Trans-arterial chemoembolization and radioembolization for treatment of intrahepatic cholangiocarcinoma
Abstract
Trans-arterial therapies performed by interventional radiology, including chemoembolization and radioembolization, have been increasingly utilized for the treatment of unresectable intrahepatic cholangiocarcinoma. There is increasing evidence demonstrating the safety and efficacy of these interventions in patients with advanced disease. This review provides an overview of trans-arterial chemoembolization and radioembolization for unresectable intrahepatic cholangiocarcinoma, summarizes current evidence, and explores future directions for locoregional therapies.
Keywords
INTRODUCTION
Cholangiocarcinoma is a primary hepatic malignancy that originates from the epithelial cells of the biliary system. The incidence of cholangiocarcinoma is relatively low, representing only 3% of gastrointestinal tumors[1]. However, cholangiocarcinoma is the second most common primary hepatic malignancy, trailing only hepatocellular carcinoma. Molecular mechanisms for the development of intrahepatic cholangiocarcinoma are not well understood, but comparison of chromosomal aberrations between intrahepatic cholangiocarcinoma and hepatocellular carcinoma suggests that they share oncogenic pathways[2]. There are multiple risk factors for developing cholangiocarcinoma, including cirrhosis related to alcohol use, primary sclerosing cholangitis, chronic viral hepatitis, and biliary parasitic infection[3]. In recent decades, the incidence of cholangiocarcinoma has been on the rise, particularly for intrahepatic cholangiocarcinoma[1,4].
Intrahepatic cholangiocarcinoma has a poor overall prognosis, with less than a 10% median 5-year survival after diagnosis[5]. R0 surgical resection is a curative treatment option for intrahepatic cholangiocarcinoma. The role of liver transplantation as a potential curative option for intrahepatic cholangiocarcinoma has also been revisited in recent years[6]. However, the majority of patients are not eligible for resection at the time of diagnosis and recurrent disease is common following surgery, often occurring in the remnant liver[7,8]. Early detection of intrahepatic cholangiocarcinoma is difficult since many patients do not develop symptoms in the early stages.
Systemic chemotherapy has limited efficacy in patients with unresectable disease. First-line chemotherapy with cisplatin and gemcitabine has demonstrated median survival of less than 12 months in multiple studies[9,10]. Some improvement over these results has been seen with the addition of durvalumab to gemcitabine and cisplatin in the TOPAZ-1 study, as well as gemcitabine, cisplatin, and nab-paclitaxel, although this regimen has a high grade III/IV adverse event rate[11,12]. Recent advances have also been made in targeted systemic therapy options against IDH1 and FGFR2 mutations for intrahepatic cholangiocarcinoma[13-15]. Treatment options offered by interventional radiology, including trans-arterial radioembolization (TARE) and trans-arterial chemoembolization (TACE), are increasingly utilized in current practice for unresectable diseases. Both TACE and TARE are minimally invasive procedures that allow for endovascular delivery of chemotherapy or radiation selectively to liver tumors.
The rationale for TACE and TARE relies on the dual blood supply of the liver from the hepatic arteries and portal veins. In healthy individuals, the liver receives approximately 75% of its blood flow from the portal veins and 25% from the hepatic arteries[16]. Hepatic tumors including hepatocellular carcinoma and intrahepatic cholangiocarcinoma are predominantly vascularized by the hepatic arteries[17]. This vascular pattern allows trans-arterial interventions including chemoembolization and radioembolization to deliver concentrated doses of chemotherapy or radiation directly to tumors while partially sparing normal liver parenchyma and decreasing the risk of non-target systemic side effects.
This review provides an overview of trans-arterial chemoembolization and radioembolization for intrahepatic cholangiocarcinoma, summarizes current evidence, and explores future directions for locoregional therapies.
TRANS-ARTERIAL RADIOEMBOLIZATION FOR INTRAHEPATIC CHOLANGIOCARCINOMA
Overview
TARE is an endovascular intervention that delivers radioactive isotope-coated microspheres to tumors via selective catheterization of the hepatic arteries[18]. TARE may be performed in settings with interventional radiology and nuclear medicine capabilities. Selective internal radiation therapy (SIRT) is another common name for this procedure.
TARE was first described in 1965 and has demonstrated efficacy in the setting of primary and metastatic hepatic tumors[19]. Radioembolization is performed with microspheres impregnated with the radioactive isotope Yttrium-90 (90Y), a high-energy beta emitter. 90Y decays to stable element Zirconium-90 with a half-life of 64.2 hours. Tumor necrosis is mediated by irreversible DNA damage caused by oxygen free radicals generated by the beta radiation emitted by the radioactive microspheres. The radiation emitted by the microspheres penetrates approximately 2.5 mm into the surrounding tissues, minimizing non-target radiation exposure beyond microsphere deposition within tumor[20,21].
While the goal of TARE is to selectively deliver high doses of radiation to tumors, there is a risk of non-target radioactive microsphere deposition and associated side effects. The anticipated distribution of 90Y microspheres may be assessed with a “mapping” angiogram prior to treatment[22]. During the mapping procedure, 99mTechnicium-macroaggregated albumin is delivered trans-arterially to the vessels supplying the tumor, and SPECT-CT is performed to assess the distribution. In addition, extrahepatic vessels with potential communication with the tumoral vessels are also interrogated and may be embolized if necessary to prevent non-target radiation. 9mTc-MAA particles and 90Y microspheres are approximately the same size, so the distribution of injected 9mTc-MAA may be used as a surrogate for the radiation treatment dose distribution.
