The future direction of liver transplantation for intrahepatic cholangiocarcinoma
Abstract
Liver transplantation has emerged as a potential therapeutic option for select patients with intrahepatic cholangiocarcinoma (iCCA) who are not amenable to curative resection. Recent studies have challenged the traditional notion that liver transplantation is contraindicated for iCCA, leading to a paradigm shift in its management. This review provides a comprehensive synthesis of the evidence regarding the role of liver transplantation in the treatment of very early or advanced iCCA and discusses the key challenges and future directions in this rapidly evolving field. For patients with cirrhosis and very early iCCA, liver transplantation has demonstrated excellent long-term survival rates, rivaling those of patients with hepatocellular carcinoma. However, the current transplantation criteria based on tumor size and number may be overly restrictive, excluding potential candidates who could benefit from this treatment. The incorporation of tumor markers into selection criteria may improve prognostic prediction and patient outcomes. In advanced iCCA, liver transplantation remains controversial but holds promise, especially when combined with neoadjuvant and adjuvant therapies. Donor organ scarcity necessitates the consideration of living donor liver transplantation as an alternative, while strategies such as utilizing marginal donors and exploring xenotransplantation offer potential solutions to address the shortage of donor livers. Overall, the evolving understanding of iCCA and the development of novel treatment strategies promise to refine and enhance the role of liver transplantation in the management of this challenging malignancy.
Keywords
INTRODUCTION
In recent years, the landscape of liver transplantation for treating intrahepatic cholangiocarcinoma (iCCA) has undergone significant changes. iCCA, a primary liver malignancy, constitutes the second most common form of liver cancer, accounting for 10%-15% of all primary liver malignancies[1-3]. Its incidence has consistently risen worldwide, posing a considerable challenge to healthcare systems[4]. Despite advances in surgical techniques and systemic therapies, the prognosis for patients with iCCA remains unfavorable, particularly for patients who cannot be a candidate for curative resection due to background liver cirrhosis or advanced stages of the disease[5,6]. Liver transplantation has emerged as a potential therapeutic option for a subset of these patients, offering an opportunity for long-term survival and improved quality of life[7-9].
Traditionally, liver transplantation has been considered a contraindication for iCCA, due to the high recurrence rates and poor post-transplant outcomes associated with this malignancy[10,11]. Nonetheless, accumulating evidence from recent studies has led to a paradigm shift in the management of iCCA, with liver transplantation increasingly recognized as a viable option for select patients[3,8]. This change has been driven primarily by the identification of acceptable post-transplant outcomes in incidentally discovered small iCCA in explant pathology[7], as well as the development of effective neoadjuvant treatment capable of controlling advanced iCCA[9].
However, the role of liver transplantation in the management of very early or advanced iCCA remains a subject of ongoing debate and investigation. While two studies have reported encouraging outcomes following transplantation for early-stage iCCA[7,8], there are concerns about the potential risk of tumor recurrence and the allocation of scarce donor organs to this patient population. Moreover, the optimal selection criteria and treatment strategies for patients with advanced iCCA have yet to be established. In this context, this review aims to provide a current synthesis of the evidence regarding the role of liver transplantation in managing very early or advanced iCCA, as well as to discuss the key challenges and future directions in this rapidly evolving field.
LIVER CIRRHOSIS PATIENTS WITH INTRAHEPATIC CHOLANGIOCARCINOMA
Liver cirrhosis, a consequence of chronic liver injury, is characterized by fibrosis, nodule formation, and subsequent loss of liver function, predisposing patients to the development of primary liver malignancies[12]. iCCA is known to arise in the context of liver cirrhosis in a subset of patients, with the prevalence of cirrhosis in iCCA patients ranging from 20% to 50%[13,14]. The population of liver cirrhosis patients with iCCA exhibits specific characteristics in terms of etiology, clinical presentation, and prognosis compared to non-cirrhotic patients with iCCA. The etiology of liver cirrhosis in iCCA patients is often multifactorial, with several risk factors contributing to its development. Common etiologies include chronic viral hepatitis (hepatitis B and C), alcohol-induced liver disease, nonalcoholic steatohepatitis (NASH), primary sclerosing cholangitis (PSC), and certain metabolic disorders[15,16]. Viral hepatitis, in particular, has been identified as a significant risk factor for iCCA in cirrhotic patients[17].
