Combined hepatocellular-cholangiocarcinoma: morpho-molecular updates and considerations
Abstract
Combined Hepatocellular-Cholangiocarcinoma is a heterogenous primary malignant epithelial tumor of the liver with variable morphological and immunophenotypical features. Although the biology of this tumor has been described in the literature, changes in classification and its heterogeneity imply difficulties in collecting reliable homogenous groups to compare. The article aims to review available data on morphology and immunohistochemistry for practicing pathologists integrated with original data from our referral Center.
Keywords
INTRODUCTION
Combined hepatocellular-cholangiocarcinoma (cHCC-CCA) is a heterogenous primary liver carcinoma (around 2%-5%)[1] in which both hepatocellular and cholangiocellular differentiation is present. Diagnosis relies on morphological aspects with routine haematoxylin-eosin (H&E) which can be confirmed, if needed, by immunohistochemistry (IHC). Classification has changed and evolved through the years due to the heterogenous presentation of this tumor type. In the nineteen-forties, Allen and Lisa[2] proposed to differentiate between tumors with features of both hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (iCCA), and distinct morphological lesions of HCC and iCCA in the same liver in which three different entities could be described in the liver: (1) Distinct concomitant HCC and iCCA lesions; (2) Contiguous HCC and iCCA lesions; and (3) a single lesion with cellular characteristics of both hepatocytes and cholangiocytes[2]. Only five years later, Edmondson and Steiner reported 4 cases of morphologically distinct cHCC-CCA tumors among 100 cases in an autopsy series[3].
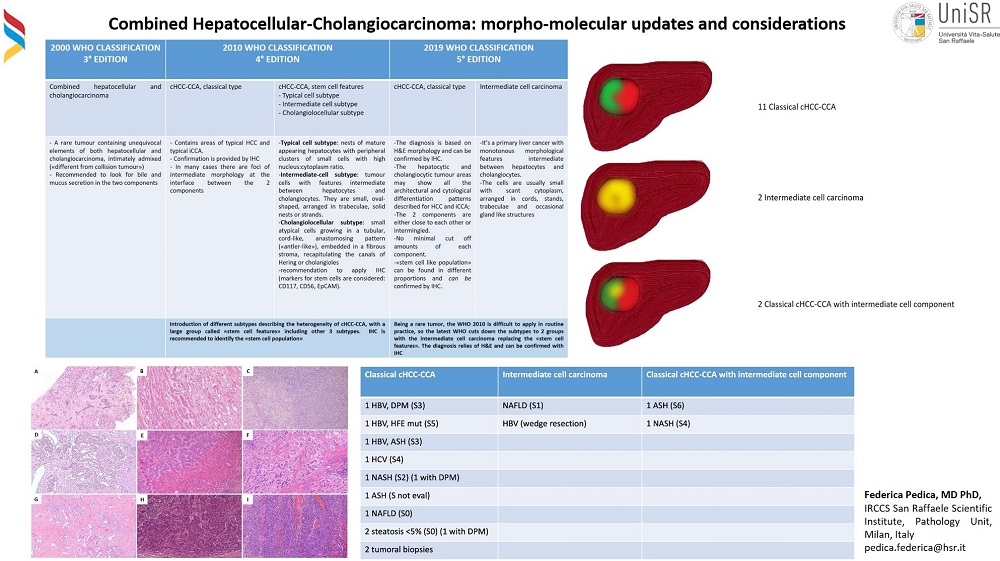
With the advent of IHC, classification was integrated to include this additional descriptor, and with the turn of the century, the WHO suggested novel assays, pCEA and HepPar1 markers, to better describe and classify hepatocellular differentiation within mixed HCC-CCA, which they first defined as tumor characterized by both HCC and iCCA intimately admixed[4].The 4th edition, published in 2010[5], further refined the definition of this tumor type, subdividing cHCC-CCA into classical cHCC-CCA and cHCC-CCA with stem cell features; three subtypes, typical, intermediate-cell, and cholangiolocellular, were also identified.