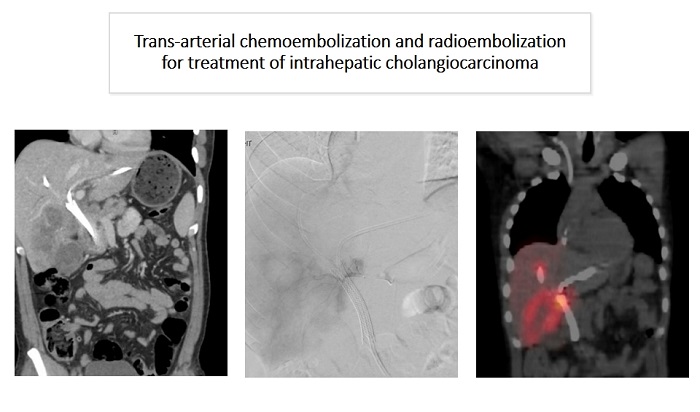
Non-target deposition of radioactive microspheres to the lungs or gastrointestinal tract may result in radiation pneumonitis or enteritis[23-25]. The mapping procedure performed with 99mTc-MAA prior to treatment allows for quantification of potential non-target microsphere deposition to the lungs and bowel and adjustments to the dose administration to limit the potential for radiation-induced complications. The mapping procedure is typically performed 1 to 2 weeks prior to the later radioembolization procedure. In Figure 1, the lung shunt fraction is being assessed following a mapping procedure prior to TARE treatment of a right-sided intrahepatic cholangiocarcinoma. In addition, the mapping procedure helps define the appropriate radiation dose to deliver to the tumor.
Figure 1. A patient with a right-sided intrahepatic cholangiocarcinoma undergoing a mapping procedure with 99mTc-MAA prior to TARE. (A) Coronal CT images demonstrating the intrahepatic cholangiocarcinoma in the right hepatic lobe; (B) Angiographic images in the delayed arterial phase following contrast injection via the right hepatic artery with contrast opacification of the tumor; (C) SPECT-CT performed following injection of 99mTc-MAA via the right hepatic artery, demonstrating radiotracer uptake in the distribution of the tumor. TARE: trans-arterial radioembolization.
In Figure 2, we show illustrative images of the TARE workflow from an example patient who presented with a large left-sided intrahepatic cholangiocarcinoma occupying the entirety of the left lobe with extension into the right lobe and involving the porta hepatis.
Figure 2. A patient with multifocal right-sided intrahepatic cholangiocarcinoma undergoing evaluation of lung shunt fraction prior to TARE. (A) Coronal CT images demonstrating multiple lesions in the right hepatic lobe consistent with patient’s known non-resectable intrahepatic cholangiocarcinoma; (B) Planar images performed in nuclear medicine following injection of 99mTc-MAA via the right hepatic artery demonstrate minimal radiotracer uptake to the lungs, which implies a low risk for radiation pneumonitis with TARE. TARE: trans-arterial radioembolization.
Clinical efficacy of TARE
TARE has demonstrated clinical efficacy in the setting of unresectable intrahepatic cholangiocarcinoma. Median overall survival has been reported between 9.3 and 22 months following TARE for unresectable intrahepatic cholangiocarcinoma in several retrospective studies[26-31]. A 2015 meta-analysis with 298 patients who underwent TARE for unresectable intrahepatic cholangiocarcinoma demonstrated a median survival of 15.5 months with a 3-month partial response rate of 28% and a stable disease rate of 54%[32]. Similarly, a study by Hoffman et al. reported partial response in 36% of patients and stable disease in 52% of patients at 3-month follow-up post TARE[29]. Increased overall survival post TARE was associated with ECOG performance status of 0 to 1, increased radiation treatment dose, and evidence of tumor response on imaging post treatment[27,29]. A summary of studies examining TARE for intrahepatic cholangiocarcinoma is included in Table 1. Factors associated with poor survival post TARE included impaired liver function (elevated INR and bilirubin), elevated MELD post treatment, and increased time from diagnosis to TARE treatment[33].
Prior studies investigating TARE in treatment of intrahepatic cholangiocarcinoma
| Author | Study type | Sample size | Technique | Outcomes |
| Rafi et al. (2012)[26] | Retrospective | n = 19 | TARE with y90 resin microspheres | Median OS = 11.5 months |
| Bargellini et al. (2020)[31] | Retrospective | n = 81 | TARE | Median OS = 14.5 months |
| Saxena et al. (2010)[28] | Retrospective | n = 25 | TARE with y90 resin microspheres | Median OS = 9.3 months |
| Hoffman et al. (2012)[29] | Retrospective | n = 33 | TARE with y90 resin microspheres | Median PFS = 9.8 months Median OS = 22 months |
| Buettner et al. (2020)[30] | Retrospective | n = 114 | TARE | Median OS = 29 months |
| Gangi et al. (2018)[33] | Retrospective | n = 85 | TARE with y90 glass microspheres | Median OS = 12 months |
| Mouli et al. (2013)[34] | Retrospective | n = 46 | TARE | Median OS = 14.6 months (solitary disease) |
Patients who undergo TARE for unresectable intrahepatic cholangiocarcinoma are sometimes able to be down-staged to surgical resection post-treatment. Mouli et al. studied 46 patients who underwent TARE for unresectable intrahepatic cholangiocarcinoma and found that 5/46 (11%) were able to qualify for surgical resection due to partial treatment response following TARE[34]. Patients who can be down-staged to surgical resection post TARE have a significant survival benefit, with a study by Bourien et al. demonstrating a median overall survival of 51.9 months in patients who were able to be down-staged to surgical resection post TARE[27]. Similarly, a study with 136 patients who underwent TARE found that 8.1% were down-staged to resection and 1.5% to transplant[35].
