Liver Transplantation for perihilar cholangiocarcinoma. Do we need to move forward?
Abstract
Perihilar cholangiocarcinoma (pCCA) is a challenging disease with limited options. Surgical resection and adjuvant therapy remain the only established treatment for those with resectable disease. Since the publication of the Mayo protocol in 2000, neoadjuvant chemoradiation and liver transplantation have become the standard of care in selected patients with unresectable de novo pCCA or resectable pCCA arising under primary sclerosing cholangitis. However, its application is diverse worldwide, and the need for donor organs is one of the main limitations. Also, differences in the neoadjuvant regimen used were observed. In this review, we discuss the latest results of this approach, the recommended tools for diagnostic work-up, and advances in systemic therapy to improve patient selection and long-term survival.
Keywords
INTRODUCTION
Transplant Oncology is an emerging, albeit not a new concept, since neoadjuvant chemoradiotherapy combined with liver transplantation (LT) showed great promise, according to the Nebraska group and the Mayo Clinic in 2000 for patients with unresectable pCCA[1,2].
Surgical resection remains the primary treatment for CCA; however, its infiltrative nature may involve the second-order biliary ducts defined as Bismuth-Corlette (BC) classification type 4, considered a locally-advanced tumor amenable to surgical treatment for highly selected patients[3,4]. Nevertheless, results in Western countries showed 42% 5-year overall survival (OS) for BC type 4 pCCA in N0/R0 patients with a 53% rate of Clavien-Dindo ≥ type 3 complications, 9% 30-day mortality, and 39% R1 resection[5]. Eastern countries reported slightly better results. Recently, Mizuno et al. published their results in combined vascular resection for locally-advanced pCCA, and the 5-year OS for those N0/R0 was 53% with similar Clavien-Dindo ≥ type 3 complications (51%) and R1 resection (36%); however, postoperative mortality (4%) was lower compared to Western countries[6].
Unfortunately, among lymph node-negative patients with positive surgical margins, the 5-year OS rate dropped to 13% for BC type 4 in Western countries[5] and 23% after vascular resection in Eastern countries[6]. This demonstrates that the indications for resection in BC type 4 pCCA patients should be assessed adequately in the context of the significant postoperative mortality and morbidity, with post-hepatectomy liver failure being one of the leading causes (25%-40%)[7] and provision of positive surgical margins one of the main risk factors associated with poor prognosis aside from positive lymph nodes[8,9]. For these reasons, there is a rational indication for LT in selected patients with locally-advanced pCCA and no extrahepatic disease or negative lymph node metastases, with an R1 margin, insufficient future liver remnant being expected, or underlying liver disease (i.e., primary sclerosing cholangitis).
In the late 90s, a survival benefit was demonstrated in patients with unresectable pCCA treated with chemoradiation alone[10,11]. Thereafter, the 2-year survival rate for patients who received a combination of external beam radiation therapy (EBRT), brachytherapy, and 5-fluorouracil (5-FU) was 30%. This was compared to a 2-year survival rate of only 17% for those who received brachytherapy and 5-FU without EBRT. These findings were statistically significant (P = 0.01)[12].
Based on that, the Mayo Clinic developed a new concept of neoadjuvant chemoradiation combined with liver transplantation, publishing their first promising results in 2000[1]. The protocol and indications have evolved over recent decades, and the Mayo Clinic recently published the largest sequential series[13]. Between 1993 and 2018, 211 patients underwent LT, and 5-year OS for de novo unresectable pCCA was 58%. However, its clinical application differs from American, European, and Asian centers.
The current main challenge in the management of locally-advanced pCCA is next-generation sequencing, with the identification of several actionable molecular alterations and the use of immune checkpoint inhibitors targeting programmed cell death 1 (PD-1)[14-17]. Their role is still to be established as part of conversion treatment in those who became resectable or even transplantable.
The present review aimed to analyze the different aspects of the Mayo protocol and offer suggestions for future treatment development.
PATIENT SELECTION
The foremost requisite for accomplishing good outcomes is strict adherence to selection criteria, which is challenging. Controversies have centered on establishing the correct pCCA diagnosis, which is difficult in most cases owing to the location and desmoplastic nature of the tumor[18].
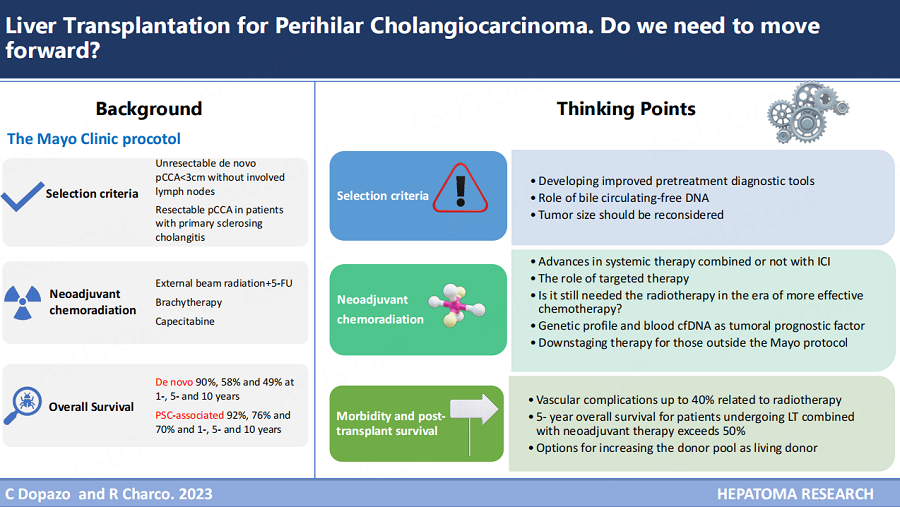
Eligibility criteria, when the first Mayo protocol was published in 2000 by Vreede et al., were early-stage pCCA arising under primary sclerosing cholangitis (PSC) disease or considered unresectable by the multidisciplinary team (MDT) committee[1]. The pCCA diagnosis was based on at least one of the following criteria: (1) positive brush cytology or biopsy result obtained at the time of cholangiography; or (2) a serum Ca 19.9 value greater than 100 U/mL in the absence of cholangitis. Of course, patients with intrahepatic or evidence of extrahepatic disease, including local lymph node metastases, were excluded from the study. The criteria for anatomical unresectability were better established by Jarnagin et al. in 2001 and included bilateral segmental duct extension to second-order biliary radicals (BC type 4) or unilateral extension to second-order biliary radicles with contralateral portal vein involvement or unilateral extension to second-order biliary radicals with contralateral hepatic lobar atrophy or main or bilateral portal vein involvement (Blumgart stage 3)[19].