Differentiating hepatocellular carcinoma (HCC) and iCCA in a cirrhotic liver poses a diagnostic challenge. The mainstay for this diagnosis hinges on imaging techniques. HCC, which originates from hepatocytes, often demonstrates an arterial enhancement pattern. In contrast, iCCA, stemming from hepatic parenchyma and showcasing desmoplastic features, relies primarily on the portal system for its blood supply. Consequently, iCCA is often characterized by a peripheral rim-like contrast enhancement during arterial and portal phases, with a more attenuated center during the delayed phase[18,19].
Due to the challenges in diagnosis, a growing number of patients undergoing liver transplantation because of end-stage liver disease or suspected HCC have either HCC or iCCA upon pathological examination, potentially impacting patient outcomes negatively and leading to the suboptimal utilization of scarce organs[20]. In light of the increasing recognition that iCCA is more prevalent than previously believed, it may be prudent to place greater emphasis on liver biopsy for the pre-liver transplantation diagnosis of iCCA. This is especially pertinent for nodules emerging in cirrhosis that do not exhibit typical contrast enhancement patterns in imaging studies.
LIVER RESECTION FOR LIVER CIRRHOSIS PATIENTS WITH INTRAHEPATIC CHOLANGIOCARCINOMA
Liver resection is considered a potentially curative treatment for iCCA in patients with preserved liver function, and it remains the first-line therapeutic option for liver cirrhosis patients with iCCA[21,22]. The indication for liver resection in these patients is determined by several factors, including the extent of cirrhosis, the presence and severity of portal hypertension, the patient's overall performance status, and the location and size of the tumor[23,24]. A comprehensive assessment of liver function, typically using the Child-Pugh classification and the Model for End-stage Liver Disease (MELD) score, is crucial for determining the patient's suitability for liver resection[25].
In patients with well-compensated cirrhosis (Child-Pugh class A) and without clinically significant portal hypertension, liver resection can be safely performed with acceptable perioperative outcomes[26]. However, patients with more advanced cirrhosis (Child-Pugh class B or C) or significant portal hypertension are at a higher risk of postoperative complications, including liver failure, and may not be considered suitable candidates for liver resection[27]. Recent advances in surgical techniques, such as laparoscopic and robotic approaches, may contribute to reduced surgical morbidity and improved outcomes in selected patients[28].
The surgical outcomes of liver resection for liver cirrhosis patients with iCCA have been shown to be influenced by various factors, such as the extent of liver resection, the presence of microvascular invasion, node metastases status, and the tumor differentiation grade[29]. Although liver resection can provide favorable long-term survival rates in well-selected patients, tumor recurrence remains a significant challenge[30]. The reported 5-year overall survival rate after liver resection for iCCA in patients with liver cirrhosis is approximately 20%[30,31]. The strategy of salvage liver transplantation, which involves conducting a liver transplant after hepatectomy if cancer recurs, is a concept worth exploring. However, in the context of iCCA and donor scarcity, further comprehensive investigations are needed to establish the efficacy and survival benefit of this approach. Moreover, patients with cirrhosis may encounter a higher risk of postoperative complications, such as liver decompensation, bleeding, and infection, in comparison to non-cirrhotic patients[32]. Liver resection represents a viable therapeutic option for well-selected liver cirrhotic patients with iCCA. On the other hand, numerous decompensated cirrhosis patients with iCCA lack curative treatment options, even if the tumor is small enough to have a favorable prognosis if completely removed.