Side effects of TARE
The side effect profile of TARE is typically minor, with most patients tolerating the procedure in the outpatient setting. Mild symptoms of fatigue, nausea, and malaise are common in up to 20-40% of patients post radioembolization, with these symptoms being referred to as “post-radioembolization syndrome”. Non-target deposition of radioactive microspheres to the lungs and gastrointestinal tract is relatively rare, but has the potential to cause gastrointestinal ulceration or radiation pneumonitis[36,37]. Radioembolization-induced liver disease (REILD) is another rare but potentially serious post-radioembolization complication. REILD occurs due to excessive radiation exposure to normal hepatic parenchyma and the risk for development increases with a history of severe cirrhosis, prior liver external beam radiation therapy, or multiple TARE procedures[38,39].
Overall, post-treatment symptoms from TARE are typically better tolerated compared to those following TACE procedures. A study by Mosconi et al. demonstrated an increased rate of clinical adverse events for TACE compared to TARE (58.5% vs. 43%), particularly with post-embolization syndrome and post-embolic liver abscesses[40]. This finding suggests that TARE may be preferable in patients with a history of biliary instrumentation or frail patients receiving concurrent systemic chemotherapy.
TRANS-ARTERIAL CHEMOEMBOLIZATION FOR INTRAHEPATIC CHOLANGIOCARCINOMA
Overview
TACE delivers chemotherapy and embolic material to liver tumors via the hepatic arteries. Selective delivery of chemotherapy via the hepatic arteries that supply tumor allows a concentrated and localized dose of chemotherapy to be administered with limited systemic delivery[41]. Embolization and occlusion of arterial branches supplying tumor also have the additional advantage of causing ischemia and necrosis within the targeted lesions.
There are two different approaches to performing TACE: conventional TACE (cTACE) and drug-eluting bead TACE (DEB-TACE). cTACE uses a chemotherapeutic agent mixed with ethiodized oil contrast called lipiodol. Chemotherapeutic agents commonly utilized during cTACE include doxorubicin, cisplatin, gemcitabine, and mitomycin-C[42,43]. DEB-TACE utilizes chemotherapy-coated microbeads which allow for sustained drug release within the tumor[44,45]. Irinotecan and doxorubicin are commonly utilized chemotherapeutic agents with DEB-TACE for intrahepatic cholangiocarcinoma[46]. Both TACE approaches combine the synergistic cytotoxic effects of high-dose, localized chemotherapy with the ischemic effects of occluding the tumor arterial blood supply.
Clinical efficacy of TACE
Multiple studies have demonstrated median survival between 12 to 26 months post treatment with cTACE in the setting of unresectable intrahepatic cholangiocarcinoma[40,43,47-50]. Park et al. compared patients with intrahepatic cholangiocarcinoma who underwent cTACE (n = 72) vs. supportive therapy (n = 83) and reported significantly improved survival for the cTACE cohort (12.2 months vs. 3.3 months)[51]. Cisplatin was the chemotherapy of choice utilized during chemoembolization and partial tumor response was noted in 23% of patients who underwent TACE. Among patients undergoing TACE, survival was significantly improved in patients with liver-only disease compared to those with extrahepatic metastases (13.3 months vs. 11.3 months) and among patients with partial tumor response compared to those with no response (22 months vs. 10.9 months). Factors associated with decreased survival post TACE for unresectable intrahepatic cholangiocarcinoma include increased tumor size (> 5 cm), history of prior major resection, short interval tumor progression, poor tumor differentiation, and tumor hypo-vascularity[48,49,52].
The median survival following DEB-TACE has been shown to be between 9 to 13 months for unresectable intrahepatic cholangiocarcinoma, similar to studies with cTACE[53-56]. A meta-analysis of 1091 patients who underwent cTACE and DEB-TACE for intrahepatic cholangiocarcinoma demonstrated a pooled objective response rate of 51.2% for DEB-TACE and 29.4% for cTACE. Despite this large difference in response rate, there was no statistically significant difference in survival between the two treatment methods[57]. Similarly, Wang et al. reported a prospective study comparing DEB-TACE and cTACE with irinotecan demonstrated no significant difference in median overall survival of 11.5 months vs. 9.0 months, respectively[58]. However, this study did demonstrate a statistically significant advantage for DEB-TACE over cTACE in overall response rate and progression-free survival. Venturini et al. compared DEB-TACE with doxorubicin vs. irinotecan in 10 patients and demonstrated increased overall survival in patients who underwent irinotecan DEB-TACE compared to doxorubicin DEB-TACE, although results were limited by small sample size[46]. Additional prospective studies are needed to further compare the efficacy of cTACE vs. DEB-TACE for intrahepatic cholangiocarcinoma.
Scheuermann et al. compared survival in patients undergoing both cTACE and DEB-TACE vs. surgical resection for intrahepatic cholangiocarcinoma[59]. As expected, there was significantly improved median survival in patients who underwent R0 surgical resection without lymph node metastases (37 months). However, the authors demonstrated no significant difference in median survival between patients who underwent TACE (11 months) vs. patients who had positive resection margins (11 months) or positive lymph node metastases (9 months). A summary of studies examining TACE for intrahepatic cholangiocarcinoma is included in Table 2.