The 5th and latest edition of the WHO (2019[1]) simplified subtypes and returned to descriptors used in the 3rd edition (2000[4]), which were adopted, from the conclusions of the International Consensus Group reported by Brunt et al.[6]. It defines cHCC-CCA as consisting of its two components, HCC and CCA, which must be very close or intermingled, without a clear transition and without limits in their proportion and, therefore, with no cut-off for the percentage of the two components. Furthermore, IHC is a support for the diagnosis and is not sufficient alone without an adequate morphological description. The terminology used in the previous version, with stem cell features[5], was no longer recommended since subclassification was applied with difficulty in routine practice due to disagreement between pathologists and “stem cell” features may be found in all cHCC-CCA and because immunohistochemical markers used for the confirming stem cell phenotype, such asCD56, EpCAM, CD133, and CK19[7], are not specific for stem cells. Current classification thus recognizes two broad subgroups of cHCC-CCA; a neoplastic mass made of two clear components of HCC and CCA and a mass made of tumoral cells with intermediate phenotype, morphologically and phenotypically, a mix of hepatocytes and cholangiocytes. The evolution of nomenclature is compared in Figure 1.
Figure 1. WHO classification: change over time in classification (2010 and 2019)(modified from 1,4,5).
The correct diagnosis implies extensive sampling of the liver mass (at least 1 sample for each cm of the maximum diameter) since cHCC-CCAs do not have specific macroscopic aspects and one of the two morphological components may be lost with incorrect tissue probing. Morphological patterns are very variable and include the different histological aspects described in HCC and iCCA. The iCCA component is generally characterized by a malignant glandular proliferation and is subdivided between “small duct type” and “large duct type”[1]; the first is more peripheral, the latter usually arises close to the hilum, is mucus-secreting and involves the perihilar extrahepatic tissue. The HCC component also tends to be very heterogeneous, with malignant differentiated proliferation of cells arranged in trabeculae, pseudoglands, and acinar structures[1], characterized by bile duct secretion, fat accumulation in the cytoplasm or cytoplasmic inclusions, probably due to accumulation of proteins[8]. Correct differentiation between iCCA and HCC is difficult as treatment guidelines are not well established, due to the heterogeneity of these tumors, their rare presentation, and limited data from which to draw conclusions. Biopsy differential diagnosis can be very challenging and may lead to an incorrect diagnosis of cancer of unknown primitive (CUP) due to poorly undifferentiated tissue, incomplete clinical information, inaccurate choice of correct immunohistochemical markers, and a non-specific grading system for cHCC-CCA[1], in particular for intermediate cell category tumors.
IMMUNOHISTOCHEMISTRY
To date, specific biomarkers for biliary differentiation are not able to distinguish normal and non-normal tissue; only specific markers for hepatocellular and cholangiocellular differentiation can be considered reliable [Figure 2].
If bile production is not visible on the embedded slide, hepatocyte-specific antigen/Hep-par1 (clone OCH1E5)[9] can be useful. This marker is also expressed in normal hepatocytes and associated with the urea cycle enzyme carbamoyl phosphatase synthetase 1, CPS1[10]. Unfortunately, it works well only when the tumor is well or at least moderately differentiated, but it has very poor sensitivity in poorly differentiated tumors. It also is not completely specific for hepatocellular neoplasms because occasionally gastric, esophageal, and lung adenocarcinomas show strong positive reactions and are thus called hepatoid adenocarcinomas[11]. When the tumors are less differentiated, Arginase 1 is very useful; this enzyme of the urea cycle[12], mainly expressed in the periportal hepatocytes, was first described as a powerful tool for diagnosis of origin in 2010[13]. Expressed both in normal and neoplastic hepatocytes, both in the nucleus and the cytoplasm. Detection sensitivity and specificity are superior compared to HepPar1, also in the poorly differentiated HCC[1] but can also be positive in hepatoid adenocarcinomas, similarly to HepPar1[14]. Glypican 3 is an oncofetal gene that encodes a protein of the prototypical cell-surface heparan sulfate proteoglycans and can be useful, especially in moderately and poorly differentiated HCC. It is not specific to HCC since it stains other different and very important tumors such as yolk sac tumors and squamous cell carcinoma of the lung[15]. For this reason, a strong correlation with morphology is mandatory for its interpretation. Nevertheless, testing for Arginase1 and Glypican 3 seems to increase sensitivity to approximately 100% in poorly differentiated HCC[16].