PROGNOSIS OF UNRESECTABLE ICCA PATIENTS
Owing to multiple intrahepatic lesions, local infiltration, lymph node involvement, and distant metastases, a considerable number of patients are not eligible for operative procedures[4,33]. Reportedly, approximately 60%-70% of iCCA patients present with conditions that are unresectable[4]. Without any form of treatment, the median survival time for patients with unresectable iCCA ranges from 2.5 to 7.5 months[34]. The first-line chemotherapy for unresectable iCCA, typically comprising gemcitabine and cisplatin, demonstrates limited effects on overall survival (OS)[35,36]. Although a prior study indicated that GEMOX (gemcitabine combined with oxaliplatin) chemotherapy was the recommended treatment for cholangiocarcinoma patients, its response rate was 21.4%, with the median recurrence-free survival (RFS) and OS time of 2.5 and 14.5 months, respectively[37]. Data on chemotherapy for iCCA are limited, derived from case series and retrospective studies with varied results due to the restricted sample size and absence of control groups. The effectiveness and appropriateness of these treatments are largely dictated by factors such as the patient’s overall health status, tumor characteristics, and response to therapy. Additionally, patients with iCCA confront additional challenges regarding chemotherapy due to the heightened risk of treatment-related toxicity and reduced OS[38].
Recently, immunotherapies, particularly immune checkpoint inhibitors, have shown promise in treating advanced iCCA. Checkpoint inhibitors, such as pembrolizumab and nivolumab, have exhibited durable responses and enhanced survival outcomes in patients with unresectable or recurrent biliary tract cancer[39]. These agents may offer a potential bridge to transplantation or even serve as adjuvant therapy in combination with liver transplantation. Recent studies have reported successful liver transplantation cases following neoadjuvant immunotherapy in patients with advanced iCCA, suggesting a potential role for this approach in the management of selected patients[40].
Beyond immune checkpoint inhibitors, adoptive cell therapies such as natural killer (NK) cells and chimeric antigen receptor T (CAR-T) cells have emerged as promising immunotherapeutic strategies for advanced iCCA. Combing liver transplantation with adoptive cell therapies may improve patient outcomes by augmenting immune surveillance and targeting residual cancer cells[41,42]. Additional research is required to ascertain the safety and efficacy of this combined approach in advanced iCCA patients[43].
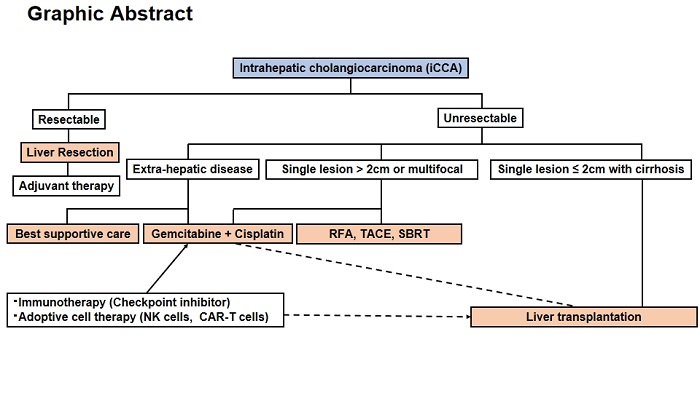
Meanwhile, therapies that target the local region, such as radiofrequency ablation (RFA), transarterial chemoembolization (TACE), and stereotactic body radiation therapy (SBRT), have demonstrated some promise in the treatment of unresectable iCCA[44,45]. A multi-center retrospective study revealed that 25% of patients with advanced iCCA achieved complete or partial response after intra-arterial embolization therapy (IAET), and 61% achieved disease stability[29]. The median survival duration for this cohort was 13.2 months, with 1, 3, and 5-year OS of 54.0%, 22.2%, and 16.2%, respectively. Meanwhile, a study that compared outcomes in incidental iCCA patients who underwent either IAET or RFA to those who did not indicates that locoregional therapies developed for HCC are not significantly effective treatments for iCCA[46]. Retrospective studies suggest that tumor size is a key determinant of RFA effectiveness and patient outcomes in iCCA, as one study found RFA ineffective on lesions larger than 4 cm[47,48]. A significant proportion of iCCA-related deaths occur due to local and locoregional progression rather than distant metastases. Despite their potential to provide local tumor control and extend survival in selected cases, they are not curative and might be associated with significant complications. Unresectable iCCA patients who are not suitable for chemotherapy or locoregional therapies may be managed with palliative care[49]. The current treatment strategy for iCCA is depicted in Graphical Abstract.