Prior studies investigating TACE in treatment of intrahepatic cholangiocarcinoma
| Author | Study type | Sample size | Technique | Outcomes |
| Burger et al. (2005)[50] | Retrospective | n = 17 | cTACE | Median OS = 23 months |
| Vogl et al. (2012)[48] | Retrospective | n = 115 | cTACE | Median OS = 13 months |
| Mosconi et al. (2021)[40] | Meta-analysis | n = 906 | cTACE and DEB-TACE | Median OS = 14.2 months |
| Aliberti et al. (2008)[54] | Prospective | n = 11 | DEB-TACE | Median OS = 13 months |
| Liu et al. (2022)[55] | Retrospective | n = 39 | DEB-TACE | Median PFS = 8.0 months Median OS = 11.0 months |
| Zhou et al. (2020)[56] | Retrospective | n = 88 | DEB-TACE | Median PFS = 3.0 months Median OS = 9.0 months |
Although TACE has the potential to improve survival in patients with unresectable intrahepatic cholangiocarcinoma, the intervention remains palliative rather than curative. In a study of patients with intrahepatic cholangiocarcinoma who underwent TACE prior to liver transplant, viable residual tumor was demonstrated in 100% of explants (n = 13)[60]. In addition, tumor necrosis was observed in only 7.6% of the tumor volume post-TACE treatment for intrahepatic cholangiocarcinoma, which was significantly lower than the degree of tumor necrosis observed in patients with hepatocellular carcinoma who underwent TACE pre-transplant (75.1%). This finding may be secondary to the hypo-vascular nature of intrahepatic cholangiocarcinoma relative to hepatocellular carcinoma, which may make the tumors less susceptible to ischemic necrosis following TACE.
Side effects of TACE
Potential side effects of TACE include abdominal pain, nausea, fever, and liver enzyme elevation in up to 20% -40% of cases[61,62]. Additional systemic side effects such as anemia, alopecia, and myelosuppression are uncommon, but have also been observed post-TACE due to systemic translocation of chemotherapeutic agents[63]. Together, these symptoms are referred to as “postembolization syndrome” and often self-resolve in one to two days. TACE is often performed as an outpatient procedure; however, some patients require overnight observation for symptom management. Major complications from TACE are relatively uncommon, but may be secondary to non-target embolization and/or induced liver ischemia. Potential major complications include hepatic abscess, cholecystitis, or gastrointestinal tract ulceration, which occur in approximately 2%-5% of patients[63,64].
SYSTEMIC CHEMOTHERAPY COMBINED WITH LOCOREGIONAL INTERVENTIONS
Palliative systemic chemotherapy remains the main treatment option for patients with unresectable intrahepatic cholangiocarcinoma[9,65]. However, there is increasing evidence that locoregional therapies including TACE and TARE in combination with palliative systemic chemotherapy may provide additional survival benefits for patients with unresectable intrahepatic cholangiocarcinoma.
TACE combined with chemotherapy
Multiple retrospective studies have demonstrated survival benefits for TACE in combination with palliative chemotherapy[53,66]. A study by Gairing et al. demonstrated improved median overall survival in patients receiving gemcitabine/cisplatin combined with TACE compared to palliative chemotherapy alone (26.2 vs. 13.1 months)[66]. Kiefer et al. similarly reported improved survival in patients who received both systemic chemotherapy and TACE compared to those who received TACE alone (28 months vs. 16 months)[43].
These findings have been further supported in a prospective, phase II study by Martin et al. in patients randomized to gemcitabine/cisplatin with DEB-TACE (irinotecan) vs. gemcitabine/cisplatin alone[67]. In this study with 48 patients, the overall response rate was significantly greater in the chemotherapy with DEB-TACE group compared to the chemotherapy alone group at 2, 4, and 6 months. In addition, there was improved overall survival in the combined DEB-TACE with systemic chemotherapy group compared to systemic chemotherapy alone (33.7 vs. 12.6 months). An added benefit of the combined DEB-TACE and chemotherapy group was an increased rate of downsizing of tumor to resection or curative ablation compared to the chemotherapy alone group. There are currently no phase 3, randomized controlled clinical trials assessing the clinical efficacy of TACE in combination with systemic chemotherapy.
TARE combined with chemotherapy
Prior studies have also demonstrated a survival benefit for TARE in combination with systemic chemotherapy for intrahepatic cholangiocarcinoma. A meta-analysis performed by Cucchetti et al. analyzed 224 patients from nine retrospective studies and demonstrated improved median survival in patients who underwent SIRT with concomitant chemotherapy (19.5 months) compared to those not receiving chemotherapy (5.5 months)[68]. This meta-regression study also found that SIRT was most likely to be beneficial in mass-forming CCA. An additional study retrospectively studied 24 patients who underwent SIRT with chemotherapy given either prior to SIRT or concomitantly with SIRT. This study demonstrated longer progression-free survival when chemotherapy was given concomitantly with SIRT compared to before SIRT (20.0 vs. 8.8 months)[69].
