Treatment of intrahepatic cholangiocarcinoma: evidence for the role of percutaneous ablation
Abstract
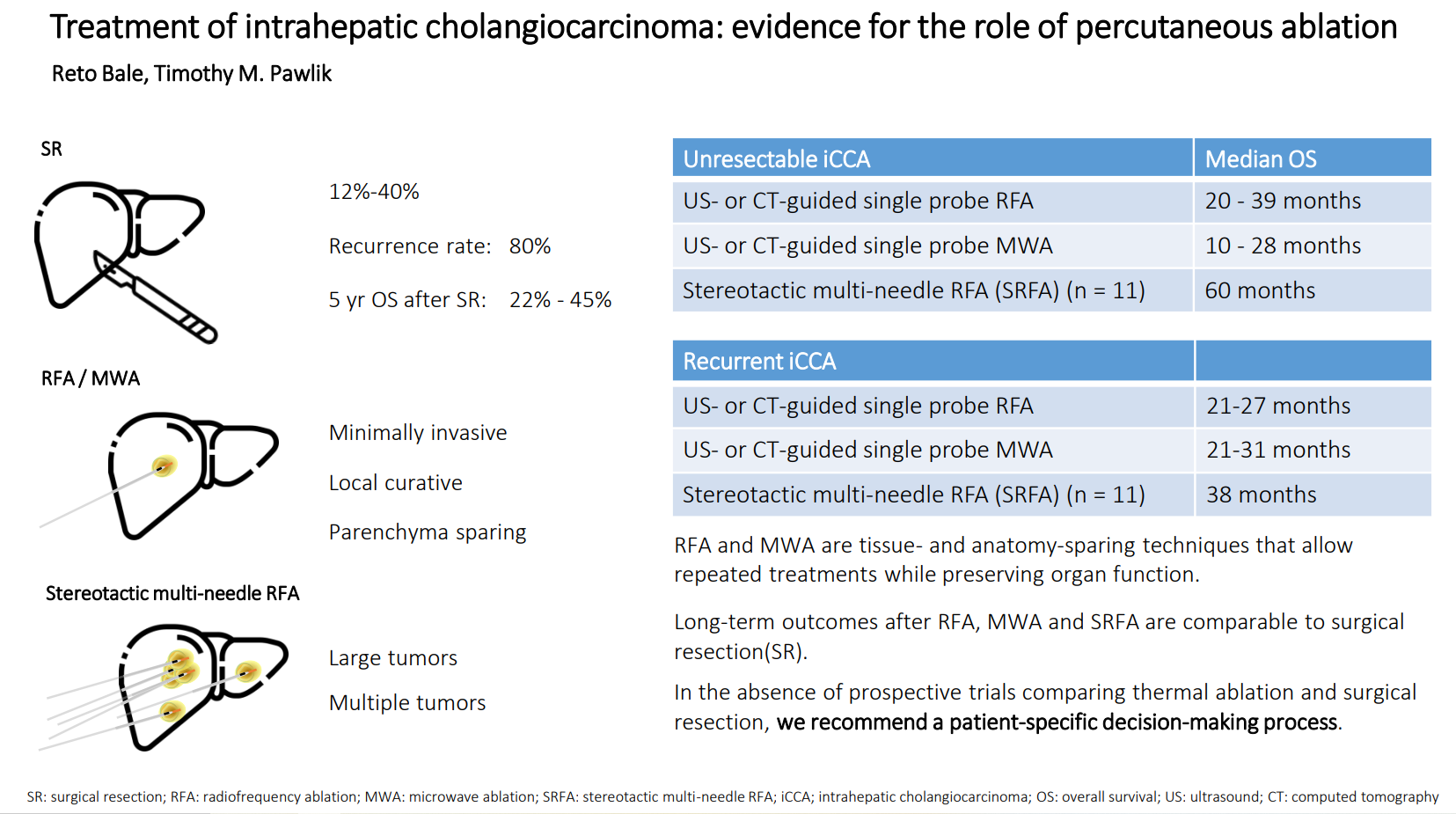
Intrahepatic cholangiocarcinoma (iCCA) is a rare cancer with generally poor prognosis. In this narrative review, we examine the role of thermal ablation and summarize the current literature. Radiofrequency ablation (RFA) and microwave ablation (MWA) are both safe and well-tolerated as a minimally invasive local curative treatment option for patients suffering from primary and secondary liver tumors. Both methods can be used in patients with medical morbidities that would preclude surgery, as well as individuals with anatomical or functional constraints that impede liver resection. In unresectable iCCA, the median OS after conventional percutaneous US- or CT-guided RFA and MWA is between 20 and 39 months and 10 and 28 months, respectively. In recurrent iCCA, percutaneous RFA and MWA achieved a median OS of 21-27 months and 21-31 months, respectively. These data are comparable to long-term outcomes after surgical resection (SR), with the number of nodules and tumor size affecting prognosis. Stereotactic radiofrequency ablation (SRFA) allows for effective treatment of large and multiple iCCA nodules within one session and achieves short- and long-term results in inoperable patients compared with resection. With the addition of SRFA as an alternative treatment option, the proportion of patients who can be treated with curative treatment has significantly increased. In the absence of prospective trials comparing thermal ablation and surgical resection, we recommend a patient-specific decision-making process. Future research to identify technical and clinical prognostic criteria, as well as molecular markers of tumor biology, may help select patients for ablation and subsequent outcomes.
Keywords
INTRODUCTION
Incidence of iCCA
Intrahepatic cholangiocarcinoma (iCCA) is the second most common primary liver cancer with a worldwide increase in incidence and mortality. Due to its late diagnosis, aggressive biology, and resistance to therapy, iCCA is generally associated with a poor 5-year OS of less than 10%[1].
Histopathology and risk factors for iCCA
The large and small duct types of iCCA exhibit different clinicopathological characteristics and mutation profiles[2]. Small duct iCCA is frequently associated with non-biliary chronic liver illnesses, such as viral hepatitis and metabolic syndrome. The large duct iCCA type occurs in chronic cholangitis and is characterized by more severe pathological symptoms like lymphatic and/or perineural invasion, and a worse long-term prognosis[3]. Primary sclerosing cholangitis (PSC) is the most important known risk factor for iCCA, which is an important cause of death among PSC patients[4]. Annually, approximately 0.6%-1.5% of PSC patients develop iCCA, with a prevalence of 6 to 13% and a lifetime risk of up to 20%[5].