LIVER TRANSPLANTATION FOR VERY EARLY ICCA IN PATIENTS WITH CIRRHOSIS
Liver transplantation has been demonstrated to provide survival benefits for patients with early-stage iCCA in the setting of cirrhosis[40]. A recent investigation identified that a subset of cirrhotic patients with very early iCCA, denoted by a single tumor ≤ 2 cm, attained an impressive 5-year survival rate of up to 73% following liver transplantation[50]. The recurrence rate of these early-stage iCCAs over five years was as low as 18%, in contrast to 65% for advanced iCCAs comprising multiple or larger tumors (> 2 cm)[8]. Consequently, liver transplantation should not be ruled out for patients with a single iCCA less than 2 cm, as their long-term prognosis may align with that of HCC patients. However, the current criteria for transplantation, primarily based on tumor size and number, may be overly restrictive, disqualifying some patients who could potentially benefit from this treatment. Additionally, the risk of recurrence post-transplantation remains a concern, underscoring the need for more robust selection criteria[8]. One potential approach for expanding the indications for liver transplantation in early iCCA could involve initially targeting smaller tumors, such as those measuring 2 cm or less. This would facilitate the gradual incorporation of criteria to encompass multiple tumors (up to two) or solitary tumors measuring up to 3 or 4 cm with low tumor marker levels (i.e., carbohydrate antigen 19-9).
The Milan Criteria, which have been widely adopted for determining liver transplant eligibility in HCC, concentrate primarily on tumor morphology. However, this approach may be insufficient for iCCA, as tumor biology plays a pivotal role in determining prognosis and the risk of recurrence following transplantation. Potential candidates for tumor biology markers include molecular profiling, immune cell infiltration, and the tumor microenvironment. Integrating these factors into the selection criteria could result in more accurate prognostic predictors and improved patient outcomes[4].
LIVER TRANSPLANTATION FOR ADVANCED ICCA
Historically, liver transplantation has been regarded as a high-risk treatment option for advanced iCCA due to elevated rates of tumor recurrence and suboptimal OS outcomes. In contrast, perihilar CCA (phCCA) is now increasingly recognized as an indication for liver transplantation under the Mayo Protocol[51,52]. In early efforts at liver transplantation for CCA, the specific subtypes were often not distinguished, which included iCCA in the transplantations. However, the results remained disappointing until the introduction of neoadjuvant protocols, such as the Mayo Protocol applied solely to phCCA. Studies that incorporate neoadjuvant therapy before liver transplantation, similar to the Mayo Protocol, are currently limited to small case series. In general, it can be inferred that while liver transplantation can enhance outcomes compared to palliative therapy for unresectable iCCA, the outcomes, compared to transplantation for other indications, including phCCA, are presently inferior, albeit evidence remains limited. Nevertheless, recent promising studies suggest that liver transplantation may be a viable option for patients with non-resectable iCCA. An exploratory study by Hong et al. analyzed the data from patients who received liver transplants for locally advanced iCCA to identify potential beneficiaries[53], revealing that patients in the group without any predictive recurrence factors (e.g., multiple tumors, perineural invasion, invasive growth patterns, absence of neoadjuvant and adjuvant therapy, and lymphovascular invasion) could achieve a high 5-year RFS rate of up to 78%. Liver transplantation appeared to provide superior RFS compared to radical liver resection accompanied by bile duct resection in cases of locally advanced iCCA[54]. Notably, transplant patients who underwent neoadjuvant and adjuvant treatment demonstrated a more pronounced survival advantage (5-year RFS: 47% vs. 20%). In a recent prospective study, six patients with non-resectable locally advanced iCCA who demonstrated at least 6 months of stable or regressing disease after neoadjuvant chemotherapy underwent liver transplantation[9]. The median duration of follow-up for transplant recipients was 36 months, with 5-year OS and RFS rates of 83.3% and 50%, respectively. Nonetheless, liver transplantation following a regimen of gemcitabine and cisplatin should be reserved for highly selected cases only.