These findings were further supported in a prospective, phase 2 clinical trial by Edeline et al. in which SIRT was performed concomitantly with first-line gemcitabine/cisplatin[70]. The overall response rate was 39% at 3 months based on RECIST criteria, with a disease control rate of 98%. Median overall survival was also noted to be 22 months, which was favorable compared to historical controls receiving palliative chemotherapy alone. This study also had 9/41 (22%) patients who were able to be down-staged to surgical resection following treatment with SIRT plus chemotherapy. These findings suggest that SIRT with concomitant systemic chemotherapy is a viable first-line treatment option for unresectable intrahepatic cholangiocarcinoma.
A phase three clinical trial comparing TARE preceding gemcitabine/cisplatin with gemcitabine/cisplatin alone (SIRCCA) was closed in 2022; however, preliminary results are not yet available[71]. Further prospective, randomized controlled clinical trials should be performed to define the clinical benefit of TARE and systemic chemotherapy in the context of intrahepatic cholangiocarcinoma.
TARE and TACE in the neoadjuvant setting
TARE and TACE for intrahepatic cholangiocarcinoma are most often utilized in the palliative setting. However, several retrospective studies have demonstrated a small percentage of patients initially with unresectable diseases that were able to be down-staged to resectability following locoregional therapy with TARE and TACE[72]. Burger et al. performed a retrospective analysis of 17 patients with unresectable intrahepatic cholangiocarcinoma who underwent TACE with cisplatin, doxorubicin, and mitomycin-C. Two patients (12%) were able to undergo tumor resection following downstaging post-TACE therapy[50]. Similarly, Mouli et al. conducted a retrospective analysis involving 60 patients who underwent TARE for unresectable intrahepatic cholangiocarcinoma and found that 5 patients (8%) underwent R0 resection following down-staging post-TARE[34]. However, downstaging to surgical resection post TACE/TARE appears to be an uncommon occurrence overall based on available retrospective data. Future clinical trials are warranted to help clarify the role of locoregional therapies such as TACE and TARE in the neoadjuvant setting.
Hepatic artery infusion therapy
Hepatic artery infusion pump therapy is another form of locoregional therapy that has a role in unresectable intrahepatic cholangiocarcinoma. Hepatic artery infusion pumps are surgically implanted catheters that deliver high-dose chemotherapy directly to the hepatic arterial circulation while reducing systemic effects and toxicity. Hepatic artery infusion therapy for cholangiocarcinoma is most commonly performed with floxuridine, a precursor of the chemotherapeutic agent fluorouracil.
A meta-analysis of nine studies with 478 patients with intrahepatic cholangiocarcinoma who underwent hepatic artery infusion therapy demonstrated a three-year overall survival of 39.5%, which outperformed systemic chemotherapy[73]. An additional study by Franssen et al. comparing 141 patients who underwent hepatic arterial infusion with 178 patients who underwent surgical resection demonstrated similar overall survival between groups (20.3 vs. 18.9 months, respectively)[74]. These findings suggest that hepatic arterial infusion with floxuridine may also be considered an effective locoregional therapy option.
Locoregional therapy with immunotherapy
Immunotherapy has an increasing role in the treatment of unresectable intrahepatic cholangiocarcinoma. The TOPAZ-1 trial demonstrated improved survival and response rates for patients who received durvalumab plus gemcitabine/cisplatin compared to placebo plus gemcitabine/cisplatin[75]. This study has led to durvalumab being the first immunotherapy to be FDA-approved for the treatment of intrahepatic cholangiocarcinoma. However, limited data are available for TACE and TARE combined with immunotherapy in the context of intrahepatic cholangiocarcinoma. A single retrospective study with 49 patients who underwent DEB-TACE combined with immune checkpoint inhibitors demonstrated improved objective response rate and overall survival compared to patients who received gemcitabine/cisplatin alone[76]. Additional studies are necessary to assess the potential additional benefit that TACE and TARE may provide in combination with immunotherapy.
CONCLUSION
Locoregional treatments, including TACE and TARE, have demonstrated clinical efficacy and safety in numerous retrospective studies in the context of non-resectable intrahepatic cholangiocarcinoma. Both TACE and TARE have been shown to provide an additional survival benefit in the setting of concomitant systemic chemotherapy and should be considered in the first-line setting in combination with systemic chemotherapy or in chemotherapy-refractory patients. However, the lack of quality data from well-controlled, prospective trials limits the ability to drive formal recommendations for these interventions in the context of intrahepatic cholangiocarcinoma.
Based on currently available data, there is no clinically significant difference in efficacy between TARE and TACE for intrahepatic cholangiocarcinoma. A systemic review performed by Yang et al. in 929 patients with unresectable intrahepatic cholangiocarcinoma demonstrated similar overall survival of 12.5 months for TARE and 13 months for TACE[76]. However, patient-specific factors such as a history of bilio-enteric anastomosis or poor functional status may make TARE the preferred intervention due to decreased infection risk and better short-term tolerability.
The majority of literature on TACE and TARE for intrahepatic cholangiocarcinoma are retrospective in design and are limited by potential selection bias or differences in treatment protocols between treatment groups. For both interventions, significant variations in chemotherapeutic agents, radio-embolic dosing protocols, and operator technique/experience limit the interpretation of the available data and make comparison with additional second-line therapeutic options challenging. However, given the promising available clinical data for TACE and TARE for unresectable intrahepatic cholangiocarcinoma, more prospective trials assessing the efficacy of these interventions are highly warranted.