Diagnosis of iCCA
According to the recent EASL guidelines[6], a tumor biopsy is recommended in order to confirm the iCCA diagnosis, distinguish subtypes, and investigate molecular parameters. In terms of imaging, MRI is superior to CT to stage iCCA within the liver[7]. Distinguishing iCCA from cHCC-iCCA (combined hepatocellular-cholangiocarcinoma), which is characterized by both hepatocytic and cholangiocytic phenotypes, remains a difficult task[8]. Therefore, if there are multiple suspicious nodules with varying imaging characteristics, we recommend biopsies of at least one nodule with each different imaging appearance. PET scanning is recommended to identify metastatic lymph nodes and distant metastases. PET scan has a sensitivity and specificity of 37% and 97%, respectively, to detect lymph node metastasis[7]. In addition, endoscopy-guided lymph node sampling may be helpful to inform treatment decisions[9].
Treatment options for iCCA
Surgical resection
According to recent guidelines[10-12] surgical resection (SR) with negative margins (R0) remains the first-line treatment for iCCA. However, only 12%-40% of patients who are referred for treatment are resectable[13,14]. Reported overall survival (OS) at 5 years ranges from 22% to 45%, and recurrence rates are as high as 80%[15]. Due to the early and high incidence of tumor recurrence in the postoperative setting, multifocal iCCA portends a poorer prognosis versus solitary lesions[6]. Recently, liver transplantation (LT) was proposed as an alternative to SR in cirrhotic patients with small iCCA. In a retrospective multicenter study[16], 49 LT and 26 SR patients with iCCA/cHCC-CCA ≤ 5 cm in cirrhosis were compared. LT patients had a markedly lower incidence of recurrence (18% vs. 46%; P = 0.01). Among patients in the LT group, 5-year survival was 69% and 65% (P = 0.40) among patients with lesions ≤ 2 cm and > 2-5 cm, respectively. In another retrospective analysis, 1-, 3-, and 5-year actuarial survival of 82%, 61%, and 61% were achieved in six patients with single tumors up to 3 cm[17]. According to the most recent EASL guidelines, early-stage iCCA ( ≤ 3 cm) arising in the context of cirrhosis is eligible for liver transplantation, preferably under study protocols[6].
Systemic treatment
In two randomized controlled trials, the combination of gemcitabine and cisplatin (GemCis) was associated with a median OS of 11.6 months[18]. GemCis is currently the standard first-line treatment for patients with advanced iCCA. The addition of durvalumab to GemCis increased the median OS to 12.8 months among patients with good performance status[19]. Immune checkpoint inhibitors and molecularly targeted therapies, which may have the potential to revolutionize systemic treatment of iCCA, are being tested in multiple clinical trials[20].
Locoregional treatment
Locoregional treatments (LRTs) include hepatic arterial infusion of chemotherapy (HAI)[21], transarterial chemoembolization (TACE)[22], radioembolization (TARE)[23,24], and radiation therapy (RT)[25].
Reported median OS and median PFS after HAI ranged from 10.1-31.1 months and 5-11.8 months, respectively[26]. One recent cohort study[27]reported that HAI floxuridine chemotherapy achieved similar OS in patients with multifocal iCCA compared with RX. In particular, 5-year OS among patients with 4 or more lesions was 5.0% (95%CI: 1.7%-14.3%) in the HAIP group compared with 6.8% (95%CI: 1.8%-25.3%) in the RX group. In turn, treatment of multifocal iCCA with RX should be considered with care as the risk of complications with major liver resection can be high. Moreover, evidence for the treatment of iCCA with LRT is scarce. The reported median OS for TACE, TARE, and RT ranged from 6-30 months, 5.7-33.6 months, and 7-39.5 months, respectively[26]. However, despite a low level of evidence and a wide variability in outcomes, these treatments are safe and feasible and can be reasonable alternatives or adjuncts to systemic therapy for some patients with unresectable disease.
Thermal ablation is regarded as a potentially local curative treatment option. In this narrative review, we summarized the most relevant articles related to conventional, US- or CT-guided, percutaneous thermal ablation for iCCA that were published between 2000 and 2023. In addition, we highlight studies reporting the application of sophisticated stereotactic planning and guidance techniques.
PERCUTANEOUS ABLATION
Techniques
Radiofrequency ablation (RFA) and microwave ablation (MWA) are minimally invasive procedures for the local curative treatment of liver tumors. The objective is to obliterate the entire tumor, including a margin of safety that is 0.5-1 cm[28-31]; the needle tract should also be cauterized during probe removal to avoid tumor seeding. The principle of RFA is based on high-frequency alternating electrical current that is emitted by the tip of the RFA electrode, which causes frictional heat in the surrounding tissue[32]. Cell death is achieved at temperatures of 60-100 °C. Microwave ablation (MWA) is considered a valid alternative to RFA. Direct heating in the tissue volume around the antenna is induced by an oscillating electromagnetic field. Compared with RFA, MWA is less susceptible to the heat sink effect, and larger and more predictable ablation zones can be achieved in a shorter time[33]. Interventional radiologists typically perform RFA and MWA percutaneously under computed tomography (CT)[31] or ultrasound (US) guidance[28]. Additionally, thermal ablation can be performed during open or laparoscopic liver surgery[34].
Exclusion criteria and complications
In most studies, exclusion criteria for conventional single probe RFA and MWA include severe coagulopathy, severe thrombocytopenia, vascular invasion, large tumor size (> 3 or > 5 cm), multiple hepatic lesions (> 3-5), progressive extrahepatic metastases, or poor performance status[28,29,31,34,35].
Both RFA and MWA are well tolerated. Post-ablation syndrome is common and characterized by fever and flu-like symptoms. Major complications after thermal ablation of iCCA include bleeding, liver abscess, biloma, biliary stricture, hepatic failure, pleural effusion, ascites, and tumor seeding. Minor complications include elevated liver function tests, thrombocytopenia, portal vein thrombosis, asymptomatic pleural effusions, or hematomas.