Alpha-fetoprotein (AFP) is a sugar-containing protein produced by the yolk sac and liver in the fetus. It is one of the most widely used serum biomarkers for the early detection and follow-up of HCCs[17]. AFP is expressed in only about one-third of cases of HCC[18] but can be useful in poorly differentiated HCCs[19].
Other commonly used markers are polyclonal antibodies against carcinoembryonic antigen (p-CEA) and CD10, with low sensitivity and specificity (less than 50%) in poorly differentiated HCC[20]. Moreover, CD10 is not lineage-specific; it is also expressed in numerous normal tissues, and also in epithelioid hemangioendothelioma[21], which can be tricky to diagnose on liver biopsy.
Albumin was considered as a potential tool for the identification of HCC almost 40 years ago[22], but only recently, with branched chain in situ hybridization assay[23], has been shown to be applicable in routine diagnostics. Albumin is produced by hepatocytes, so it should be specific for hepatocellular origin, but like anti-hepatocyte specific antigen/HepPar1, it is also expressed in acinar cell carcinoma[24] and together with Arginase 1, can also be expressed in hepatoid carcinomas[25].
Other markers for hepatocellular origin are ATP-binding cassette (ABC) transporters which are the members of efflux pumps called bile salt export pump (BSEP) and the multidrug-resistance protein 3 (MDR3) which metabolize and remove cytotoxic agents. Both have high sensitivity and are specific for HCC, but not useful in poorly differentiated HCC[26]. In a recent study[26], their expression was investigated in hepatoid carcinomas, where they were found to be overexpressed in this subset of tumors, suggesting their value in this differential diagnosis.
To date, there are no specific markers for cholangiocytes, and the most commonly used remain CK7 and CK19 which, though, can also be expressed in HCC and other tumors. Poor prognosis remains correlated to CK 19 expression[27]; up to 10% of HCC can co-express CK7 and CK19 and approximately 18% can express at least CK7 or CK19[28]. Thus, diagnosis always relies on the combination between morphology and immunophenotype.
MOC31 is an antibody that targets EpCAM, a surface glycoprotein. Initially, it was considered as a good marker for differentiating CCA from HCC[11,29], but it is specific for diagnosis since it stains carcinomas of different origins[30]. In addition, it can be expressed in poorly differentiated HCC[31]. Another marker that is used for biliary differentiation is CA19.9, which was found to be positive in around 60% of CCA, but not present in HCC[32].
MOLECULAR STUDIES
Molecular characteristics of cHCC-CCA are described only in a few reports in literature, mainly due to the rarity of these tumors. Changes in classification definitions have also complicated the collection of reliable series since the family of cHCC-CCA is very heterogeneous in terms of molecular alterations.
The larger series come from Eastern countries where the main etiology is still HBV infection, which is not frequent in western countries. In fact, one of the most recent and extensive studies by Xue et al. is a pan-Asia multi-center study including 8 hospitals with 133 cases[33]. Of these, 75% were HBV positive, 5.3% HCV positive, 18.8% double negative and only one double positive. The authors used whole-exome and whole-genome RNA with single-cell nucleus sequencing to investigate the different molecular aspects. It is noteworthy that 22.9% of cases had the same hotspot mutation C228T in the TERT promoter and FGFR fusions were found in 6.5% of cases. Authors subdivided the cases between cHCC-CCA, characterized by clearly defined areas of HCC and CCA components in the same tumor, and mixed HCC-CCA, in which the two components were deeply intermingled without clear boundaries. They interestingly found that the first subgroup is more like CCA in terms of different gene pathways, while the second group is closer to HCC[33]. Another important result in this series was identifying a monoclonal cell of origin both for combined and mixed type[2], also supported by other studies (Fujii et al. 2000[34] and Moeini et al.[35]). Moeni et al studied a series of 18 cHCC-CCA including 6 cholangiolocarcinoma, 8 stem cell features, and 4 classical combined, according to WHO 2010. Cholangiolocarcinoma was characterized by chromosomal stability and activation of TGF pathway which was not found in the other categories. They also confirmed that the classical combined tumor shared features of both HCC and CCA and that the stem cell category had different molecular signatures, mainly regarding activation of proliferation[35], and characteristically expressed SALL4 as progenitor phenotype in 75% of cases.