DONOR LIVER FOR LIVER TRANSPLANTATION FOR ICCA
The demand for donor livers far exceeds the available supply, resulting in patients with HCC and other malignancies often encountering extended waiting times for transplantation[55]. This scarcity is particularly problematic in transplant oncology, as patients with advanced-stage cancers may have a limited timeframe before their disease progresses beyond the point where transplantation remains a viable option. Studies have suggested that hepatectomy and liver transplantation show comparable postoperative outcomes and survival rates in patients with iCCA[56]. Consequently, given the considerable resources and the requirement for chronic immunosuppression associated with transplantation, the option of liver resection should be given serious consideration, particularly in cirrhotic patients with well-preserved liver function. Living donor liver transplantation (LDLT) is an alternative to deceased donor liver transplantation that can help alleviate the organ shortage. In LDLT, a healthy living donor donates a portion of their liver to the recipient, allowing both the donor’s and recipient’s livers to regenerate to near-normal size and function[57]. Although LDLT has been successfully utilized in patients with HCC, its application in iCCA patients remains limited and warrants further investigation. A 2021 meta-analysis by Ziogas et al. indicated that merely 6.6% of all liver transplantations for iCCA were performed with LDLT[40]. LDLT should only be considered when the potential risk to the donor can be justified by a reasonable outcome for the recipient. As the outcome for the donor improves, even after right-sided hepatectomy, an ideal 5-year OS post-liver transplantation of 83.3%, as reported by Lunsford et al., can be justified[9]. Given the low median OS of 10.3 months for patients with advanced cholangiocarcinoma, LDLT could be a valuable option for tumors otherwise deemed non-resectable[58]. Strategies to expand the donor pool include the utilization of marginal donors, such as donation after circulatory death (DCD) livers, aged donors, or donors initially discarded but subsequently recovered using machine perfusion techniques[59]. Additionally, donors with a history of cancer and moderate/high transmission risks, such as neuroblastoma, breast cancer, and colon cancer, could be considered in select cases[60].
Xenotransplantation, the transplantation of organs from animals to humans, is an emerging field with the potential to address the shortage of donor livers. Preclinical studies have demonstrated the feasibility of employing pig livers for transplantation; however, significant immunological and ethical challenges must be overcome before implementing this approach in clinical practice[61].
Patients with iCCA face unique challenges within the context of liver transplantation, as they often fail to meet traditional criteria for transplantation based on tumor size and number. The use of exception points, which grant additional priority on the waiting list to patients with specific medical conditions, could help to address these challenges and ensure that iCCA patients have access to transplantation when appropriate[62].
CONCLUSION
The prognosis of iCCA remains challenging due to the complexity and aggressiveness of the disease. While liver transplantation provides a potentially curative option for a select group of patients with early-stage iCCA, particularly those with liver cirrhosis, its application in advanced iCCA lacks robust evidence. Notably, studies suggest that integrating neoadjuvant and adjuvant therapies may improve post-transplant outcomes, opening avenues for further research. However, in the context of donor scarcity, careful evaluation and balance of risk and benefit become paramount. The evolving understanding of iCCA and the continual development of novel treatment strategies promise to refine and enhance the current therapeutic landscape.
DECLARATIONS
Authors’ ContributionsParticipated in research design; Akabane M, Sasaki K
Participated in the writing of the paper; Akabane M, Sasaki K
Participated in the critical review: Akabane M, Imaoka Y, Sasaki K
Availability of Data and MaterialsNot applicable.
Financial Support and SponsorshipNone.