Follow-up after thermal ablation
Patient follow-up usually includes an early treatment response assessment by contrast-enhanced CT scan or magnetic resonance imaging (MRI) performed within 1 month after treatment, followed by imaging at 3-6 month intervals[31-37]. Assessment at one month after the intervention defines technical effectiveness. When no suspicious contrast enhancement is observed in the periphery or the ablated area, then tumor necrosis is deemed complete. Nodular intralesional or peripheral contrast enhancement on CT or MRI imaging and/or an increase in tumor size define recurrence[31-37].
Outcomes after conventional US- and CT- guided thermal ablation [Table 1]
Radiofrequency ablation
In 2002, Slakey[38] reported the first successful use of RFA in a patient with recurrent iCCA after SR.
The authors reported a LTPFS of 9.3 months and a median OS of 27.5 months. The major complication rate was 7%. Butros et al. treated seven patients with nine iCCAs with percutaneous US-guided RFA. Local tumor control was achieved in 8/9 tumors[43]. The mean OS was 39 months (range: 12-69 months).
Outcomes of conventional US- and CT-guided percutaneous thermal ablation of iCCA
| Author (Year) | Technique | n | Diagnosis | Tumor size (cm) Median/Range; Mean/SD | Median OS | OS 1 yr | OS 3 yr | OS 5 yr | Major Complications | LTPFS / PFS / LR |
| RFA | ||||||||||
| Chu et al. (2021)[39] | percutaneous US-guided RFA | 40 | Recurrent iCCA | 1.5 (0.6-4.4) | 27 mo | 67% | 36% | 18% | 4.7% | N/A |
| Kim et al. (2011)[40] | percutaneous US-guided RFA | 20 | Recurrent iCCA | 1.5 (0.7-4.4) | 27 mo | 70% | 60% (2 yr) | 21% (4yr) | 7% | median LTPFS 40 mo |
| Kim et al. (2011)[41] | percutaneous US-guided RFA | 13 | Unresectable iCCA (Primary) | 3.2 (0.9-8) | 39 mo | 85% | 51% | 15% | 6% | median LTPFS 32 mo |
| Carrafiello et al. (2010)[42] | percutaneous US-guided RFA | 6 | Unresectable iCCA (Primary) | 3.5 (1-5.8) | 20 mo | N/A | N/A | N/A | 0% | N/A |
| Brandi et al. (2020)[28] | percutaneous US-guided RFA | 29 | Unresectable iCCA (Primary) | 1.8 (0.5-4.8) | 28 mo | 89% | 45% (2 yr) | 11% (4yr) | 7% | median LTPFS 9 mo |
| Butros et al. (2014)[43] | percutaneous US/CT-guided RFA | 7 | Unresectable and recurrent iCCA | 2.4 (1.3-3.3) | 39 mo | 85% | 100% | 60% | 20% | median LTPFS 36 mo |
| MWA | ||||||||||
| Yu et al. (2011)[44] | percutaneous US-guided MWA | 15 | Unresectable iCCA (Primary) | 3.2 ± 1.9 (1.3-9.9 ) | 10 mo | 60% | 60% (2 yr) | N/A | 20% | N/A |
| Zhang et al. (2018)[48] | percutaneous US-guided MWA | 107 | Unresectable/ Recurrent iCCA | < 5 | 28 mo | 94% | 40% | 8% | 2.8% | median LTPFS 9 mo |
| Ni et al. (2019)[46] | percutaneous US-guided MWA | 78 | Unresectable iCCA (Primary) | 3.1 ± 0.7 (0.8-50) | N/A | 90% | 52% | 35% | 3.8% | LTPFS 1,3,5 yr: 79%,20%,0% |
| Yang et al. (2021)[47] | percutaneous US-guided MWA | 55 | Unresectable iCCA (Primary) | 3.1 ± 0.7 (0.8-5.0) | N/A | 87% | 51% | 35% | 3.8% | LTPFS 1,3,5 yr: 69%,57%,57% |
| Wang et al. (2022)[45] | percutaneous US-guided MWA | 29 | Unresectable iCCA (Primary) | 0.5-8.1 cm | 18 mo | N/A | N/A | N/A | N/A | median LTPFS 18 mo |
| MWA vs. RFA | ||||||||||
| Giorgio et al. (2019)[49] | percutaneous US-guided MWA | 35 | Unresectable iCCA (Primary) | 3.6 (2.2-7.2 ) | N/A | 95% | 75% | 68% | 0% | PFS 1,3,5 yr: 79%, 59%, 55% |
| percutaneous US-guided RFA | 36 | Unresectable iCCA (Primary) | 3.1 (2-8 ) | N/A | 86% | 53% | 26% | 0% | PFS 1,3,5 yr: 9%, 51%, 8.5% | |
| Ablation vs. HR | ||||||||||
| Xu et al. (2019)[51] | percutaneous US-guided MWA | 56 | Recurrent iCCA | 2.7 ± 0.5 (0.8-5.0) | 31 mo | 81% | 42% | 24% | 5.3% | N/A |
| SR | 65 | Recurrent iCCA | 2.8 ± 0.4 (1.0-5.0) | 29 mo | 77% | 36% | 22% | 13.8% | N/A | |
| Zhang et al. (2013) [50] | percutaneous US-guided MWA/ RFA | 77 | Recurrent iCCA | > 3 (44%) max 5 | 21 mo | 70% | 21% | 0% | 3.9% | N/A |
| HR | 32 | Recurrent iCCA | > 3 (32%) max 6.7 | 20 mo | 84% | 17% | 0% | 46.9% | N/A | |
| Xiang et al. (2020) [29] | percutaneous US-guided RFA | 34 | Resectable iCCA (Primary) | 3.2 (2.5-4) | 39 mo | 90% | 42% | 24% | N/A | N/A |
| HR | 150 | Resectable iCCA (Primary) | 3.5 (2.5-4.2) | 38 mo | 87% | 73% | 62% | N/A | N/A |
Microwave ablation
In an early series, Yu et al. treated 15 patients with 24 iCCA lesions (mean tumor size, 3.2 ± 1.9 cm) with ultrasound-guided MWA in 38 sessions[44]. Major complications occurred in three patients, including two liver abscesses (13.3%) and one needle seeding (6.7%). During a mean follow-up of 12.8 months, 6/24 lesions (25%) demonstrated local tumor progression. The cumulative 6-, 12-, and 24-months OS were 78.8%, 60.0%, and 60.0%, respectively. Wang et al. treated 29 patients with 58 iCCAs (mean diameter: 2.7 cm) with US-guided MWA[45]. With 18 months of median follow-up, cumulative 1- and 3-year OS was 64.4% and 48.1%, respectively. Postoperative extrahepatic metastasis was associated with worse long-term survival (P = 0.006).