The eligibility criteria for the Mayo protocol enrolment were modified and required at least one of the following[20]: (1) endoscopic intraluminal brushings or tissue biopsy positive for pCCA; (2) Ca 19.9 level greater than 100 U/mL in the absence of cholangitis, and/or a mass on cross-sectional imaging with a malignant-appearing stricture on cholangiography; (3) biliary ploidy by fluorescence in situ hybridization with a malignant stricture on cholangiography [Table 1]. The protocol excludes patients with more than
The Mayo protocol enrolment inclusion and exclusion criteria[20]
| Inclusion criteria | Exclusion criteria |
| Diagnosis of cholangiocarcinoma (1 of the following criteria): ● Transcatheter biopsy or brush cytology ● Ca19-9 > 100 mg/mL and/or mass on cross-sectional imaging with a malignant-appearing stricture on cholangiography ● Biliary ploidy by FISH with a malignant-appearing stricture on cholangiography | Intrahepatic cholangiocarcinoma Intra o extrahepatic metastases Lymph node metastases Tumor 3 cm Gall bladder involvement |
| Unresectable tumor above the cystic duct | Transperitoneal biopsy |
| Resectable cholangiocarcinoma in patients with primary sclerosing cholangitis | Uncontrolled infection |
| Candidate for liver transplantation | Prior radiation or chemotherapy |
| Tumor < 3 cm | Prior biliary resection |
| No involved lymph nodes on staging diagnostics | History of other malignancies within 5 years |
One controversial issue is the lack of tissue-confirmed pCCA to be included in the protocol. Conventional endoscopic retrograde cholangiopancreatography (ERCP), together with standard brush cytology and biopsy, is the most used technique for its diagnosis. However, it is well known that sensitivity is < 60%, and plus, FISH could reach 70% with a high false-negative rate[22]. This is a great handicap since it delays diagnosing and treating patients with suspected pCCA.
As discussed by the Mayo group[23], some proportion of transplant patients did not have pCCA, which was more evident in those with PSC, in whom the indeterminate biliary strictures could be confused with malignant disease. The question of whether pretreatment biopsy confirmation is necessary was widely explained by Rosen et al. in 2012[24]. They analyzed 144 patients who were finally transplanted. Pathological confirmation before LT was achieved in 52% of PSC patients and 45% of de novo pCCA patients. For patients with PSC, 5-year OS was worse among those with biopsy-confirmed pCCA (50% vs. 80%,
To avoid unclear assumptions, a better understanding of this disease depends on developing improved pretreatment diagnostic tools.
Advances in magnetic resonance imaging (MRI) have yielded excellent tissue contrast based on the difference in the diffusion of water molecules among tissues [Figure 1]. Diffusion-weighted imaging (DWI) MRI is rising used for tumor diagnoses and characterization in hepato-biliary malignance, and the apparent diffusion coefficient (ADC) proved helpful in the preoperative diagnosis of lymph node metastasis with greater specificity and sensitivity compared to positron emission tomography (PET)[25]. The capacity to accurately diagnose and stage pCCA also includes technological advances such as next-generation three-dimensional modeling software to reproduce tumors, size and extension, and vasculature relationships[26].
Figure 1. Morphology of pCCA with magnetic resonance cholangiopancreatography (MRCP). (A) On T2-weighted axial image, a nodular mass is noted within the bile duct bifurcation. (B) On T1-weighted axial image, the same lesion is seen as an enhancing nodular mass. (C) MRCP with 3D reconstruction shows the lesion involving the confluence of the bile duct.
In recent years, image resolution of direct visualization and targeted biopsy via cholangioscopy have improved, being more useful in diagnosing malignant stenosis. The sensitivity of malignancy diagnosed by digital single-operator cholangioscopy-guided biopsies (80%-100%) appeared to be higher than brush cytology alone (45%)[22] [Table 2]. However, cost and availability are the main obstacles to its widespread usage. Next-generation sequencing (NGS) has already demonstrated great capability to enhance the accuracy of ERCP-brushing diagnosis. Recently, a Spanish group developed a mutational analysis of bile circulating-free DNA (cfDNA) collected during ERCP using an NGS panel[27]. Sensitivity and specificity for malignancy were 96% and 69%, respectively, similar to a previous study[28]. Mutations in potentially-actionable genes were detected in 54% of positive samples. However, the mutation analysis is limited to a defined panel of genes and is not always concordant with the mutations detected in tissue samples. These results, which still need to be validated, are promising and could be incorporated into the diagnostic work-up of patients evaluated for pCCA.