Another important study by Joseph et al. from the University of California studied a series of 30 cases classified according to WHO 2010[5] which included 20 classical types cHCC-CCA and 10 CCA, with variable etiology; in particular, 60% of the cHCC-CCA were concomitant with HCV-related cirrhosis[36]. They applied broad capture-based sequencing of the genomic DNA collected, separating the HCC and CCA components. Eighty percent of cHCC-CCA showed TERT promoter mutations and 70% also had p53 alterations. iCCA in cirrhosis showed different alterations, mostly IDH1/2, FGFR2, CDKN2A, and those involving chromatin regulators, demonstrating that cHCC-CCA were more similar to HCC than CCA[36].
FOCUS ON OUR CASES
We retrospectively reviewed our internal histopathological series from 2012 to September 2022 and found 21 cases of cHCC-CCA in the 1263 intrahepatic resected liver tumors (1,7%) available. Tumor tissue was reclassified according to WHO 2019[1]; 6 were reclassified as cholangiolocarcinoma, considered iCCA, and 15 as cHCC-CCA, representing 1,2% of the intrahepatic liver tumors resected. The median age was 71 years and only 2 were female subjects (13,3%). Eleven were classified as classical type (73,3%) [Figure 3 A-F], 2 were intermediate cell phenotype (13,3%, Figure 3 G and H) and 2 showed both subtypes, including mature components of HCC and CCA but also a minor group of cells with intermediate phenotype (13,3%)
Figure 3. Morphological aspects of cHCC-CCA in our series. H&E, classical HCC-CCA can show different aspects, depending on how the cells intermingle with each other. There can be a sharp transition (10x, A) or a deep intermingle (20x, B,C,D,E,F,G); intermediate cell carcinoma is composed of small cells with glandular or solid organization and hyperchromatic nuclei (20x, H,I).
Figure 4. A case of classical HCC-CCA. This case shows an adenocarcinoma component (20x, A), a solid hepatocellular carcinoma part ( 20x, C), and an area of transition (20x, B) made of smaller cells which are partially positive for CK7 (40x, 1), glypican 3 (40x, 2), alpha-fetoprotein (40x, 3) and arginase 1 (40x, 4).
Regarding liver condition, four patients had HBV hepatitis (26%) and were associated with hemochromatosis in one case and non-alcoholic steatohepatitis (NASH) in another. Six patients had background liver fibrosis grade S3 (according to Ishak,[37]) or more; 5 cases showed no or mild fibrosis. In 4 cases, fibrosis could not be evaluated histologically because collected tissue was from tumor biopsies or resections of the nodule [Figure 5].
Figure 5. A detailed description of background liver function in our cases. S: staging of fibrosis according to Ishak[44], HFE mut-homeostatic iron regulator mutation; NASH- non-alcoholic steatohepatitis; ASH-alcoholic steatohepatitis.
Another interesting feature to report is that in 3 cases of classical HCC-CCA, the tumor had aspects of ductal plate malformation (DPM). One case [Figure 6A], in particular, had areas resembling DPM
Figure 6. Case of cHCC-CCA with features of biliary adenofibroma. (A) Gross features of the 2.5 cm nodule with yellow-greyish color, in an HBV-positive patient with S3 fibrosis. (B) H&E, 10x, areas resembling ductal plate malformation (DPM). (C) H&E, 10x, areas resembling biliary adenofibroma. (D) H&E 40x, Peculiar neoplastic glandular component (blu arrow) with intraglandular growth of HCC (green arrow), confirmed by IHC with arginase-1. (E) 20x (green arrow).