Ni et al. reported that ALBI grade predicted long-term outcomes of CT-guided MWA of iCCAs in 78 patients[46]. With 23 months of median follow-up, cumulative 1-, 3-, and 5-year OS was 89.5%, 52.2%, and 35.0%, respectively. OS was higher among patients with ALBI grade 1 compared with patients who had ALBI grade 2 (P < 0.001). In a similar study, Yang et al. reported 52 patients with 74 iCCA lesions who underwent MWA[47]. The incidence of major complications was 3.8% and the 1-, 3-, and 5-year OS was 87.4%, 51.4%, and 35.2%, respectively. Older age (P = 0.002), tumor size > 3 cm (P = 0.021), and albumin bilirubin (ALBI) grade (P = 0.004) were negative predictors of OS.
Zhang et al. treated 107 patients with 171 iCCAs with a maximum size of 5 cm and a maximum tumor number of three with US-guided MWA[48]. The reported incidence of major complications was 2.8%. With 20 months of median follow-up, median PFS and OS were 9 months and 28 months, respectively. Corresponding OS after 1, 3, and 5 years was 93.5%, 39.6%, and 7.9%, respectively.
Study comparing radiofrequency ablation and microwave ablation
Giorgio et al. compared the long-term results of percutaneous US-guided RFA (n = 36) and US-guided MWA (n = 35) in 71 iCCA patients with a total of 98 nodules in an Italian retrospective multicenter study[49]. No major complications occurred. OS among the entire series at 1-, 3-, and 5-year was 88%, 65%, and 45%, respectively. OS, disease-free survival (DFS), and PFS were superior in the MWA group
Studies comparing thermal ablation with surgical resection
Xiang et al. utilized the Surveillance, Epidemiology, and End Results (SEER) database and compared the outcomes of RFA and SR for 184 patients with small (≤ 5 cm) T1 stage primary iCCA[29]. RFA was associated with significantly worse 1-, 3-, and 5-year OS compared with SR (P < 0.001). The OS associated with the RFA group at 1-, 3-, and 5-years was 89.9, 42.4, and 23.9%, and 87.4, 73.3, and 61.5%, respectively, for the SR group.
Zhang et al. compared the effectiveness, safety, and outcome of repeated SR
In a similar study, Xu et al. noted that the OS (P = 0.405) and RFS (P = 0.589) of patients with recurrent iCCA were similar after RS in 65 patients and US-guided MWA in 56 patients[51]. 5-year OS was 22% after SR and 24% after MWA, and 3-year RFS was 31 % after SR and 33% after MWA. The incidence of major complications was lower in the MWA group than in the SR group (P < 0.001). Tumor number (P = 0.012), ALBI grade (P = 0.007), and metastasis (P = 0.016) were independent predictors of OS rate.
FUTURE PERSPECTIVE
Local recurrence is associated with a worse long-term outcome. Tumor size is considered the most relevant factor associated with obtaining complete ablation in primary and secondary liver tumors, including iCCA[41,42,50,52]. The discrepancy between tumor geometry and geometry of the necrosis induced by RFA accounts for incomplete ablations. After thermal ablation of hepatocellular carcinoma (HCC), a minimal ablative margin (MAM) of at least 5 mm should be attained to prevent local tumor growth[53]. For colorectal liver metastases (CRLM), a safety margin of at least 1 cm has been proposed[54]. A single ablation probe position can ablate only a limited volume, regardless of the specific ablation technology. Percutaneous thermal segmentectomy, which combines balloon-occluded single probe MWA and then balloon-occluded TACE, seems to be a promising approach for the treatment of large tumors (> 2-3 cm)[55]. Alternately, ideal coverage of the tumor including a MAM must be achieved by overlapping ablation zones. To achieve reliable results, careful three-dimensional placement planning and a method for precise execution of the planning are required. Conventional US- or CT-guided punctures might not be able to meet this requirement.
Stereotactic thermal ablation
Neurosurgeons have utilized stereotaxy for years to extract tumors and conduct biopsies. It employs a 3D coordinate system that enables precise insertion of instruments within patients[56,57]. Frame-based stereotaxy includes screwing a frame to the patient's skull and employing computer-aided technology to calculate instrument trajectories and distances in a Cartesian coordinate system. Invasive fixation and surgical access restrictions limit this method[56]. Frameless stereotactic three-dimensional navigation devices have addressed these issues. These technologies, now standard in neurosurgery operating rooms, allow surgeons to identify spots within the patient using a real-time 3D coordinate CT or MR system[58]. Modern navigation systems are used in many different clinical settings, including the liver. Using a Cartesian coordinate system, the software allows for the planning of the needle trajectory. Adjusting the aiming device in accordance with the virtual pre- or intraoperative plan enables the puncture of almost any part of the body through the skin[59]. Robot-assisted navigation systems have the advantage of providing semi-automatic adjustments compared with passive navigation systems that necessitate manual aiming[60-63].
Single-probe stereotactic MWA
Stereotactic systems have been utilized in conjunction with MWA (SMWA). Kim-Fuchs et al. used single-probe SMWA to treat 10 patients with 5 primary and 6 recurrent iCCA lesions (mean tumor size: 2.1 cm) and demonstrated that it is safe, with short hospital stays and a low complication rate[64]. The reported local recurrence rate was 27% (3/11).
Multi-needle stereotactic RFA
Three-dimensional planning and precise needle placement is facilitated by stereotactic methods that enhance the efficacy of multi-needle RFA[65], which can define overlapping ablation areas[56]. Using a multi-needle coaxial technique, multiple lesions[65] can be targeted in a single session. Moreover, coaxial needles allow for tumor biopsies right before ablation. As the needles are positioned prior to the start of the ablation[66], this technique facilitates the customization of the ablation site to a virtually arbitrary size[67]. Decreasing the distance between needles close to vessels can decrease the heat sink effect[68].