Sensitivity, specificity, and accuracy of visual impression and cholangioscopy-guided biopsy, reproduced from Angsuwatcharakon et al. [22]
| Visual impression | Targeted biopsy | |||||
| Sensitivity (%) | Specificity (%) | Accuracy (%) | Sensitivity (%) | Specificity (%) | Accuracy (%) | |
| All types of scope | 67-100 | 49-100 | 50-94 | 38-100 | 75-100 | 61-100 |
| -Fiberoptic SOC | 78-100 | 77-93 | 80-94 | 49-100 | 94-100 | 73-100 |
| -Digital SOC | 83-90a 94b (89-97) | 89-96a 95b (90-98) | 87-93a 94b (90-98) | 80-85 | 100 | 93 |
| -DPOC | 78-89 | 73-91 | 75-90 | 80-100 | 75-100 | 79-93 |
| -DOC | 67-100 | 49-100 | 50-97 | 38-100 | 100 | 61-100 |
| History of prior negative or unconfirmed diagnosis | 90-100 | 79-96 | 89-97 | 38-88 | 94-100 | 61-93 |
The final controversial point is the maximum tumor size as one of the main inclusion criteria. In 2012, the Mayo Clinic and 11 other US centers published the results from the first multicenter study[29]. Despite the considerable heterogeneity among centers regarding the neoadjuvant protocol used, it was demonstrated that patients with increasing tumor diameter, de novo pCCA, and rising Ca 19.9 levels were related to drop-out. Moreover, patients with tumors over 3 cm had a 2-3-fold increased risk of recurrence.
By contrast, Ito et al. recently published their three-decade single experience. They concluded that the results in the last era (2008-2019) had improved mainly due to the use of combined neoadjuvant plus local therapy[30]. Although 33 patients had large-size tumors (pCCA ≥ 30 mm n = 12 or intrahepatic
NEOADJUVANT CHEMORADIATION. MOLECULAR PROFILING
There is no doubt that the use of neoadjuvant chemoradiation in pCCA has improved results after LT. A recent meta-analysis[31] demonstrated that neoadjuvant therapy reduces the risk of recurrence at 3 years (51.7% vs. 24.1%). This review also identified a substantial variation in these patients’ neoadjuvant regimens and how chemoradiation is crucial for achieving the assumed 50% 5-year survival after LT. However, this standard is not met if transplantation proceeds directly.
In 1993, the Mayo Clinic started the protocol which combined high-dose brachytherapy (60Gy) with continuous intravenous 5-FU infusion[1,2]. The current protocol[20] utilizes a lower radiation dose of EBRT with 45Gy in 30 fractions with continuous 5-FU infusion for three weeks plus brachytherapy with 20 Gy to reduce the risk of radiation-induced complications. Afterward, the patient received oral capecitabine maintenance until transplantation [Figure 2].
Figure 2. The Mayo protocol, created by Rosen et al.[20].
The rationale for using radiotherapy is that we deal with very radiosensitive-no-chemosensitive tumors. Nevertheless, the high perioperative morbidity associated with neoadjuvant local irradiation may complicate the postoperative course, as will be discussed later, and the question of whether radiotherapy is still needed in the era of more effective chemotherapy becomes potentially more significant. To date, there is insufficient data to respond to that; however, alternatives for safer dose escalation include hypo-fractionation or stereotactic body radiotherapy (SBRT), which leads to high disease control rates and induces immune-modulatory effects[32]. The impact of SBRT is currently being prospectively assessed in several trials, such as the STRONG trial (NCT03307538) or II trial ABC ISRCTN10639376 (https://doi.org/10.1186/ISRCTN10639376) in the setting of unresectable pCCA. The other dose escalation alternative is proton beam radiation therapy (PBRT). Initial studies included patients treated with palliative intent and showed promising results with local control at one year, similar to SBRT; however, toxicities were still described (i.e., cholangitis)[33]. The other main limitation of PBRT is the narrow range to prevent underdosing of the treatment target or overdosing an organ at risk[33].
Several retrospective studies evaluated the role of EBRT with a brachytherapy boost as a palliative treatment in extrahepatic biliary tract cancer. They concluded that brachytherapy provides no significant benefit and can increase the incidence of toxicities such as the risk of cholangitis, pain, or bleeding[34,35]. While most US centers still follow the Mayo protocol, the use of brachytherapy was abandoned at most of the non-US centers.
Regarding the chemotherapy regimen used, the maintenance of oral capecitabine until transplantation is under debate. Cisplatin and gemcitabine (Cis/Gem) became the first-line standard of care following the results of the ABC-02 study published in 2010[36] for patients with unresectable biliary tract tumors. Its efficacy was corroborated in a Japanese population in the BT22 study[37]. In fact, three recent papers substituted 5-FU/capecitabine for Cis/Gem[29,38,39] in their pre-transplant protocol. The Toronto group[38] included 18 patients who started neoadjuvant chemoradiation, eleven entered the maintenance chemotherapy phase, and only six were finally transplanted (one on the waiting list). Disease progression was the leading cause of drop-out, and the median OS of those who remained on protocol was 15.3 months. Ito et al. published their series, including iCCA and pCCA, using SBRT and Cis/Gem until LT[30]. There are no data regarding the drop-out rate; however, the OS of pCCA patients receiving combined therapy (n = 8) was 88% at five years. Finally, Abdelrahim et al. published their series of 10 patients (7 iCCA and three pCCA) using long-term Cis/Gem with no radiation, resulting in excellent outcomes[39].