HCC with partial biliary differentiation following transarterial chemoembolization (TACE) [Figure 8], as described in the literature[38], was found in two cases. They have the typical presentation of and HCC, but in the proximity of the embolized foreign material, a neoplastic glandular component [Figure 8A and B] showing biliary differentiation (CK7 positive, Figure 8C) can be seen.
Figure 8. A case of biliary differentiation post-TACE. (A) H&E 20x, TACE foreign material, on the left, with close neoplastic glandular component and hepatocellular carcinoma on the right. (B) H&E, 20x, Another view of the adenocarcinoma component. (C) IHC for CK7 demonstrating clear biliary differentiation.
In cases in which lymphadenectomy was performed (in patients with biopsy diagnosis of adenocarcinoma, n = 5), two poorly differentiated cHHCC-CCA were metastatic; of these, one also had a sarcomatoid component.
FINAL CONSIDERATIONS
cHCC-CCA is a very heterogeneous malignant primary liver tumor that can be difficult to diagnose, especially on biopsies, and whose classification has changed over the years, complicating correct reporting and case presentations.
Clinical management is still not “standardized” and is left to personalized approaches in which the role of the pathologist is fundamental in order to aid in correct sampling procedures and accurate diagnosis of all the components, according to WHO 2019[1]. According to the literature, the main prognostic factors for cHCC-CCA are size (> 5 cm), multiple satellite nodules, lymph node metastases, vascular invasion, levels of tumoral markers (CA19-9 and CEA), and resection margins[39].
cHCC-CCA are fascinating tumors because, in the same mass, many different histological aspects can be found, from a classical HCC to a classical iCCA with different grades of differentiation and intermediate cells. These aspects recapitulate molecular characteristics[40]. The “stem cell-like” components, no longer recommended in reporting, can be nevertheless recognized by pathologists as smaller cells with scant cytoplasm and hyperchromatic nucleus, organized in ductular structures (different presentation from ductular reactions) and generally associated with a desmoplastic stroma. IHC can help with several markers [Figure 2], but morphology drives the diagnosis as no immunohistochemical staining is entirely specific. Reviewing our cases, we found that HBV infection was present in 26% of cases with cHCC-CCA, and approximately 5% in patients with iCCA. This interesting result could be the consequence of HBV integration in the DNA of the hepatocytes as previously investigated in the literature[41], but its biological meaning needs further investigation and the collection of well characterized cases of cHCC-CCA can help to better understand its biology.
The incidence of lymph node metastases in cHCC-CCA is around 20%, similar to that of intrahepatic CCA[40]; in our cases, it reached 33%, considering that only 5/12 patients performed lymphadenectomy.
Molecular analyses have a fundamental role as a descriptor of individualized, targetable alterations, but also in terms of confirmation of diagnosis. In fact, in our reported case above, both a mutation in the promoter of TERT, typically found in HCC[36], and ERBB2 amplification, described in cHCC-CCA but more frequent in biliary tract cancer found in extrahepatic biliary adenocarcinomas[40], were present.
The molecular profiles of cHCC-CCA seem to recapitulate the two different histological populations, yet the current WHO classification does not contemplate this, mostly due to the limited data available for this rare tumor type. The lack of data[42] also hinders official recommendations on lymphadenectomy, which has been shown to improve survival in patients with iCCA[43]. Correct differential diagnosis (HCC vs. CCA vs. cHCC-CCA) is also fundamental in terms of locoregional treatments for disease control, such as microwave ablation or transarterial chemoembolization[44] in HCC, or liver transplant[45], still debated but available treatment option in patients with HCC[46].
Correct sampling methods for unclear cases, precise morphological description, adequate molecular analysis, and up-to-date knowledge of the current scenario of hepatic mixed tumor types must be all proper to the pathologist in order to best describe the tumor and aid in offering the most adequate therapeutical option for these patients.