Outcomes after multi-needle thermal ablation with stereotactic guidance [Table 2]
Local recurrence and OS after multi-needle SRFA for different types of primary[69-71] and secondary liver tumors[72-75] have been reported to be similar to those after surgery, even when the tumor was large and close to major vessels[68]. The first study published in 2011[31] reported outcomes after multi-needle SRFA of 18 primary iCCAs and 16 recurrent ICCAs in 11 consecutive patients. Despite a median lesion diameter of 3.0 cm (range: 0.5 to 10 cm) only three local recurrences (8%) were observed after a mean follow-up time of 35 months. Three major complications (13%) were noted and treated by the interventional radiologist. The resulting 1-year and 3-year OS and median OS (Kaplan Meier) were 91% and 71%, and 60 months, respectively. The 1- and 3-year DFS rates were 62 and 22%, respectively, with a median DFS time of 24.3 months.
Outcomes of stereotactic CT-guided percutaneous thermal ablation of iCCA
| Author (Year) | Technique | n | Diagnosis | Tumor size (cm) Median/Range; Mean/SD | Median OS | OS 1 yr | OS3 yr | OS 5 yr | Major Complications | LR |
| Stereotactic RFA | ||||||||||
| Haidu et al. (2012)[31] | Multi-needle SRFA | 11 | Unresectable/Recurrent iCCA | 3 (0.5-10) | 60 mo | 91% | 71% | N/A | 13% | LR SRFA: 3/36 (8%) |
| Kim-Fuchs et al. (2021)[64] | Single-probe SMWA | 10 | Unresectable/ Recurrent iCCA | 2 (0.6-3.2) | N/A | N/A | N/A | N/A | 10% | LR SMWA: 3/11 (27%) |
| SRFA / HR vs. CTX | ||||||||||
| Braunwarth et al. (2022)[77] | SRFA (n = 11)/ HR (n = 5) | 16 | Recurrent iCCA | N/A | 38 mo | 88% | 57% | 49% | LR SRFA: 1/11 (9%), LR HR: 1/5 (20%) | |
| Palliative treatment | 27 | Recurrent iCCA | N/A | 17 mo | 65% | 17% | 0% |
Patient morbidity or anatomical or functional limitations can preclude repeated hepatic resection. Thermal ablation is a tissue- and anatomy-sparing technique that allows repeated treatments while preserving organ function. In 2010, we described a case of a 72-year-old male patient with a 13-centimeter-diameter, unresectable iCCA with intrahepatic metastases. Initially, the patient underwent three SRFA sessions[66]. The same patient received seven additional ablation sessions over nine years for ten recurrent intrahepatic lesions in all eight Coinaud segments[76]. Hospitalization periods were short, and procedure-related discomfort was mild. The patient's liver function remained within the physiological range in spite of multiple sessions one year after the last SRFA.
Another recent study[77] from our group demonstrated that the application of multi-needle SRFA in recurrent iCCA significantly increases the number of patients that can be re-treated with curative intent. The efficacy, safety, and outcome of local versus palliative treatment for recurrent iCCA after SR were compared in a total of 43 consecutive patients. Five patients underwent hepatic resection (1-2 sessions), eleven patients underwent SRFA (1-9 sessions) with curative intent, and the other 27 patients had palliative care. Patients who underwent repeated liver-directed therapy had OS similar to patients without recurrence (P = 0.938) and better outcomes than patients who had palliative care (P = 0.018). 5-year OS among patients without recurrence after initial resection versus patients who underwent repeated local curative liver-directed therapy versus individuals who had palliative care were 54.3%, 47.7%, and 12.2%, respectively. The rate of curative re-treatment increased from 11.9% to 37.0% when SRFA was added to SR as another treatment option. Unresectable patients undergoing multi-needle SRFA had fewer local recurrences (1/11, 9%) vs. SR
Despite the consistently good outcomes, stereotactic approaches are still utilized in only a small number of centers worldwide[78]. This is likely due to the requirement for additional investments, such as a 3D navigation system, a dedicated CT, and the availability of general anesthesia. Additionally, the stereotactic approach necessitates the training of a specialized team composed of an interventional oncologist, a radiation technician, and an anesthesiologist.
Combination of thermal ablation with lymph node dissection
The presence of nodal metastasis in iCCA patients is associated with a poor prognosis, with a median survival of < 20 months[79]. Therefore, adequate lymphadenectomy (at least 6) during surgical resection is recommended for accurate staging[80]. However, it is still unclear if the additional morbidity related to lymphadenectomy during SR is justified because the impact on survival remains uncertain[81]. We therefore recommend combining thermal ablation with laparoscopic LND only in cases with highly suspicious lymph nodes on cross-sectional imaging and/or PET scan, as there is no clear evidence of a survival benefit to removing LN in the setting of an ablation.
SUMMARY
Percutaneous image-guided thermal ablation techniques such as RFA and MWA are safe and well-tolerated local curative treatment options. These treatment modalities are associated with a lower risk of complications and a shorter hospital stay compared with resection. In addition, these techniques spare healthy tissue and may also be applied in unresectable patients. The reported median OS after conventional US- or CT-guided RFA and MWA in patients with unresectable or recurrent iCCA ranges from 10-39 months. For the treatment of patients with primary iCCA, retrospective studies indicate that SR is more effective than conventional US- and CT-guided thermal ablation. Among patients with recurrent iCCA after SR, two recent studies reported similar long-term outcomes for thermal ablation and repeated SR, with the risk of severe complications being in favor of thermal ablation. The number of nodules and tumor size are relevant prognostic factors. Technical developments such as stereotaxy, image fusion, and robotics improve the efficacy and outcome of thermal ablation procedures. Multi-needle SRFA with intraprocedural control of the ablation result by means of image fusion allows for effective and reliable treatment of large and multiple iCCA nodules within one session with excellent short- and long-term results that are comparable to resection. With the addition of SRFA as an alternative treatment option for recurrent iCCAs, the rate of curative re-treatment can be increased significantly.