DECLARATIONS
Authors’ contributionsMade substantial contributions to manuscript draft preparation, editing, and submission: An T, Wehrenberg-Klee E
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. Bergquist A, von Seth E. Epidemiology of cholangiocarcinoma. Best Pract Res Clin Gastroenterol 2015;29:221-32.
2. Andersen JB, Thorgeirsson SS. Genetic profiling of intrahepatic cholangiocarcinoma. Curr Opin Gastroenterol 2012;28:266-72.
3. Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology 2013;145:1215-29.
4. Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-year trends in cholangiocarcinoma incidence in the U.S.: intrahepatic disease on the rise. Oncologist 2016;21:594-9.
6. Sapisochin G, Javle M, Lerut J, et al. Liver transplantation for cholangiocarcinoma and mixed hepatocellular cholangiocarcinoma: working group report from the ILTS transplant oncology consensus conference. Transplantation 2020;104:1125-30.
7. Hyder O, Hatzaras I, Sotiropoulos GC, et al. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery 2013;153:811-8.
8. Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg 2014;149:565-74.
9. Valle J, Wasan H, Palmer DH, et al. ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81.
10. Okusaka T, Nakachi K, Fukutomi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer 2010;103:469-74.
11. Oh D, He AR, Qin S, et al. A phase 3 randomized, double-blind, placebo-controlled study of durvalumab in combination with gemcitabine plus cisplatin (GemCis) in patients (pts) with advanced biliary tract cancer (BTC): TOPAZ-1. JCO 2022;40:378.
12. Shroff RT, Javle MM, Xiao L, et al. Gemcitabine, cisplatin, and nab-paclitaxel for the treatment of advanced biliary tract cancers: a phase 2 clinical trial. JAMA Oncol 2019;5:824-30.
13. Zhu AX, Macarulla T, Javle MM, et al. Final overall survival efficacy results of ivosidenib for patients with advanced cholangiocarcinoma with IDH1 mutation: the phase 3 randomized clinical ClarIDHy trial. JAMA Oncol 2021;7:1669-77.
14. Jain A, Borad MJ, Kelley RK, et al. Cholangiocarcinoma with FGFR genetic aberrations: a unique clinical phenotype. JCO Precis Oncol 2018;2:1-12.
15. Goyal L, Meric-Bernstam F, Hollebecque A, et al. FOENIX-CCA2 Study Investigators. Futibatinib for FGFR2-Rearranged intrahepatic cholangiocarcinoma. N Engl J Med 2023;388:228-39.
16. Kan Z, Madoff DC. Liver anatomy: microcirculation of the liver. Semin Intervent Radiol 2008;25:77-85.
18. Tong AK, Kao YH, Too CW, Chin KF, Ng DC, Chow PK. Yttrium-90 hepatic radioembolization: clinical review and current techniques in interventional radiology and personalized dosimetry. Br J Radiol 2016;89:20150943.
19. Ariel IM. Treatment of inoperable primary pancreatic and liver cancer by the intra-arterial administration of radioactive isotopes (Y90 radiating microspheres). Ann Surg 1965;162:267-78.
20. Kennedy A, Nag S, Salem R, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys 2007;68:13-23.
21. Lewandowski RJ, Geschwind JF, Liapi E, Salem R. Transcatheter intraarterial therapies: rationale and overview. Radiology 2011;259:641-57.
22. Leung WT, Lau WY, Ho SK, et al. Measuring lung shunting in hepatocellular carcinoma with intrahepatic-arterial technetium-99m macroaggregated albumin. J Nucl Med 1994;35:70-3.
23. Leung TW, Lau WY, Ho SK, et al. Radiation pneumonitis after selective internal radiation treatment with intraarterial 90yttrium-microspheres for inoperable hepatic tumors. Int J Radiat Oncol Biol Phys 1995;33:919-24.
24. Collins J, Salem R. Hepatic radioembolization complicated by gastrointestinal ulceration. Semin Intervent Radiol 2011;28:240-5.
25. Estes DJ, Wideroff GA, Sendzischew Shane MA, Sussman DA. Yttrium-90 radioembolization: an unusual cause of radiating abdominal pain. ACG Case Rep J 2019;6:1-2.
26. Rafi S, Piduru SM, El-Rayes B, et al. Yttrium-90 radioembolization for unresectable standard-chemorefractory intrahepatic cholangiocarcinoma: survival, efficacy, and safety study. Cardiovasc Intervent Radiol 2013;36:440-8.
27. Bourien H, Palard X, Rolland Y, et al. Yttrium-90 glass microspheres radioembolization (RE) for biliary tract cancer: a large single-center experience. Eur J Nucl Med Mol Imaging 2019;46:669-76.
28. Saxena A, Bester L, Chua TC, Chu FC, Morris DL. Yttrium-90 radiotherapy for unresectable intrahepatic cholangiocarcinoma: a preliminary assessment of this novel treatment option. Ann Surg Oncol 2010;17:484-91.
29. Hoffmann RT, Paprottka PM, Schön A, et al. Transarterial hepatic yttrium-90 radioembolization in patients with unresectable intrahepatic cholangiocarcinoma: factors associated with prolonged survival. Cardiovasc Intervent Radiol 2012;35:105-16.
30. Buettner S, Braat AJAT, Margonis GA, et al. Yttrium-90 radioembolization in intrahepatic cholangiocarcinoma: a multicenter retrospective analysis. J Vasc Interv Radiol 2020;31:1035-43.e2.