DECLARATIONS
AcknowledgmentsWe would like to thank Stephanie Steidler (U.O. Radiologia IRCCS Ospedale San Raffaele - Milano) for her careful critical reading of the manuscript and for the English Language review
Authors’ contributionsMade substantial contributions to study conception and design, data analysis and interpretation: Ahmed N, Falcinelli F, Rimini M, Burgio V, Casadei-Gardini A, Aldrighetti L, Ratti F, Pedica F
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declare that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. Sempoux C, Kakar S, Kondo F, Schirmacher P. Combined hepatocellular-cholangiocarcinoma and undifferentiated primary liver carcinoma. In: Arends MJ, Fukayama M, Klimstra DS, Lam AKY, Nagtegaal ID, Odze RD, et al., editors. WHO classification of tumours: digestive system tumours. 5th ed. Lyon: IARC; 2019. p. 260-2.
3. Edmondson HA, Steiner PE. Primary carcinoma of the liver. A study of 100 cases among 48,900 necropsies. Cancer 1954;7:462-503.
4. Hamilton SR, Lauri MD, Aaltonen A. WHO classification of tumours of the digestive system. 3 ed. Lyon, France: IARC press; 2000. Available from: https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/Pathology-And-Genetics-Of-Tumours-Of-The-Digestive-System-2000 [Last accessed on 28 Apr 2023].
5. Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. 4 ed.Lyon, France: IARC press; 2010. Available from: https://www.cabdirect.org/cabdirect/abstract/20113051318 [Last accessed on 28 Apr 2023].
6. Brunt E, Aishima S, Clavien PA, et al. cHCC-CCA: consensus terminology for primary liver carcinomas with both hepatocytic and cholangiocytic differentation. Hepatology 2018;68:113-26.
7. Beaufrère A, Calderaro J, Paradis V. Combined hepatocellular-cholangiocarcinoma: an update. J Hepatol 2021;74:1212-24.
9. Mivervini MI, Demetris AJ, Lee RG, et al. Utilization of hepatocyte-specific antibody in the immunocytochemical evaluation of liver tumors. Mod Pathol 1997;10:686-92.
10. Butler SL, Dong H, Cardona D, et al. The antigen for Hep Par 1 antibody is the urea cycle enzyme carbamoyl phosphate synthetase 1. Lab Invest 2008;88:78-88.
11. Kakar S, Gown AM, Goodman ZG, Ferrell LD. Best practices in diagnostic immunohistochemistry: hepatocellular carcinoma versus metastatic neoplasms. Arch Pathol Lab Med 2007;131:1648-54.
12. Multhaupt H, Fritz P, Schumacher K. Immunohistochemical localisation of arginase in human liver using monoclonal antibodies against human liver arginase. Histochemistry 1987;87:465-70.
13. Yan BC, Gong C, Song J, et al. Arginase-1: a new immunohistochemical marker of hepatocytes and hepatocellular neoplasms. Am J Surg Pathol 2010;34:1147-54.
14. Chandan VS, Shah SS, Torbenson MS, Wu TT. Arginase-1 is frequently positive in hepatoid adenocarcinomas. Human Pathology 2016; 55:11-6.
15. Mounajjed T, Zhang L, Wu TT. Glypican-3 expression in gastrointestinal and pancreatic epithelial neoplasms. Hum Pathol 2013;44:542-50.
16. Nguyen T, Phillips D, Jain D, et al. Comparison of 5 immunohistochemical markers of hepatocellular differentiation for the diagnosis of hepatocellular carcinoma. Arch Pathol Lab Med 2015;139:1028-34.
17. Debruyne EN, Delanghe JR. Diagnosing and monitoring hepatocellular carcinoma with alpha-fetoprotein: new aspects and applications. Clin Chim Acta 2008;395:19-26.
18. Brumm C, Schulze C, Charels K, Morohoshi T, Klöppel G. The significance of alpha-fetoprotein and other tumour markers in differential immunocytochemistry of primary liver tumours. Histopathology 1989;14:503-13.