As a result of the rarity of early iCCA, evidence regarding the efficacy of all different local treatment options remains scant. There are no prospective studies comparing thermal ablation and SR for the treatment of primary and recurrent iCCA available. More research, including validation of technical and clinical predictors and a better understanding of the molecular biology of tumors, should help to stratify patients for a combination of local and systemic treatments including promising immunotherapies and targeted therapies[82].
DECLARATIONS
Authors' Contributions
Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Writing-original draft, Writing-review & editing: Bale R
Methodology, Supervision, Validation, Writing-original draft, Writing-review & editing: Pawlik TM
Availability of Data and Materials
Not applicable.
Financial Support and Sponsorship
None.
Conflicts of Interest
The authors declared that there are no conflicts of interest.
Ethical Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Copyright
© The Author(s) 2023.
REFERENCES
1. Beal EW, Tumin D, Moris D, et al. Cohort contributions to trends in the incidence and mortality of intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr 2018;7:270-6.
2. Ma B, Meng H, Tian Y, et al. Distinct clinical and prognostic implication of IDH1/2 mutation and other most frequent mutations in large duct and small duct subtypes of intrahepatic cholangiocarcinoma. BMC Cancer 2020;20:318.
3. Akita M, Fujikura K, Ajiki T, et al. Dichotomy in intrahepatic cholangiocarcinomas based on histologic similarities to hilar cholangiocarcinomas. Mod Pathol 2017;30:986-97.
4. Chapman MH, Thorburn D, Hirschfield GM, et al. British society of gastroenterology and UK-PSC guidelines for the diagnosis and management of primary sclerosing cholangitis. Gut 2019;68:1356-78.
5. Clements O, Eliahoo J, Kim JU, Taylor-Robinson SD, Khan SA. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a systematic review and meta-analysis. J Hepatol 2020;72:95-103.
6. European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver. EASL-ILCA clinical practice guidelines on the management of intrahepatic cholangiocarcinoma. J Hepatol 2023;79:181-208.
7. Kim YY, Yeom SK, Shin H, et al. Clinical staging of mass-forming intrahepatic cholangiocarcinoma: computed tomography versus magnetic resonance imaging. Hepatol Commun 2021;5:2009-18.
8. Kim TH, Kim H, Joo I, Lee JM. Combined hepatocellular-cholangiocarcinoma: changes in the 2019 World Health Organization histological classification system and potential impact on imaging-based diagnosis. Korean J Radiol 2020;21:1115-25.
9. Malikowski T, Levy MJ, Gleeson FC, et al. Endoscopic ultrasound/fine needle aspiration is effective for lymph node staging in patients with cholangiocarcinoma. Hepatology 2020;72:940-8.
10. Fong ZV, Brownlee SA, Qadan M, Tanabe KK. The clinical management of cholangiocarcinoma in the united states and europe: a comprehensive and evidence-based comparison of guidelines. Ann Surg Oncol 2021;28:2660-74.
11. Cholangiocarcinoma Working Group. Italian clinical practice guidelines on cholangiocarcinoma - part ii: treatment. Dig Liver Dis 2020;52:1430-42.
12. Benson AB, D'Angelica MI, Abbott DE, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2021;19:541-65.
13. Amini N, Ejaz A, Spolverato G, Kim Y, Herman JM, Pawlik TM. Temporal trends in liver-directed therapy of patients with intrahepatic cholangiocarcinoma in the United States: a population-based analysis. J Surg Oncol 2014;110:163-70.
14. Mazzaferro V, Gorgen A, Roayaie S, Droz Dit Busset M, Sapisochin G. Liver resection and transplantation for intrahepatic cholangiocarcinoma. J Hepatol 2020;72:364-77.
15. Lamarca A, Ross P, Wasan HS, et al. Advanced intrahepatic cholangiocarcinoma: post Hoc analysis of the ABC-01, -02, and -03 clinical trials. J Natl Cancer Inst 2020;112:200-10.
16. Sha M, Jeong S, Xia Q. Analysis of liver resection versus liver transplantation on outcome of small intrahepatic cholangiocarcinoma and combined hepatocellular-cholangiocarcinoma in the setting of cirrhosis. Liver Transpl 2020;26:1202-3.
17. Sapisochin G, Facciuto M, Rubbia-Brandt L, et al. iCCA International Consortium. Liver transplantation for "very early" intrahepatic cholangiocarcinoma: International retrospective study supporting a prospective assessment. Hepatology 2016;64:1178-88.
18. Valle JW, Furuse J, Jitlal M, et al. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Ann Oncol 2014;25:391-8.
19. Oh DY, Lee KH, Lee DW, et al. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: an open-label, single-centre, phase 2 study. Lancet Gastroenterol Hepatol 2022;7:522-32.
20. Rizzo A, Brandi G. First-line chemotherapy in advanced biliary tract cancer ten years after the ABC-02 trial: "and yet it moves! Cancer Treat Res Commun 2021;27:100335.
21. Cercek A, Boerner T, Tan BR, et al. Assessment of hepatic arterial infusion of floxuridine in combination with systemic gemcitabine and oxaliplatin in patients with unresectable intrahepatic cholangiocarcinoma: a phase 2 clinical trial. JAMA Oncol 2020;6:60-7.
22. Zhou TY, Zhou GH, Zhang YL, et al. Drug-eluting beads transarterial chemoembolization with CalliSpheres microspheres for treatment of unresectable intrahepatic cholangiocarcinoma. J Cancer 2020;11:4534-41.
23. Gupta AN, Gordon AC, Gabr A, et al. Yttrium-90 radioembolization of unresectable intrahepatic cholangiocarcinoma: long-term follow-up for a 136-patient cohort. Cardiovasc Intervent Radiol 2022;45:1117-28.
24. Bargellini I, Mosconi C, Pizzi G, et al. Yttrium-90 Radioembolization in unresectable intrahepatic cholangiocarcinoma: results of a multicenter retrospective study. Cardiovasc Intervent Radiol 2020;43:1305-14.
25. Jonczyk M, Collettini F, Schnapauff D, et al. Cholangiocarcinoma: CT-guided high-dose rate brachytherapy (CT-HDRBT) for limited (<4 cm) and large (>4 cm) tumors. Anticancer Res 2018;38:5843-52.