The toxicity associated with Cis/Gem, the number of cycles before transplant, the need for radiotherapy, and the best neoadjuvant protocol are unanswered and need to be correctly addressed in prospective multicenter studies, mainly in the era of targeted therapy.
The molecular landscape of pCCA is growing, and genomic profiling has become increasingly apparent. Genomic sequencing also demonstrated that around 30% of patients have targetable mutations[40]. Targeted therapies relevant to pCCA comprise Ivosidenib for IDH-1 mutant disease, pembrolizumab in patients with MSI-H, and the trastuzumab/pertuzumab combination for HER-2-positive disease[41,42]. Recently, the US Food and Drug Administration (FDA) approved using an immune checkpoint inhibitor targeting PD-1 (durvalumab) in combination with systemic therapy as the first line in locally-advanced tumors[16,17]. Moreover, an increased understanding of pCCA genetics could identify subtypes with prognostic importance. In fact, genetic alterations in the FGFR and DNA damage repair alteration pathways were associated with less aggressive disease, a superior response to first-line chemotherapy, and improved OS in LT for iCCA[43].
Nevertheless, the main obstacle in pCCA is obtaining sufficient tissue pretransplant for molecular typing and diagnostic histology. The unique nature of the disease has led to very small and underpowered trials with imprecise results, none in the transplant field. The biliary tract circulating-tumor DNA (ctDNA) that could alter this paradigm is still in its infancy, as discussed previously. The lack of standardized diagnostic procedures currently limits the use of non-blood ctDNA for specific research intentions. Furthermore, a recent study[44] described the blood cfDNA alteration in more than 1,671 patients with advanced biliary tract cancer and found that genetic alterations were detected in 84% of patients, with targetable mutations identified in 44%. The concordance between blood cfDNA and tissue was high for IDH1 mutations (87%) and BRAF V600E (100%), still very low, however, for FGFR2 fusions (18%).
In future years, continued evolution in diagnostic modalities and advancing treatment options in patients with locally-advanced pCCA will facilitate a better understanding of this disease and the decision of which patients may benefit from the combined neoadjuvant treatment and liver transplantation approach.
Another interesting point to explore is “downstaging therapy” in patients with locally-advanced pCCA outside the Mayo protocol, which means tumor size ≥ 3 cm or involved regional lymph nodes. The experience is limited to retrospective case series with a small sample size from the Medical College Wisconsin[45] reporting the potential effectiveness of combined neoadjuvant therapy in patients with unresectable CCA, including tumor size ≤ 3.5 cm for pCCA and ≤ 8 cm for iCCA. The presence of positive lymph nodes on staging was not an exclusion criterion. The pre-transplant drop-out rate was 61% (11 of 18 patients), higher than previously reported. Five patients were transplanted, and three of them were pCCA. Of the two patients who had demonstrated PET-positive lymph nodes before starting neoadjuvant therapy, one had a post-treatment PET scan showing resolution of the adenopathy before transplantation, and all patients had negative portal lymphadenectomies in the explant. The median OS of the whole group was 44.1 months (range: 35.8-55.2). The authors concluded that neoadjuvant therapy provides a period for tumor biology selection. In fact, tumors with aggressive biology usually resist treatment, whereas those with favorable biology could be downstaged. Nevertheless, considering the high risk of recurrence after LT, it should be considered only in very selected patients, under very well-designed protocols, and with the possibility of offering a living donation.
MORBIDITY AND SURVIVAL OF PATIENTS UNDERGOING LIVER TRANSPLANTATION
The main outstanding issues regarding post-transplant morbidity and survival in this group of patients are the vascular complications related to the irradiated hilar hilum and the OS differences between patients with de novo vs. PSC-associated pCCA.
Mantel et al. from the Mayo Clinic had already described the high incidence of vascular complications after LT in the setting of neoadjuvant therapy for pCCA in 2007[46]. At least 40% of patients (27/68) had post-LT vascular complications: 21% developed hepatic artery, and 22% had portal complications. Smaller vessels seem to be especially sensitive to radiation injury; however, veins are more resistant to the injurious effects of ionizing radiation, but stenotic and thromboembolic complications have also been reported in the literature[47,48]. Based on their findings, the authors suggested that donor iliac artery grafts between the donor hepatic artery and the recipient's infrarenal or supraceliac abdominal aorta would be an ideal option for reconstruction during deceased donor liver transplantation, as opposed to the native artery[46]. This technique tries to avoid the potential adverse effect of neoadjuvant therapy on arterial inflow.
However, it failed to work for living donor liver transplantation (LDLT) owing to the significant arterial size mismatch, so they used the native common hepatic artery with close observation and early intervention for patients who developed vascular complications[46]. Also, portal vein length is usually insufficient in the case of living donors. In some cases, a segment of the deceased donor iliac vein is required as an interposition graft which, at the same time, is a dominant risk factor for late portal vein stenosis (HR 13.51, 95%;
The second point is the difference in OS of patients with pCCA in the setting of PSC compared with de novo tumors. Tan et al. published the updated results from the Mayo Clinic in 2020, the largest series to date[13]. The intention-to-treat survival for patients with PSC-associated pCCA (n = 211) was 78, 60, and 52% at 1, 5, and 10 years compared to 83, 39, 32% for patients with de novo pCCA (n = 138) (P = 0.03). Overall survival after transplantation for patients with PSC-associated pCCA (n = 138) was 92%, 76%, and 70% at 1, 5, and 10 years compared to 90%, 58%, and 49% for patients with de novo pCCA (n = 73) (P = 0.02). These differences were observed in multiple publications from the Mayo Clinic and others, reflecting that patients with PSC are more likely to be monitored for disease and have less advanced disease at diagnosis than patients with de novo pCCA. PSC patients may also have less frequent pretreatment tissue-confirmed diagnoses or different biological behavior[13,24,49-51]. What is clear is that the distinct outcomes for intention-to-treat survival and survival after transplantation warrants independent reporting.