19. Chu PG, Ishizawa S, Wu E, Weiss LM. Hepatocyte antigen as a marker of hepatocellular carcinoma: an immunohistochemical comparison to carcinoembryonic antigen, CD10, and alpha-fetoprotein. Am J Surg Pathol 2002;26:978-88.
20. Morrison C, Marsh W Jr, Frankel WL. A comparison of CD10 to pCEA, MOC-31, and hepatocyte for the distinction of malignant tumors in the liver. Mod Pathol 2002;15:1279-87.
21. Weinreb I, Cunningham KS, Perez-Ordoñez B, Hwang DM. CD10 is expressed in most epithelioid hemangioendotheliomas: a potential diagnostic pitfall. Arch Pathol Lab Med 2009;133:1965-8.
22. Kinoyama S, Yamada G, Okushin H, Mimura H, Kobayashi T, Tsuji T. Immunoelectron microscopic observation of alpha-fetoprotein synthesis in human non-malignant liver tissues using immunoperoxidase methods. Gastroenterol Jpn 1988;23:414-22.
23. Shahid M, Mubeen A, Tse J, et al. Branched chain in situ hybridization for albumin as a marker of hepatocellular differentiation: evaluation of manual and automated in situ hybridization platforms. Am J Surg Pathol 2015;39:25-34.
24. Askan G, Deshpande V, Klimstra DS, et al. Expression of markers of hepatocellular differentiation in pancreatic acinar cell neoplasms: a potential diagnostic pitfall. Am J Clin Pathol 2016;146:163-9.
25. Chandan VS, Shah SS, Torbenson MS, Wu TT. Arginase-1 is frequently positive in hepatoid adenocarcinomas. Hum Pathol 2016;55:11-6.
26. Fujikura K, Yamasaki T, Otani K, et al. BSEP and MDR3: useful immunohistochemical markers to discriminate hepatocellular carcinomas from intrahepatic cholangiocarcinomas and hepatoid carcinomas. Am J Surg Pathol 2016;40:689-96.
27. Durnez A, Verslype C, Nevens F, et al. The clinicopathological and prognostic relevance of cytokeratin 7 and 19 expression in hepatocellular carcinoma. A possible progenitor cell origin. Histopathology 2006;49:138-51.
28. Jain R, Fischer S, Serra S, Chetty R. The use of Cytokeratin 19 (CK19) immunohistochemistry in lesions of the pancreas, gastrointestinal tract, and liver. Appl Immunohistochem Mol Morphol 2010;18:9-15.
29. Karabork A, Kaygusuz G, Ekinci C. The best immunohistochemical panel for differentiating hepatocellular carcinoma from metastatic adenocarcinoma. Pathol Res Pract 2010;206:572-7.
30. Proca DM, Niemann TH, Porcell AI, DeYoung BR. MOC31 immunoreactivity in primary and metastatic carcinoma of the liver. Report of findings and review of other utilized markers. Appl Immunohistochem Mol Morphol 2000;8:120-5.
31. Morrison C, Marsh W Jr, Frankel WL. A comparison of CD10 to pCEA, MOC-31, and hepatocyte for the distinction of malignant tumors in the liver. Mod Pathol 2002;15:1279-87.
32. Tsuji M, Kashihara T, Terada N, Mori H. An immunohistochemical study of hepatic atypical adenomatous hyperplasia, hepatocellular carcinoma, and cholangiocarcinoma with alpha-fetoprotein, carcinoembryonic antigen, CA19-9, epithelial membrane antigen, and cytokeratins 18 and 19. Pathol Int 1999;49:310-7.
33. Xue R, Chen L, Zhang C, et al. Genomic and transcriptomic profiling of combined hepatocellular and intrahepatic cholangiocarcinoma reveals distinct molecular subtypes. Cancer Cell 2019;35:932-947.e8.
34. Fujii H, Zhu XG, Matsumoto T, et al. Genetic classification of combined hepatocellular-cholangiocarcinoma. Hum Pathol 2000;31:1011-7.