31. Bargellini I, Mosconi C, Pizzi G, et al. Yttrium-90 radioembolization in unresectable intrahepatic cholangiocarcinoma: results of a multicenter retrospective study. Cardiovasc Intervent Radiol 2020;43:1305-14.
32. Al-Adra DP, Gill RS, Axford SJ, Shi X, Kneteman N, Liau SS. Treatment of unresectable intrahepatic cholangiocarcinoma with yttrium-90 radioembolization: a systematic review and pooled analysis. Eur J Surg Oncol 2015;41:120-7.
33. Gangi A, Shah J, Hatfield N, et al. Intrahepatic cholangiocarcinoma treated with transarterial yttrium-90 glass microsphere radioembolization: results of a single institution retrospective study. J Vasc Interv Radiol 2018;29:1101-8.
34. Mouli S, Memon K, Baker T, et al. Yttrium-90 radioembolization for intrahepatic cholangiocarcinoma: safety, response, and survival analysis. J Vasc Interv Radiol 2013;24:1227-34.
35. Gupta AN, Gordon AC, Gabr A, et al. Yttrium-90 radioembolization of unresectable intrahepatic cholangiocarcinoma: long-term follow-up for a 136-patient cohort. Cardiovasc Intervent Radiol 2022;45:1117-28.
37. Sangro B, Iñarrairaegui M, Bilbao JI. Radioembolization for hepatocellular carcinoma. J Hepatol 2012;56:464-73.
38. Young JY, Rhee TK, Atassi B, et al. Radiation dose limits and liver toxicities resulting from multiple yttrium-90 radioembolization treatments for hepatocellular carcinoma. J Vasc Interv Radiol 2007;18:1375-82.
39. Sangro B, Gil-Alzugaray B, Rodriguez J, et al. Liver disease induced by radioembolization of liver tumors: description and possible risk factors. Cancer 2008;112:1538-46.
40. Mosconi C, Solaini L, Vara G, et al. Transarterial chemoembolization and radioembolization for unresectable intrahepatic cholangiocarcinoma-a systemic review and meta-analysis. Cardiovasc Intervent Radiol 2021;44:728-38.
42. Gusani NJ, Balaa FK, Steel JL, et al. Treatment of unresectable cholangiocarcinoma with gemcitabine-based transcatheter arterial chemoembolization (TACE): a single-institution experience. J Gastrointest Surg 2008;12:129-37.
43. Kiefer MV, Albert M, McNally M, et al. Chemoembolization of intrahepatic cholangiocarcinoma with cisplatinum, doxorubicin, mitomycin C, ethiodol, and polyvinyl alcohol: a 2-center study. Cancer 2011;117:1498-505.
44. Lencioni R, Petruzzi P, Crocetti L. Chemoembolization of hepatocellular carcinoma. Semin Intervent Radiol 2013;30:3-11.
45. Lencioni R, de Baere T, Burrel M, et al. Transcatheter treatment of hepatocellular carcinoma with doxorubicin-loaded DC bead (DEBDOX): technical recommendations. Cardiovasc Intervent Radiol 2012;35:980-5.
46. Venturini M, Sallemi C, Agostini G, et al. Chemoembolization with drug eluting beads preloaded with irinotecan (DEBIRI) vs doxorubicin (DEBDOX) as a second line treatment for liver metastases from cholangiocarcinoma: a preliminary study. Br J Radiol 2016;89:20160247.
47. Herber S, Otto G, Schneider J, et al. Transarterial chemoembolization (TACE) for inoperable intrahepatic cholangiocarcinoma. Cardiovasc Intervent Radiol 2007;30:1156-65.
48. Vogl TJ, Naguib NN, Nour-Eldin NE, et al. Transarterial chemoembolization in the treatment of patients with unresectable cholangiocarcinoma: results and prognostic factors governing treatment success. Int J Cancer 2012;131:733-40.
49. Ge Y, Jeong S, Luo GJ, et al. Transarterial chemoembolization versus percutaneous microwave coagulation therapy for recurrent unresectable intrahepatic cholangiocarcinoma: development of a prognostic nomogram. Hepatobiliary Pancreat Dis Int 2020;19:138-46.
50. Burger I, Hong K, Schulick R, et al. Transcatheter arterial chemoembolization in unresectable cholangiocarcinoma: initial experience in a single institution. J Vasc Interv Radiol 2005;16:353-61.
51. Park SY, Kim JH, Yoon HJ, Lee IS, Yoon HK, Kim KP. Transarterial chemoembolization versus supportive therapy in the palliative treatment of unresectable intrahepatic cholangiocarcinoma. Clin Radiol 2011;66:322-8.
52. Kim JH, Yoon HK, Sung KB, et al. Transcatheter arterial chemoembolization or chemoinfusion for unresectable intrahepatic cholangiocarcinoma: clinical efficacy and factors influencing outcomes. Cancer 2008;113:1614-22.
53. Schiffman SC, Metzger T, Dubel G, et al. Precision hepatic arterial irinotecan therapy in the treatment of unresectable intrahepatic cholangiocellular carcinoma: optimal tolerance and prolonged overall survival. Ann Surg Oncol 2011;18:431-8.
54. Aliberti C, Benea G, Tilli M, Fiorentini G. Chemoembolization (TACE) of unresectable intrahepatic cholangiocarcinoma with slow-release doxorubicin-eluting beads: preliminary results. Cardiovasc Intervent Radiol 2008;31:883-8.