A recent meta-analysis[31] demonstrated the heterogeneity in published reports combining data from PSC and non-PSC patients, pCCA and intrahepatic CCA, or different neoadjuvant regimens, which undoubtedly reflects the large variability in results. Other variables such as tumor size, lymph node metastases, pretransplant Ca 19.9, intention-to-treat survival, percentage of patients with pretreatment-positive biopsy, or patients with a tumor on the explant are also infrequently reported[52]. Nevertheless, this meta-analysis[31] confirmed that the pooled 5-year survival for patients undergoing LT combined with neoadjuvant therapy exceeds the 50% 5-year survival threshold (65.1%, 95%CI: 55.1%-74.4%), and disease recurrence in the studies which followed patients for ≥ 36 months was 24% (95%CI: 17.9%-30.9%). The living donation rate was 6.3% (27 patients), too small to assess whether LDLT may offer survival benefits over cadaveric donors. Whether LDLT will result in reduced disease progression owing to a shorter waiting list time or, in contrast, losing the “observed time for disease progression” when waiting for a cadaveric donor to exclude patients destined to progress remains unsolved. An outstanding conclusion of this study was the importance of the quality of data collected, establishing a minimum dataset for all patients evaluated and enrolled in combined neoadjuvant-LT programs to achieve robust results not only in outcomes but also for patient selection and the best neoadjuvant protocols.
The characteristics and main outcomes of the more salient papers published in the field of the combined treatment of chemoradiation and LT for unresectable pCCA are summarized in Table 3[2,13,23,29,38,39,45,51,53-59].
Study characteristics and main outcomes of the patients undergoing liver transplantation after neoadjuvant therapy (Review of the main papers published to date)
| Year/author | Country | Patients recluted/ patients transplanted | Time in waiting list | LDLT/DDLT | Post-LT morbidty Clavien-Dindo 3 | Post-LT Mortality | Retransplant | Recurrence | 1st y - 5th y post-LT OS (%) | Follow-up (months) |
| Sudan et al.[2], 2002 | USA | 17/11 | 3.4 (3-14) months | 5/6 | 4/11 (36%) | 18%-30day | 1/11 (9%) | 2/11 (18%) | 54%-36% | N/A |
| Wu et al.[53], 2008 | USA | 8/6 | 87 (7-151) days | 0/6 | 3/6 (50%) | 17% | 0% | 0% | 100%-66% | 90 |
| Rosen et al.[23], 2012 | USA | 215/136 | N/A | 45/91 | N/A | N/A | N/A | 29/136 (21%) | 92%-74% | N/A |
| Duignan et al.[54], 2014 | IRLAND | 27/20 | 1-7 months | 0/20 | 4/20 (20%) | 20% hospital mortality | 3/20 (15%) | 7/20 (35%) | 75%-51% | N/A |
| Welling et al.[55], 2014 | USA | 12/6 | 88 (29-201) days | 0/6 | 2/6 (33%) | 16.6%-30 day | 0 | 2/6 (33%) | 83%-N/A | 14 |
| Marchan et al.[56], 2016 | USA | 10/8 | N/A | 0/8 | 2/8 (25%) | 25% | N/A | N/A | 87.5%-N/A | 27 |
| Loveday et al.[38], 2017 | CANADA | 18/6 | 22 (20-48) weeks | 3/3 | N/A | N/A | N/A | 1/6 (16%) | 83% -N/A | 17.6 (5-58) |
| Ethun et al.[51], 2018 | USA | 70/41 | N/A | N/A | 14/41 (34%) | 4.8%-90 day | N/A | 10/41 (24%) | 93%-64% | 58 (3-127) |
| Wong et al.[45], 2019 | USA | 16/5 (2iCCA+3pCCA) | 13 (8.8-19) months | N/A | 1/5 (20%) | 20% | N/A | 1/5 (20%) | 80%-N/A | 44 (36-55) |
| Tan et al.[13], 202 | USA | 349/211 | N/A | 76/135 | N/A | N/A | N/A | N/A | 91%-62% | N/A |
| Zaborowski et al.[57], 2020 | IRLAND | 37/26 | N/A | 0/26 | 8/26 (31%) | 15% hospital mortality | 5/26 (19%) | 6/26 (23%) | 81%-55% | 67 (24-158) |
| Dopazo et al.[58], 2021 | SPAIN | 13/8 | 122 (5-192) days | 0/8 | 4/8 (50%) | 12%-90 day | 0% | 3/8 (37%) | 87%-62% | 24 (2-103) |
| Ahmed et al.[59], 2022 | USA | 58/38 | 3.72 months | 0/38 | 15/38 vascular complications 9/38 relaparotomy | 5% | 4/38 (10%) | 13/38 (34%) | 91%-52% | 40 (0.7-157) |
| Ito et al.[29], 2022 | USA | N/A / 49 (30iCCA+19pCCA) | N/A | N/A | N/A | N/A | N/A | N/A | pCCA under combined NAT 88%-88% vs. 91%-9% | 29 (1-252) |
| Abdelrahim et al.[39], 2022 | USA | N/A/10 (7iCCA+3pCCA) | N/A | N/A | N/A | 10% | N/A | 1/10 (10%) | 100%-75% | 28 (27-32) |
The present review did not aim to compare survival in transplanted versus resected patients with pCCA since, once again, the heterogeneity among studies and inherent limitations in study design, comparisons, and data made it difficult to reach a conclusion about which approach is superior[60].
