immunother Cancer 2018;6:145.
51. Wang X, Shen H, Zhangyuan G, et al. 14-3-3ζ delivered by hepatocellular carcinoma-derived exosomes impaired anti-tumor function of tumor-infiltrating T lymphocytes. Cell Death Dis 2018;9:159.
52. Pu J, Xu Z, Nian J, et al. M2 macrophage-derived extracellular vesicles facilitate CD8+T cell exhaustion in hepatocellular carcinoma via the miR-21-5p/YOD1/YAP/β-catenin pathway. Cell Death Discov 2021;7:182.
53. Xue D, Han J, Liang Z, et al. Current perspectives on the unique roles of exosomes in drug resistance of hepatocellular carcinoma. J Hepatocell Carcinoma 2022;9:99-112.
54. El-Awady R, Saleh E, Hashim A, et al. The Role of eukaryotic and prokaryotic ABC transporter family in failure of chemotherapy. Front Pharmacol 2016;7:535.
55. Chen Z, Shi T, Zhang L, et al. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: a review of the past decade. Cancer Lett 2016;370:153-64.
56. Bebawy M, Combes V, Lee E, et al. Membrane microparticles mediate transfer of P-glycoprotein to drug sensitive cancer cells. Leukemia 2009;23:1643-9.
57. Levchenko A, Mehta BM, Niu X, et al. Intercellular transfer of P-glycoprotein mediates acquired multidrug resistance in tumor cells. Proc Natl Acad Sci U S A 2005;102:1933-8.
58. Sousa D, Lima RT, Vasconcelos MH. Intercellular transfer of cancer drug resistance traits by extracellular vesicles. Trends Mol Med 2015;21:595-608.
61. Wu Y, Song Y, Wang R, Wang T. Molecular mechanisms of tumor resistance to radiotherapy. Mol Cancer 2023;22:96.
62. Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer 2015;15:409-25.
63. Fan Q, Yu Y, Zhou Y, Zhang S, Wu C. An emerging role of radiation‑induced exosomes in hepatocellular carcinoma progression and radioresistance (Review). Int J Oncol 2022;60:46.
64. He D, Zhao Z, Fu B, et al. Exosomes participate in the radiotherapy resistance of cancers. Radiat Res 2022;197:559-65.
65. Ni J, Bucci J, Malouf D, Knox M, Graham P, Li Y. Exosomes in cancer radioresistance. Front Oncol 2019;9:869.
66. Ohri N, Dawson LA, Krishnan S, et al. Radiotherapy for hepatocellular carcinoma: new indications and directions for future study. J Natl Cancer Inst 2016;108:djw133.
67. Arnold CR, Mangesius J, Skvortsova II, Ganswindt U. The role of cancer stem cells in radiation resistance. Front Oncol 2020;10:164.
68. Pu X, Ma S, Gao Y, Xu T, Chang P, Dong L. Mesenchymal stem cell-derived exosomes: biological function and their therapeutic potential in radiation damage. Cells 2020;10:42.
69. Yang Z, Zhong W, Yang L, Wen P, Luo Y, Wu C. The emerging role of exosomes in radiotherapy. Cell Commun Signal 2022;20:171.
70. Fang X, Yan Q, Liu S, Guan XY. Cancer stem cells in hepatocellular carcinoma: intrinsic and extrinsic molecular mechanisms in stemness regulation. Int J Mol Sci 2022;23:12327.
71. Lee TK, Guan XY, Ma S. Cancer stem cells in hepatocellular carcinoma - from origin to clinical implications. Nat Rev Gastroenterol Hepatol 2022;19:26-44.
72. Ma S, Lee TK, Zheng BJ, Chan KW, Guan XY. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene 2008;27:1749-58.
73. Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol 2019;20:69-84.
74. Syn N, Wang L, Sethi G, Thiery JP, Goh BC. Exosome-mediated metastasis: from epithelial-mesenchymal transition to escape from immunosurveillance. Trends Pharmacol Sci 2016;37:606-17.
75. Chen L, Guo P, He Y, et al. HCC-derived exosomes elicit HCC progression and recurrence by epithelial-mesenchymal transition through MAPK/ERK signalling pathway. Cell Death Dis 2018;9:513.
76. Karaosmanoğlu O, Banerjee S, Sivas H. Identification of biomarkers associated with partial epithelial to mesenchymal transition in the secretome of slug over-expressing hepatocellular carcinoma cells. Cell Oncol 2018;41:439-53.
77. Shi CJ, Lv MY, Deng LQ, Zeng WQ, Fu WM, Zhang JF. Linc-ROR drive adriamycin resistance by targeting AP-2α/Wnt/β-catenin axis in hepatocellular carcinoma. Cell Biol Toxicol 2023;39:1735-52.
78. Lin Q, Zhou CR, Bai MJ, et al. Exosome-mediated miRNA delivery promotes liver cancer EMT and metastasis. Am J Transl Res 2020;12:1080-95.
79. Sun H, Wang C, Hu B, et al. Exosomal S100A4 derived from highly metastatic hepatocellular carcinoma cells promotes metastasis by activating STAT3. Signal Transduct Target Ther 2021;6:187.
80. He R, Wang Z, Shi W, et al. Exosomes in hepatocellular carcinoma microenvironment and their potential clinical application value. Biomed Pharmacother 2021;138:111529.
81. Wu Y, Zhang J, Li Q. Autophagy, an accomplice or antagonist of drug resistance in HCC? Cell Death Dis 2021;12:266.
82. Liu L, Liao JZ, He XX, Li PY. The role of autophagy in hepatocellular carcinoma: friend or foe. Oncotarget 2017;8:57707-22.