Conflicts of InterestAll authors declared that there are no conflicts of interest.
Ethical Approval and Consent to ParticipateNot applicable.
Consent for PublicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. Banales JM, Cardinale V, Carpino G, et al. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European network for the study of cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol 2016;13:261-80.
2. Bertuccio P, Malvezzi M, Carioli G, et al. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol 2019;71:104-14.
3. Mauro E, Ferrer-Fàbrega J, Sauri T, et al. New challenges in the management of cholangiocarcinoma: the role of liver transplantation, locoregional therapies, and systemic therapy. Cancers 2023;15:1244.
4. Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 2014;60:1268-89.
5. Khan SA, Davidson BR, Goldin RD, et al; British society of gastroenterology. guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut 2012;61:1657-69.
6. Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol 2018;15:95-111.
7. Sapisochin G, de Lope CR, Gastaca M, et al. Intrahepatic cholangiocarcinoma or mixed hepatocellular-cholangiocarcinoma in patients undergoing liver transplantation: a Spanish matched cohort multicenter study. Ann Surg 2014;259:944-52.
8. Sapisochin G, Facciuto M, Rubbia-Brandt L, et al; iCCA International Consortium. Liver transplantation for "very early" intrahepatic cholangiocarcinoma: international retrospective study supporting a prospective assessment. Hepatology 2016;64:1178-88.
9. Lunsford KE, Javle M, Heyne K, et al; Methodist–MD Anderson Joint Cholangiocarcinoma Collaborative Committee (MMAJCCC). Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: a prospective case-series. Lancet Gastroenterol Hepatol 2018;3:337-48.
10. Pichlmayr R. Is there a place for liver grafting for malignancy? Transplant Proc 1988;20:478-482.
11. Pichlmayr R, Weimann A, Tusch G, et al. Indications and role of liver transplantation for malignant tumors. Oncologist 1997;2:164-170.
14. Bergquist A, von Seth E. Epidemiology of cholangiocarcinoma. Best Pract Res Clin Gastroenterol 2015;29:221-32.
15. Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? J Hepatol 2012;57:69-76.
16. Welzel TM, Mellemkjaer L, Gloria G, et al. Risk factors for intrahepatic cholangiocarcinoma in a low-risk population: a nationwide case-control study. Int J Cancer 2007;120:638-41.
17. Li M, Li J, Li P, et al. Hepatitis B virus infection increases the risk of cholangiocarcinoma: a meta-analysis and systematic review. J Gastroenterol Hepatol 2012;27:1561-8.
18. Kang Y, Lee JM, Kim SH, Han JK, Choi BI. Intrahepatic mass-forming cholangiocarcinoma: enhancement patterns on gadoxetic acid-enhanced MR images. Radiology 2012;264:751-60.
19. Kodali S, Shetty A, Shekhar S, Victor DW, Ghobrial RM. Management of intrahepatic cholangiocarcinoma. J Clin Med 2021;10:2368.
20. Sapisochin G, Fidelman N, Roberts JP, Yao FY. Mixed hepatocellular cholangiocarcinoma and intrahepatic cholangiocarcinoma in patients undergoing transplantation for hepatocellular carcinoma. Liver Transpl 2011;17:934-42.
21. Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg 2014;149:565-74.
22. Reese T, Pagel G, Bause BA, von Rittberg Y, Wagner KC, Oldhafer KJ. Complex liver resections for intrahepatic cholangiocarcinoma. J Clin Med 2021;10:1672.
23. Mullen JT, Ribero D, Reddy SK, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg 2007;204:854-62; discussion 862.
24. Hackl C, Schlitt HJ, Renner P, Lang SA. Liver surgery in cirrhosis and portal hypertension. World J Gastroenterol 2016;22:2725-35.
25. D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 2006;44:217-31.
26. Gerber DA. Safely expanding surgical resection for patients with hepatocellular carcinoma and cirrhosis. Dig Med Res 2020;3:24-24.