The only randomized control trial comparing resection vs. transplantation in patients with resectable pCCA (NCT02232932, TRANSPHIL Study) was recently conducted at 18 French centers. It was stopped prematurely since it failed to recruit the required number of patients, and an unexpected drop-out rate of 55% in the LT arm was observed (personal communication: E Vivert, Paris, France, and comment appear in a recent paper[61]). However, a recently published international benchmarking study highlighted the potential superiority of LT over resection[61]. Benchmark patients were defined as those operated on at high volume centers ≥ 50 overall LT/year, transplanted following neoadjuvant chemoradiotherapy, with tumor diameter < 3 cm and negative lymph nodes. Seventeen centers were included, and 134 patients were analyzed. The benchmark for R0-resection margin and 90-day mortality rates were ≥ 80% and ≤ 5.2%, respectively, while the benchmark value for severe (grade ≥ 3) complications was ≤ 66.7%. After the exclusion of all PSC patients and when comparing the transplanted unresectable cohort to a matched cohort of curatively-resected BC type 4 without neoadjuvant treatment, better 5-year disease-free survival was observed in the transplanted group (49.9% vs. 18.1%, P = 0.005) with no differences in 5-year OS (56% vs. 39.7%, P = 0.4), major complications (72.7% vs. 74.6%, P = 0.8) or 90-day mortality (3% vs. 7%, P = 0.17).
Therefore, although most studies demonstrated improved survival for transplanted patients with unresectable pCCA after neoadjuvant therapy, the data must be interpreted with caution. Only large multicenter registries with appropriate datasets will provide more accurate information on survival benefits.
STUDIES ONGOING. NEED FOR AN INTERNATIONAL MULTICENTER STUDY?
Only three active ongoing trials are registered on www.clinicaltrials.gov and two in the German Clinical Trials Register (www.drks.de), as summarized in Table 4. Two trials (NCT02178280, NCT01549795) never achieved results, and two were terminated early because of poor accrual (NCT01151761, NCT02232932). This reveals that, given the rarity of the disease and the very selected patients meeting the restricted criteria for LT, a conclusive randomized control trial comparing PSC vs. non-PSC, resection vs. LT, or different chemoradiation options will never be performed owing to the slow recruitment causing a long study period.
Active ongoing trials in the field of liver transplantation and perihilar CCA (pCCA)
| Study title | Brief description | Neoadjuvant therapy | Type of study | Status |
| Liver Transplantation for Non-resectable perihilar cholangiocarcinoma (TESLA II) NCT04993131 Norway | 5-year overall survival Unresectable pCCA Patients who received chemotherapy for at least 6 months with at least 10% response according to RECIST criteria and with no progression of disease at the time of liver transplant Negative nodes No size available | N/A | Exploratory | Recruiting n = 15 patients |
| Liver Transplant Combined with neoadjuvant chemo-radiotherapy in the treatment of unresectable hilar cholangiocarcinoma. A prospective multicenter study NCT04378023 Spain | 5-year overall survival Unresectable pCCA ≤ 3cm in radial diameter, without evidence of lymph node or distant metastases with no progression under neoadjuvant therapy | Cisplatin/Gemcitabine External Beam radiotherapy | Prospective Observational Multicenter Study Biomarker analyses | Recruiting n = 34 patients |
| Prospective registry study of neoadjuvant therapy in conjunction with liver transplantation for cholangiocarcinoma NCT00301379 Washington University School of Medicine | Validate results after neoadjuvant chemoradiation and liver transplantation in pCCA previously reported by Mayo Clinic | Mayo Protocol | Prospective Observational Multicenter Study | Recruiting n = 100 patients |
| Microscopic Tumor Clearance after Liver Transplantation for Proximal Bile Duct Cancer DRKS00013276 Berlin | Rate of microscopic tumor clearance (R0) after liver transplantation for those unresectable pCCA < 3cm | No neoadjuvant chemoradiation | A prospective, multicenter, non-randomized, open-label, one-arm study evaluating | Recruiting n = 50 |
| Feasibility and efficacy of adjuvant gemcitabine chemotherapy after liver transplantation for proximal bile duct cancer DRKS00000805 Berlin | Percentage of patients completing the complete 6 cycles of chemotherapy after transplantation | Arm 1: Gemcitabine Arm 2: No chemotherapy | Prospective, multi-center, randomized, two-arm parallel group phase II trial | Recruiting n = 60 |
The use of molecular profiling and mutational analyses of ctDNA in current and future studies has the potential to offer personalized treatments and help identify patients at risk for recurrence. However, only an international network of transplant oncology centers and global clinical genomic data-sharing could achieve adequately-powered clinical studies in this context.
Furthermore, multi-institutional collaborations are needed not only to reach sufficient patients but also to clarify optimal timing and protocols for chemotherapy, aiming to develop center algorithms, including policies for organ allocation.
CONCLUSIONS
Without a doubt, neoadjuvant treatment and liver transplantation offer acceptable survival rates in selected patients with unresectable pCCA. However, it is time to move forward. Evolution in the accuracy of diagnostic modalities, redefining selection criteria, advancing treatment options for these patients, personalized medicine based on molecular profiling, and multicenter collaboration will be helpful to achieve a favorable oncological outcome and improve the understanding of tumor biology. Without a doubt, a multidisciplinary tumor board for case-by-case discussion is imperative to select those patients who will benefit from this approach.
DECLARATIONS
AcknowledgmentsThe authors thank Christine O’Hara for English-language editing and Dr. Xavier Merino for sharing MRI pictures.