26. Owen M, Makary MS, Beal EW. Locoregional therapy for intrahepatic cholangiocarcinoma. Cancers 2023;15:2384.
27. Franssen S, Soares KC, Jolissaint JS, et al. Comparison of hepatic arterial infusion pump chemotherapy vs resection for patients with multifocal intrahepatic cholangiocarcinoma. JAMA Surg 2022;157:590-6.
28. Brandi G, Rizzo A, Dall'Olio FG, et al. Percutaneous radiofrequency ablation in intrahepatic cholangiocarcinoma: a retrospective single-center experience. Int J Hyperthermia 2020;37:479-85.
29. Xiang X, Hu D, Jin Z, Liu P, Lin H. Radiofrequency ablation vs. surgical resection for small early-stage primary intrahepatic cholangiocarcinoma. Front Oncol 2020;10:540662.
30. Bale R, Schullian P, Haidu M, Widmann G. [Stereotactic radiofrequency ablation (SRFA) of intrahepatic cholangiocellular carcinomas: a minimal invasive alternative to liver resection]. Wien Med Wochenschr 2013;163:128-31.
31. Haidu M, Dobrozemsky G, Schullian P, et al. Stereotactic radiofrequency ablation of unresectable intrahepatic cholangiocarcinomas: a retrospective study. Cardiovasc Intervent Radiol 2012;35:1074-82.
32. Sweeney J, Parikh N, El-Haddad G, Kis B. Ablation of intrahepatic cholangiocarcinoma. Semin Intervent Radiol 2019;36:298-302.
33. Lubner MG, Brace CL, Hinshaw JL, Lee FT Jr. Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol 2010;21:S192-203.
34. Lee SM, Ko HK, Shin JH, Kim JH, Chu HH. Combination of intraoperative radiofrequency ablation and surgical resection for treatment of cholangiocarcinoma: feasibility and long-term survival. Diagn Interv Radiol 2020;26:45-52.
35. Shindoh J. Ablative therapies for intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr 2017;6:2-6.
36. Han K, Ko HK, Kim KW, Won HJ, Shin YM, Kim PN. Radiofrequency ablation in the treatment of unresectable intrahepatic cholangiocarcinoma: systematic review and meta-analysis. J Vasc Interv Radiol 2015;26:943-8.
37. Ahmed M, Solbiati L, Brace CL, et al. International Working Group on Image-guided Tumor Ablation; Interventional Oncology Sans Frontières Expert Panel; Technology Assessment Committee of the Society of Interventional Radiology; Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. Radiology 2014;273:241-60.
39. Chu HH, Kim JH, Shin YM, Won HJ, Kim PN. Percutaneous radiofrequency ablation for recurrent intrahepatic cholangiocarcinoma after curative resection: multivariable analysis of factors predicting survival outcomes. AJR Am J Roentgenol 2021;217:426-32.
40. Kim JH, Won HJ, Shin YM, Kim PN, Lee SG, Hwang S. Radiofrequency ablation for recurrent intrahepatic cholangiocarcinoma after curative resection. Eur J Radiol 2011;80:e221-5.
41. Kim JH, Won HJ, Shin YM, Kim KA, Kim PN. Radiofrequency ablation for the treatment of primary intrahepatic cholangiocarcinoma. AJR Am J Roentgenol 2011;196:W205-9.
42. Carrafiello G, Laganà D, Cotta E, et al. Radiofrequency ablation of intrahepatic cholangiocarcinoma: preliminary experience. Cardiovasc Intervent Radiol 2010;33:835-9.
43. Butros SR, Shenoy-Bhangle A, Mueller PR, Arellano RS. Radiofrequency ablation of intrahepatic cholangiocarcinoma: feasability, local tumor control, and long-term outcome. Clin Imaging 2014;38:490-4.
44. Yu MA, Liang P, Yu XL, et al. Sonography-guided percutaneous microwave ablation of intrahepatic primary cholangiocarcinoma. Eur J Radiol 2011;80:548-52.
45. Wang X, Liang P, Yu J, et al. Contrast-enhanced ultrasound features predict the prognosis of percutaneous microwave ablation of intrahepatic cholangiocarcinoma. Br J Radiol 2022;95:20211379.
46. Ni JY, An C, Zhang TQ, Huang ZM, Jiang XY, Huang JH. Predictive value of the albumin-bilirubin grade on long-term outcomes of CT-guided percutaneous microwave ablation in intrahepatic cholangiocarcinoma. Int J Hyperthermia 2019;36:328-36.
47. Yang H, Cheng Z, Han Z, et al. Assessment of the outcomes of intrahepatic cholangiocarcinoma after ultrasound-guided percutaneous microwave ablation based on albumin-bilirubin grade. Cardiovasc Inter Rad 2021;44:261-70.
48. Zhang K, Yu J, Yu X, et al. Clinical and survival outcomes of percutaneous microwave ablation for intrahepatic cholangiocarcinoma. Int J Hyperthermia 2018;34:292-7.
49. Giorgio A, Gatti P, Montesarchio L, et al. Intrahepatic cholangiocarcinoma and thermal ablation: long-term results of an italian retrospective multicenter study. J Clin Transl Hepatol 2019;7:287-92.
50. Zhang SJ, Hu P, Wang N, et al. Thermal ablation versus repeated hepatic resection for recurrent intrahepatic cholangiocarcinoma. Ann Surg Oncol 2013;20:3596-602.
51. Xu C, Li L, Xu W, et al. Ultrasound-guided percutaneous microwave ablation versus surgical resection for recurrent intrahepatic cholangiocarcinoma: intermediate-term results. Int J Hyperthermia 2019;36:350-7.
52. Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg 2005;242:158-71.
53. Laimer G, Schullian P, Jaschke N, et al. Minimal ablative margin (MAM) assessment with image fusion: an independent predictor for local tumor progression in hepatocellular carcinoma after stereotactic radiofrequency ablation. Eur Radiol 2020;30:2463-72.
54. Gillams A, Goldberg N, Ahmed M, et al. Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, the interventional oncology Sans Frontières meeting 2013. Eur Radiol 2015;25:3438-54.
55. Lucatelli P, Argirò R, Crocetti L, et al. Percutaneous thermal segmentectomy: proof of concept. Cardiovasc Intervent Radiol 2022;45:665-76.
56. Bale R, Widmann G, Stoffner DI. Stereotaxy: breaking the limits of current radiofrequency ablation techniques. Eur J Radiol 2010;75:32-6.