27. Abbas N, Fallowfield J, Patch D, et al. Guidance document: risk assessment of patients with cirrhosis prior to elective non-hepatic surgery. Available from: https://discovery.ucl.ac.uk/id/eprint/10167962 [Last accessed on 6 Jul 2023].
28. Coelho FF, Kruger JA, Fonseca GM, et al. Laparoscopic liver resection: experience based guidelines. World J Gastrointest Surg 2016;8:5-26.
29. Hyder O, Hatzaras I, Sotiropoulos GC, et al. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery 2013;153:811-8.
30. Spolverato G, Vitale A, Cucchetti A, et al. Can hepatic resection provide a long-term cure for patients with intrahepatic cholangiocarcinoma? Cancer 2015;121:3998-4006.
31. Lang H, Sotiropoulos GC, Sgourakis G, et al. Operations for intrahepatic cholangiocarcinoma: single-institution experience of 158 patients. J Am Coll Surg 2009;208:218-28.
32. Friedman LS. Surgery in the patient with liver disease. Trans Am Clin Climatol Assoc 2010;121:192-204.
33. Lieser MJ, Barry MK, Rowland C, Ilstrup DM, Nagorney DM. Surgical management of intrahepatic cholangiocarcinoma: a 31-year experience. J Hepatobiliary Pancreat Surg 1998;5:41-7.
34. Park J, Kim MH, Kim KP, et al. Natural history and prognostic factors of advanced cholangiocarcinoma without surgery, chemotherapy, or radiotherapy: a large-scale observational study. Gut Liver 2009;3:298-305.
35. Valle J, Wasan H, Palmer DH, et al; ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81.
36. Konstantinidis IT, Groot Koerkamp B, Do RK, et al. Unresectable intrahepatic cholangiocarcinoma: systemic plus hepatic arterial infusion chemotherapy is associated with longer survival in comparison with systemic chemotherapy alone. Cancer 2016;122:758-65.
37. André T, Tournigand C, Rosmorduc O, et al; GERCOR Group. Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol 2004;15:1339-43.
38. Constance D, Mairead GM, Samuel LS, et al. Influence of cirrhosis on outcomes of patients with advanced intrahepatic cholangiocarcinoma receiving chemotherapy. J Clin Oncol 2022;40:4_suppl, 475.
39. Ueno M, Ikeda M, Morizane C, et al. Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: a non-randomised, multicentre, open-label, phase 1 study. Lancet Gastroenterol Hepatol 2019;4:611-21.
40. Ziogas IA, Giannis D, Economopoulos KP, et al. Liver transplantation for intrahepatic cholangiocarcinoma: a meta-analysis and meta-regression of survival rates. Transplantation 2021;105:2263-71.
41. Wang Z, Cao YJ. Adoptive cell therapy targeting neoantigens: a frontier for cancer research. Front Immunol 2020;11:176.
42. Jindal V, Arora E, Masab M, Gupta S. Chimeric antigen receptor T cell therapy in pancreatic cancer: from research to practice. Med Oncol 2018;35:84.
43. Vignone A, Biancaniello F, Casadio M, et al. Emerging therapies for advanced cholangiocarcinoma: an updated literature review. J Clin Med 2021;10:4901.
44. Kuhlmann JB, Euringer W, Spangenberg HC, et al. Treatment of unresectable cholangiocarcinoma: conventional transarterial chemoembolization compared with drug eluting bead-transarterial chemoembolization and systemic chemotherapy. Eur J Gastroenterol Hepatol 2012;24:437-43.
45. Borakati A, Froghi F, Bhogal RH, Mavroeidis VK. Stereotactic radiotherapy for intrahepatic cholangiocarcinoma. World J Gastrointest Oncol 2022;14:1478-89.
46. Takahashi K, Obeid J, Burmeister CS, et al. Intrahepatic cholangiocarcinoma in the liver explant after liver transplantation: histological differentiation and prognosis. Ann Transplant 2016;21:208-15.