Authors’ contributionsContributed solely to the article: Dopazo C, Charco R
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestBoth authors declare that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. De Vreede I, Steers JL, Burch PA, et al. Prolonged disease-free survival after orthotopic liver transplantation plus adjuvant chemoirradiation for cholangiocarcinoma. Liver Transpl 2000;6:309-16.
2. Sudan D, DeRoover A, Chinnakotla S, et al. Radiochemotherapy and transplantation allow long-term survival for nonresectable hilar cholangiocarcinoma. Am J Transplant 2002;2:774-9.
3. Igami T, Nishio H, Ebata T, et al. Surgical treatment of hilar cholangiocarcinoma in the “new era”: the Nagoya University experience. J Hepatobiliary Pancreat Sci 2010;17:449-54.
4. Mansour JC, Aloia TA, Crane CH, Heimbach JK, Nagino M, Vauthey JN. Hilar cholangiocarcinoma: expert consensus statement. HPB (Oxford) 2015;17:691-9.
5. Ruzzenente A, Bagante F, Olthof PB, et al. Perihilar Cholangiocarcinoma Collaboration Group. Surgery for bismuth-corlette type 4 perihilar cholangiocarcinoma: results from a Western Multicenter Collaborative Group. Ann Surg Oncol 2021;28:7719-29.
6. Mizuno T, Ebata T, Yokoyama Y, et al. Combined vascular resection for locally advanced perihilar cholangiocarcinoma. Ann Surg 2022;275:382-90.
7. Mueller M, Breuer E, Mizuno T, et al. Perihilar cholangiocarcinoma - novel benchmark values for surgical and oncological outcomes from 24 expert centers. Ann Surg 2021;274:780-8.
8. Komaya K, Ebata T, Yokoyama Y, et al. Recurrence after curative-intent resection of perihilar cholangiocarcinoma: analysis of a large cohort with a close postoperative follow-up approach. Surgery 2018;163:732-8.
9. Groot Koerkamp B, Wiggers JK, Allen PJ, et al. Recurrence rate and pattern of perihilar cholangiocarcinoma after curative intent resection. J Am Coll Surg 2015;221:1041-9.
10. Veeze-Kuijpers B, Meerwaldt JH, Lameris JS, van Blankenstein M, van Putten WL, Terpstra OT. The role of radiotherapy in the treatment of bile duct carcinoma. Int J Radiat Oncol Biol Phys 1990;18:63-7.
11. Buskirk SJ, Gunderson LL, Schild SE, Bender CE, Williams HJJ, Mcilrath DC, et al. Analysis of failure after curative irradiation of extrahepatic bile duct carcinoma. Ann Surg 1992;215:125-31.
12. Alden ME, Mohiuddin M. The impact of radiation dose in combined external beam and intraluminal Ir-192 brachytherapy for bile duct cancer. Int J Radiat Oncol Biol Phys 1994;28:945-51.
13. Tan EK, Taner T, Heimbach JK, Gores GJ, Rosen CB. Liver transplantation for peri-hilar cholangiocarcinoma. J Gastrointest Surg 2020;24:2679-85.
14. Ricci AD, Rizzo A, Brandi G. The DNA damage repair (DDR) pathway in biliary tract cancer (BTC): a new Pandora’s box? ESMO Open 2020;5:e001042.
15. Rizzo A, Ricci AD, Brandi G. Durvalumab: an investigational anti-PD-L1 antibody for the treatment of biliary tract cancer. Expert Opin Investig Drugs 2021;30:343-50.
16. Oh DY, Lee KH, Lee DW, et al. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: an open-label, single-centre, phase 2 study. Lancet Gastroenterol Hepatol 2022;7:522-32.
17. Vogel A, Bridgewater J, Edeline J, et al. ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2023;34:127-40.
18. Cardinale V, Bragazzi MC, Carpino G, et al. Cholangiocarcinoma: increasing burden of classifications. Hepatobiliary Surg Nutr 2013;2:272-80.
19. Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg 2001;234:507-17; discussion 517.
20. Rosen CB, Heimbach JK, Gores GJ. Liver transplantation for cholangiocarcinoma. Transpl Int 2010;23:692-7.
21. Sapisochin G, Javle M, Lerut J, et al. Liver Transplantation for cholangiocarcinoma and mixed hepatocellular cholangiocarcinoma: working group report from the ILTS Transplant Oncology Consensus Conference. Transplantation 2020;104:1125-30.
22. Angsuwatcharakon P, Kulpatcharapong S, Moon JH, et al. Consensus guidelines on the role of cholangioscopy to diagnose indeterminate biliary stricture. HPB (Oxford) 2022;24:17-29.
23. Darwish Murad S, Kim WR, Therneau T, et al. Predictors of pretransplant dropout and posttransplant recurrence in patients with perihilar cholangiocarcinoma. Hepatology 2012;56:972-81.
24. Rosen CB, Darwish Murad S, Heimbach JK, Nyberg SL, Nagorney DM, Gores GJ. Neoadjuvant therapy and liver transplantation for hilar cholangiocarcinoma: is pretreatment pathological confirmation of diagnosis necessary? J Am Coll Surg 2012;215:31-8; discussion 38.
25. Hosokawa I, Hayano K, Furukawa K, et al. Preoperative diagnosis of lymph node metastasis of perihilar cholangiocarcinoma using diffusion-weighted magnetic resonance imaging. Ann Surg Oncol 2022;29:5502-10.
26. Lopez-Lopez V, Gomez-Perez B, de Vicente E, et al. Next-generation three-dimensional modelling software for personalized surgery decision-making in perihilar cholangiocarcinoma: multicentre study. Br J Surg 2021;108:e394-5.