83. Huang F, Wang BR, Wang YG. Role of autophagy in tumorigenesis, metastasis, targeted therapy and drug resistance of hepatocellular carcinoma. World J Gastroenterol 2018;24:4643-51.
84. Shi YH, Ding ZB, Zhou J, et al. Targeting autophagy enhances sorafenib lethality for hepatocellular carcinoma via ER stress-related apoptosis. Autophagy 2011;7:1159-72.
85. Zhang K, Chen J, Zhou H, et al. PU.1/microRNA-142-3p targets ATG5/ATG16L1 to inactivate autophagy and sensitize hepatocellular carcinoma cells to sorafenib. Cell Death Dis 2018;9:312.
86. Guo XL, Li D, Sun K, et al. Inhibition of autophagy enhances anticancer effects of bevacizumab in hepatocarcinoma. J Mol Med 2013;91:473-83.
87. Liu DX, Li PP, Guo JP, et al. Exosomes derived from HBV-associated liver cancer promote chemoresistance by upregulating chaperone-mediated autophagy. Oncol Lett 2019;17:323-31.
88. Tian XP, Wang CY, Jin XH, et al. Acidic microenvironment up-regulates exosomal mir-21 and mir-10b in early-stage hepatocellular carcinoma to promote cancer cell proliferation and metastasis. Theranostics 2019;9:1965-79.
89. Yao M, Liang S, Cheng B. Role of exosomes in hepatocellular carcinoma and the regulation of traditional Chinese medicine. Front Pharmacol 2023;14:1110922.
90. Huang M, Huang X, Huang N. Exosomal circGSE1 promotes immune escape of hepatocellular carcinoma by inducing the expansion of regulatory T cells. Cancer Sci 2022;113:1968-83.
91. Li C, Xu X. Biological functions and clinical applications of exosomal non-coding RNAs in hepatocellular carcinoma. Cell Mol Life Sci 2019;76:4203-19.
92. Yao Z, Jia C, Tai Y, et al. Serum exosomal long noncoding RNAs lnc-FAM72D-3 and lnc-EPC1-4 as diagnostic biomarkers for hepatocellular carcinoma. Aging 2020;12:11843-63.
93. Ji W, Bai J, Ke Y. Exosomal ZFPM2-AS1 contributes to tumorigenesis, metastasis, stemness, macrophage polarization, and infiltration in hepatocellular carcinoma through PKM mediated glycolysis. Environ Toxicol 2023;38:1332-46.
94. Hoshino A, Kim HS, Bojmar L, et al. Extracellular vesicle and particle biomarkers define multiple human cancers. Cell 2020;182:1044-61.e18.
95. Li R, Dong C, Jiang K, et al. Rab27B enhances drug resistance in hepatocellular carcinoma by promoting exosome-mediated drug efflux. Carcinogenesis 2020;41:1583-91.
96. Zhang H, Lu J, Liu J, Zhang G, Lu A. Advances in the discovery of exosome inhibitors in cancer. J Enzyme Inhib Med Chem 2020;35:1322-30.
97. Kim JH, Lee CH, Baek MC. Dissecting exosome inhibitors: therapeutic insights into small-molecule chemicals against cancer. Exp Mol Med 2022;54:1833-43.
98. Xu R, Rai A, Chen M, Suwakulsiri W, Greening DW, Simpson RJ. Extracellular vesicles in cancer - implications for future improvements in cancer care. Nat Rev Clin Oncol 2018;15:617-38.
99. Meng W, He C, Hao Y, Wang L, Li L, Zhu G. Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug Deliv 2020;27:585-98.
100. Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol 2021;16:748-59.
102. Lou G, Chen L, Xia C, et al. MiR-199a-modified exosomes from adipose tissue-derived mesenchymal stem cells improve hepatocellular carcinoma chemosensitivity through mTOR pathway. J Exp Clin Cancer Res 2020;39:4.
103. Li H, Yang C, Shi Y, Zhao L. Exosomes derived from siRNA against GRP78 modified bone-marrow-derived mesenchymal stem cells suppress Sorafenib resistance in hepatocellular carcinoma. J Nanobiotechnol 2018;16:103.
104. Deng J, Ke H. Overcoming the resistance of hepatocellular carcinoma to PD-1/PD-L1 inhibitor and the resultant immunosuppression by CD38 siRNA-loaded extracellular vesicles. Oncoimmunology 2023;12:2152635.
105. He C, Jaffar Ali D, Qi Y, et al. Engineered extracellular vesicles mediated CRISPR-induced deficiency of IQGAP1/FOXM1 reverses sorafenib resistance in HCC by suppressing cancer stem cells. J Nanobiotechnol 2023;21:154.
106. Kim MS, Haney MJ, Zhao Y, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine 2016;12:655-64.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Wang J, Yang Y, Lu J, Wang X. The role of exosomes in therapeutic resistance of hepatocellular carcinoma. Hepatoma Res 2023;9:46. http://dx.doi.org/10.20517/2394-5079.2023.85
AMA Style
Wang J, Yang Y, Lu J, Wang X. The role of exosomes in therapeutic resistance of hepatocellular carcinoma. Hepatoma Research. 2023; 9: 46. http://dx.doi.org/10.20517/2394-5079.2023.85
Chicago/Turabian Style
Wang, Jin, Yali Yang, Jingyi Lu, Xia Wang. 2023. "The role of exosomes in therapeutic resistance of hepatocellular carcinoma" Hepatoma Research. 9: 46. http://dx.doi.org/10.20517/2394-5079.2023.85
ACS Style
Wang, J.; Yang Y.; Lu J.; Wang X. The role of exosomes in therapeutic resistance of hepatocellular carcinoma. Hepatoma. Res. 2023, 9, 46. http://dx.doi.org/10.20517/2394-5079.2023.85
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 5 clicks
Cite This Article 5 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.