47. Giorgio A, Calisti G, DE Stefano G, et al. Radiofrequency ablation for intrahepatic cholangiocarcinoma: retrospective analysis of a single centre experience. Anticancer Res 2011;31:4575-80.
48. Zhang H, Yang T, Wu M, Shen F. Intrahepatic cholangiocarcinoma: epidemiology, risk factors, diagnosis and surgical management. Cancer Lett 2016;379:198-205.
49. Smith TJ, Temin S, Alesi ER, et al. American society of clinical oncology provisional clinical opinion: the integration of palliative care into standard oncology care. J Clin Oncol 2012;30:880-7.
50. Sapisochin G, Rodríguez de Lope C, Gastaca M, et al. "Very early" intrahepatic cholangiocarcinoma in cirrhotic patients: should liver transplantation be reconsidered in these patients? Am J Transplant 2014;14:660-7.
51. Darwish Murad S, Kim WR, Harnois DM, et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology 2012;143:88-98.e3; quiz e14.
52. Heimbach JK, Gores GJ, Haddock MG, et al. Liver transplantation for unresectable perihilar cholangiocarcinoma. Semin Liver Dis 2004;24:201-7.
53. Hong JC, Petrowsky H, Kaldas FM, et al. Predictive index for tumor recurrence after liver transplantation for locally advanced intrahepatic and hilar cholangiocarcinoma. J Am Coll Surg 2011;212:514-20; discussion 520.
54. Hong JC, Jones CM, Duffy JP, et al. Comparative analysis of resection and liver transplantation for intrahepatic and hilar cholangiocarcinoma: a 24-year experience in a single center. Arch Surg 2011;146:683-9.
55. Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2016 annual data report: liver. Am J Transplant 2018;18 Suppl 1:172-253.
56. Hue JJ, Rocha FG, Ammori JB, et al. A comparison of surgical resection and liver transplantation in the treatment of intrahepatic cholangiocarcinoma in the era of modern chemotherapy: an analysis of the national cancer database. J Surg Oncol 2021;123:949-56.
57. Hwang S, Lee SG, Lee YJ, et al. Lessons learned from 1,000 living donor liver transplantations in a single center: how to make living donations safe. Liver Transpl 2006;12:920-7.
58. Filippi R, Montagnani F, Lombardi P, et al. A prognostic model in patients with advanced biliary tract cancer receiving first-line chemotherapy. Acta Oncol 2021;60:1317-24.
59. Dutkowski P, Oberkofler CE, Slankamenac K, et al. Are there better guidelines for allocation in liver transplantation? Ann Surg 2011;254:745-53; discussion 753.
60. Nalesnik MA, Woodle ES, Dimaio JM, et al. Donor-transmitted malignancies in organ transplantation: assessment of clinical risk. Am J Transplant 2011;11:1140-7.
61. Ekser B, Li P, Cooper DKC. Xenotransplantation: past, present, and future. Curr Opin Organ Transplant 2017;22:513-21.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Akabane M, Imaoka Y, Sasaki K. The future direction of liver transplantation for intrahepatic cholangiocarcinoma. Hepatoma Res 2023;9:29. http://dx.doi.org/10.20517/2394-5079.2023.45
AMA Style
Akabane M, Imaoka Y, Sasaki K. The future direction of liver transplantation for intrahepatic cholangiocarcinoma. Hepatoma Research. 2023; 9: 29. http://dx.doi.org/10.20517/2394-5079.2023.45
Chicago/Turabian Style
Akabane, Miho, Yuki Imaoka, Kazunari Sasaki. 2023. "The future direction of liver transplantation for intrahepatic cholangiocarcinoma" Hepatoma Research. 9: 29. http://dx.doi.org/10.20517/2394-5079.2023.45
ACS Style
Akabane, M.; Imaoka Y.; Sasaki K. The future direction of liver transplantation for intrahepatic cholangiocarcinoma. Hepatoma. Res. 2023, 9, 29. http://dx.doi.org/10.20517/2394-5079.2023.45
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 7 clicks
Cite This Article 7 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.