57. Ortler M, Unterhofer C, Bauer R, Dobesberger J, Trinka E, Bale R. Flexibility of head positioning and head fixation provided by a novel system for non-invasive maxillary fixation and frameless stereotaxy: technical note. Minim Invasive Neurosurg 2009;52:144-8.
58. Freysinger W, Gunkel AR, Bale R, et al. Three-dimensional navigation in otorhinolaryngological surgery with the viewing wand. Ann Otol Rhinol Laryngol 1998;107:953-8.
59. Bale R, Widmann G. Navigated CT-guided interventions. Minim Invasive Ther Allied Technol 2007;16:196-204.
60. Bale R, Widmann G, Jaschke W. Stereotaxy and robotics for ablation - toy or tool? Radiologe 2012;52:56-62.
61. Kettenbach J, Kara L, Toporek G, Fuerst M, Kronreif G. A robotic needle-positioning and guidance system for CT-guided puncture: ex vivo results. Minim Invasive Ther Allied Technol 2014;23:271-8.
62. Schaible J, Pregler B, Verloh N, et al. Improvement of the primary efficacy of microwave ablation of malignant liver tumors by using a robotic navigation system. Radiol Oncol 2020;54:295-300.
63. Scharll Y, Letrari S, Laimer G, Schullian P, Bale R. Puncture accuracy of an optical tracked robotic aiming device-a phantom study. Eur Radiol 2022;32:6769-76.
64. Kim-Fuchs C, Candinas D, Lachenmayer A. The role of conventional and stereotactic microwave ablation for intrahepatic cholangiocarcinoma. J Clin Med 2021;10:2963.
65. Schullian P, Putzer D, Eberle G, Laimer G, Bale R. Simultaneous Stereotactic radiofrequency ablation of multiple (≥ 4) liver tumors: feasibility, safety, and efficacy. J Vasc Interv Radiol 2020;31:943-52.
66. Bale R, Widmann G, Haidu M. Stereotactic radiofrequency ablation. Cardiovasc Inter Rad 2011;34:852-6.
67. Schullian P, Johnston EW, Putzer D, Eberle G, Laimer G, Bale R. Safety and efficacy of stereotactic radiofrequency ablation for very large (≥8 cm) primary and metastatic liver tumors. Sci Rep 2020;10:1618.
68. Schullian P, Johnston E, Laimer G, et al. Stereotactic radiofrequency ablation of tumors at the hepatic venous confluence. HPB 2022;24:1044-54.
69. Schullian P, Laimer G, Putzer D, et al. Stereotactic radiofrequency ablation as first-line treatment of recurrent HCC following hepatic resection. Eur J Surg Oncol 2020;46:1503-9.
70. Bale R, Schullian P, Eberle G, et al. Stereotactic radiofrequency ablation of hepatocellular carcinoma: a histopathological study in explanted livers. Hepatology 2019;70:840-50.
71. Schullian P, Johnston EW, Putzer D, Eberle G, Laimer G, Bale R. Stereotactic radiofrequency ablation of subcardiac hepatocellular carcinoma: a case-control study. Int J Hyperthermia 2019;36:876-85.
72. Bale R, Widmann G, Schullian P, et al. Percutaneous stereotactic radiofrequency ablation of colorectal liver metastases. Eur Radiol 2012;22:930-7.
73. Schullian P, Johnston E, Laimer G, et al. Stereotactic radiofrequency ablation of breast cancer liver metastases: short- and long-term results with predicting factors for survival. Cardiovasc Intervent Radiol 2021;44:1184-93.
74. Putzer D, Schullian P, Bale R. Locoregional ablative treatment of melanoma metastases. Int J Hyperthermia 2019;36:59-63.
75. Schullian P, Johnston EW, Putzer D, et al. Stereotactic radiofrequency ablation (SRFA) for recurrent colorectal liver metastases after hepatic resection. Eur J Surg Oncol 2021;47:866-73.
76. Laimer G, Jaschke N, Gottardis M, et al. Stereotactic radiofrequency ablation of an unresectable intrahepatic cholangiocarcinoma (ICC): transforming an aggressive disease into a chronic condition. Cardiovasc Intervent Radiol 2020;43:791-6.
77. Braunwarth E, Schullian P, Kummann M, et al. Aggressive local treatment for recurrent intrahepatic cholangiocarcinoma-stereotactic radiofrequency ablation as a valuable addition to hepatic resection. PLoS One 2022;17:e0261136.
78. Bale R, Schullian P, Alzaga A. Narrative review of 3D navigated stereotactic liver ablation-do we still need a minimally invasive liver surgeon? Laparosc Surg 2021;5.
79. de Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol 2011;29:3140-5.
80. Sposito C, Ratti F, Cucchetti A, et al. Survival benefit of adequate lymphadenectomy in patients undergoing liver resection for clinically node-negative intrahepatic cholangiocarcinoma. J Hepatol 2023;78:356-63.
81. Koerkamp BG, Clavien PA, Polak WG. Surgical resection for intrahepatic cholangiocarcinoma - can we really improve survival by resecting more lymph nodes? J Hepatol 2023;78:235-7.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Bale R, Pawlik TM. Treatment of intrahepatic cholangiocarcinoma: evidence for the role of percutaneous ablation. Hepatoma Res 2023;9:44. http://dx.doi.org/10.20517/2394-5079.2023.71
AMA Style
Bale R, Pawlik TM. Treatment of intrahepatic cholangiocarcinoma: evidence for the role of percutaneous ablation. Hepatoma Research. 2023; 9: 44. http://dx.doi.org/10.20517/2394-5079.2023.71
Chicago/Turabian Style
Bale, Reto, Timothy M. Pawlik. 2023. "Treatment of intrahepatic cholangiocarcinoma: evidence for the role of percutaneous ablation" Hepatoma Research. 9: 44. http://dx.doi.org/10.20517/2394-5079.2023.71
ACS Style
Bale, R.; Pawlik TM. Treatment of intrahepatic cholangiocarcinoma: evidence for the role of percutaneous ablation. Hepatoma. Res. 2023, 9, 44. http://dx.doi.org/10.20517/2394-5079.2023.71
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 3 clicks
Cite This Article 3 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.