35. Moeini A, Sia D, Zhang Z, et al. Mixed hepatocellular cholangiocarcinoma tumors: cholangiolocellular carcinoma is a distinct molecular entity. J Hepatol 2017;66:952-61.
36. Joseph NM, Tsokos CG, Umetsu SE, et al. Genomic profiling of combined hepatocellular-cholangiocarcinoma reveals similar genetics to hepatocellular carcinoma. J Pathol 2019;248:164-78.
37. Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995;22:696-9.
38. Cotoi CG, Khorsandi SE, Pleşea IE, Quaglia A. Histological aspects of post-TACE hepatocellular carcinoma. Rom J Morphol Embryol 2012;53:677-82.
39. Zhao L, Wang Y, Tian T, et al. Analysis of viral integration reveals new insights of oncogenic mechanism in HBV-infected intrahepatic cholangiocarcinoma and combined hepatocellular-cholangiocarcinoma. Hepatol Int 2022;16:1339-52.
40. Wakizaka K, Yokoo H, Kamiyama T, et al. Clinical and pathological features of combined hepatocellular-cholangiocarcinoma compared with other liver cancers. J Gastroenterol Hepatol 2019;34:1074-80.
41. Murugesan K, Sharaf R, Montesion M, et al. Genomic profiling of combined hepatocellular cholangiocarcinoma reveals genomics similar to either hepatocellular carcinoma or cholangiocarcinoma. JCO Precis Oncol 2021;5:PO.
42. Sposito C, Ratti F, Cucchetti A, et al. Survival benefit of adequate lymphadenectomy in patients undergoing liver resection for clinically node-negative intrahepatic cholangiocarcinoma. J Hepatol 2023;78:356-63.
43. Kim KH, Lee SG, Park EH, et al. Surgical treatments and prognoses of patients with combined hepatocellular carcinoma and cholangiocarcinoma. Ann Surg Oncol 2009;16:623-9.
44. Renzulli M, Ramai D, Singh J, et al. Locoregional treatments in cholangiocarcinoma and combined hepatocellular cholangiocarcinoma. Cancers (Basel) 2021;13:3336.
45. Mazzaferro V, Gorgen A, Roayaie S, Droz Dit Busset M, Sapisochin G. Liver resection and transplantation for intrahepatic cholangiocarcinoma. J Hepatol 2020;72:364-77.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Ahmed N, Falcinelli F, Rimini M, Burgio V, Casadei-Gardini A, Aldrighetti L, Ratti F, Pedica F. Combined hepatocellular-cholangiocarcinoma: morpho-molecular updates and considerations. Hepatoma Res 2023;9:16. http://dx.doi.org/10.20517/2394-5079.2022.81
AMA Style
Ahmed N, Falcinelli F, Rimini M, Burgio V, Casadei-Gardini A, Aldrighetti L, Ratti F, Pedica F. Combined hepatocellular-cholangiocarcinoma: morpho-molecular updates and considerations. Hepatoma Research. 2023; 9: 16. http://dx.doi.org/10.20517/2394-5079.2022.81
Chicago/Turabian Style
Ahmed, Naghia, Francesca Falcinelli, Margherita Rimini, Valentina Burgio, Andrea Casadei-Gardini, Luca Aldrighetti, Francesca Ratti, Federica Pedica. 2023. "Combined hepatocellular-cholangiocarcinoma: morpho-molecular updates and considerations" Hepatoma Research. 9: 16. http://dx.doi.org/10.20517/2394-5079.2022.81
ACS Style
Ahmed, N.; Falcinelli F.; Rimini M.; Burgio V.; Casadei-Gardini A.; Aldrighetti L.; Ratti F.; Pedica F. Combined hepatocellular-cholangiocarcinoma: morpho-molecular updates and considerations. Hepatoma. Res. 2023, 9, 16. http://dx.doi.org/10.20517/2394-5079.2022.81
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 1 clicks
Cite This Article 1 clicks


















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.