55. Liu D, Wang J, Ma Z, et al. Treatment of unresectable intrahepatic cholangiocarcinoma using transarterial chemoembolisation with irinotecan-eluting beads: analysis of efficacy and safety. Cardiovasc Intervent Radiol 2022;45:1092-101.
56. Zhou TY, Zhou GH, Zhang YL, et al. Drug-eluting beads transarterial chemoembolization with CalliSpheres microspheres for treatment of unresectable intrahepatic cholangiocarcinoma. J Cancer 2020;11:4534-41.
57. He M, Jiang N, Yin X, Xu A, Mu K. Conventional and drug-eluting beads transarterial chemoembolization in patients with unresectable intrahepatic cholangiocarcinoma: a systematic review and pooled analysis. J Cancer Res Clin Oncol 2023;149:531-40.
58. Wang J, Xue Y, Liu R, et al. DEB-TACE with irinotecan versus C-TACE for unresectable intrahepatic cholangiocarcinoma: a prospective clinical study. Front Bioeng Biotechnol 2022;10:1112500.
59. Scheuermann U, Kaths JM, Heise M, et al. Comparison of resection and transarterial chemoembolisation in the treatment of advanced intrahepatic cholangiocarcinoma--a single-center experience. Eur J Surg Oncol 2013;39:593-600.
60. Lee DD, Croome KP, Musto KR, et al. Liver transplantation for intrahepatic cholangiocarcinoma. Liver Transpl 2018;24:634-44.
61. Pomoni M, Malagari K, Moschouris H, et al. Post embolization syndrome in doxorubicin eluting chemoembolization with DC bead. Hepatogastroenterology 2012;59:820-5.
62. Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology 2016;64:106-16.
64. Tu J, Jia Z, Ying X, et al. The incidence and outcome of major complication following conventional TAE/TACE for hepatocellular carcinoma. Medicine 2016;95:e5606.
65. Lamarca A, Palmer DH, Wasan HS, et al; Advanced Biliary Cancer Working Group. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol 2021;22:690-701.
66. Gairing SJ, Thol F, Müller L, et al. The addition of transarterial chemoembolization to palliative chemotherapy extends survival in intrahepatic cholangiocarcinoma. J Clin Med 2021;10:2732.
67. Martin RCG 2nd, Simo KA, Hansen P, et al. Drug-eluting bead, irinotecan therapy of unresectable intrahepatic cholangiocarcinoma (DELTIC) with concomitant systemic gemcitabine and cisplatin. Ann Surg Oncol 2022;29:5462-73.
68. Cucchetti A, Cappelli A, Mosconi C, et al. Improving patient selection for selective internal radiation therapy of intra-hepatic cholangiocarcinoma: a meta-regression study. Liver Int 2017;37:1056-64.
69. Edeline J, Du FL, Rayar M, et al. Glass Microspheres 90Y selective internal radiation therapy and chemotherapy as first-line treatment of intrahepatic cholangiocarcinoma. Clin Nucl Med 2015;40:851-5.
70. Edeline J, Touchefeu Y, Guiu B, et al. Radioembolization plus chemotherapy for first-line treatment of locally advanced intrahepatic cholangiocarcinoma: a phase 2 clinical trial. JAMA Oncol 2020;6:51-9.
71. SIRT followed by CIS-GEM chemotherapy versus CIS-GEM chemotherapy alone as 1st line treatment of patients with unresectable intrahepatic cholangiocarcinoma (SIRCCA). Available from: https://clinicaltrials.gov/ct2/show/study/NCT02807181 [Last accessed on 27 Sep 2023].
72. Akateh C, Ejaz AM, Pawlik TM, Cloyd JM. Neoadjuvant treatment strategies for intrahepatic cholangiocarcinoma. World J Hepatol 2020;12:693-708.
73. Holster JJ, El Hassnaoui M, Franssen S, et al. Hepatic arterial infusion pump chemotherapy for unresectable intrahepatic cholangiocarcinoma: a systematic review and meta-analysis. Ann Surg Oncol 2022;29:5528-38.
74. Franssen S, Soares KC, Jolissaint JS, et al. Comparison of hepatic arterial infusion pump chemotherapy vs resection for patients with multifocal intrahepatic cholangiocarcinoma. JAMA Surg 2022;157:590-6.
75. Oh D, Ruth He A, Qin S, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid 2022:1.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
An T, Wehrenberg-Klee E. Trans-arterial chemoembolization and radioembolization for treatment of intrahepatic cholangiocarcinoma. Hepatoma Res 2023;9:43. http://dx.doi.org/10.20517/2394-5079.2023.60
AMA Style
An T, Wehrenberg-Klee E. Trans-arterial chemoembolization and radioembolization for treatment of intrahepatic cholangiocarcinoma. Hepatoma Research. 2023; 9: 43. http://dx.doi.org/10.20517/2394-5079.2023.60
Chicago/Turabian Style
An, Thomas, Eric Wehrenberg-Klee. 2023. "Trans-arterial chemoembolization and radioembolization for treatment of intrahepatic cholangiocarcinoma" Hepatoma Research. 9: 43. http://dx.doi.org/10.20517/2394-5079.2023.60
ACS Style
An, T.; Wehrenberg-Klee E. Trans-arterial chemoembolization and radioembolization for treatment of intrahepatic cholangiocarcinoma. Hepatoma. Res. 2023, 9, 43. http://dx.doi.org/10.20517/2394-5079.2023.60
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 5 clicks
Cite This Article 5 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.