27. Arechederra M, Rullán M, Amat I, et al. Next-generation sequencing of bile cell-free DNA for the early detection of patients with malignant biliary strictures. Gut 2022;71:1141-51.
28. Singhi AD, Nikiforova MN, Chennat J, et al. Integrating next-generation sequencing to endoscopic retrograde cholangiopancreatography (ERCP)-obtained biliary specimens improves the detection and management of patients with malignant bile duct strictures. Gut 2020;69:52-61.
29. Darwish Murad S, Kim WR, Harnois DM, et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology 2012;143:88-98.e3; quiz e14.
30. Ito T, Butler JR, Noguchi D, et al. A 3-Decade, Single-Center Experience of Liver Transplantation for Cholangiocarcinoma: Impact of Era, Tumor Size, Location, and Neoadjuvant Therapy. Liver Transpl 2022;28:386-96.
31. Cambridge WA, Fairfield C, Powell JJ, et al. Meta-analysis and meta-regression of survival after liver transplantation for unresectable perihilar cholangiocarcinoma. Ann Surg 2021;273:240-50.
32. Vatner RE, Cooper BT, Vanpouille-Box C, Demaria S, Formenti C. Combinations of immunotherapy and radiation in cancer therapy. Front Oncol 2014;4:325.
33. Siddiqui O, Pollock A, Samanta Samanta, Kaiser A, Molitoris JK. Proton beam therapy in liver malignancies. Curr Oncol Rep 2020;22:30.
34. Mattiucci GC, Autorino R, Tringali A, et al. A Phase I study of high-dose-rate intraluminal brachytherapy as palliative treatment in extrahepatic biliary tract cancer. Brachytherapy 2015;14:401-4.
35. Yoshioka K, Ogawa R, Oikawa H, et al. Japanese Radiation Oncology Study Group (JROSG). Impact of intraluminal brachytherapy on survival outcome for radiation therapy for unresectable biliary tract cancer: a propensity-score matched-pair analysis. Int J Radiat Oncol Biol Phys 2014;89:822-9.
36. Valle J, Wasan H, Palmer DH, et al. ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81.
37. Okusaka T, Nakachi K, Fukutomi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer 2010;103:469-74.
38. Loveday BPT, Knox JJ, Dawson LA, et al. Neoadjuvant hyperfractionated chemoradiation and liver transplantation for unresectable perihilar cholangiocarcinoma in Canada. J Surg Oncol 2018;117:213-9.
39. Abdelrahim M, Al-Rawi H, Esmail A, et al. Gemcitabine and cisplatin as neo-adjuvant for cholangiocarcinoma patients prior to liver transplantation: case-series. Curr Oncol 2022;29:3585-94.
40. Banales JM, Marin JJG, Lamarca A, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol 2020;17:557-88.
42. Macias RIR, Cardinale V, Kendall TJ, et al. Clinical relevance of biomarkers in cholangiocarcinoma: critical revision and future directions. Gut 2022;71:1669-83.
43. McMillan RR, Javle M, Kodali S, et al. Survival following liver transplantation for locally advanced, unresectable intrahepatic cholangiocarcinoma. Am J Transplant 2022;22:823-32.
44. Berchuck JE, Facchinetti F, DiToro DF, et al. The clinical landscape of cell-free DNA alterations in 1671 patients with advanced biliary tract cancer. Ann Oncol 2022;33:1269-83.
45. Wong M, Kim J, George B, et al. Downstaging Locally Advanced Cholangiocarcinoma Pre-Liver Transplantation: A Prospective Pilot Study. J Surg Res 2019;242:23-30.
46. Mantel HT, Rosen CB, Heimbach JK, et al. Vascular complications after orthotopic liver transplantation after neoadjuvant therapy for hilar cholangiocarcinoma. Liver Transpl 2007;13:1372-81.
47. Renard R, Davaine JM, Couture T, et al. Surgical repair of radiation-induced carotid stenosis. J Vasc Surg 2020;72:959-67.
48. Patel DA, Kochanski J, Suen AW, Fajardo LF, Hancock SL, Knox SJ. Clinical manifestations of noncoronary atherosclerotic vascular disease after moderate dose irradiation. Cancer 2006;106:718-25.
49. Tan EK, Rosen CB, Heimbach JK, Gores GJ, Zamora-Valdes D, Taner Y. Living donor liver transplantation for perihilar cholangiocarcinoma: outcomes and complications. J Am Coll Surg 2020;231:98-110.
50. Rea DJ, Heimbach JK, Rosen CB, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg 2005;242:451-8; discussion 458.
51. Ethun CG, Lopez-Aguiar AG, Anderson DJ, et al. Transplantation versus resection for hilar cholangiocarcinoma: an argument for shifting treatment paradigms for resectable disease. Ann Surg 2018;267:797-805.
52. Machairas N, Kostakis DI, Prodromidou A, et al. Liver transplantation for hilar cholangiocarcinoma: A systematic review. Transplant Rev (Orlando) 2020;34:100516.
53. Wu Y, Johlin FC, Rayhill SC, et al. Long-term, tumor-free survival after radiotherapy combining hepatectomy-Whipple en bloc and orthotopic liver transplantation for early-stage hilar cholangiocarcinoma. Liver Transpl 2008;14:279-86.
54. Duignan S, Maguire D, Ravichand CS, et al. Neoadjuvant chemoradiotherapy followed by liver transplantation for unresectable cholangiocarcinoma: a single-centre national experience. HPB (Oxford) 2014;16:91-8.
55. Welling TH, Feng M, Wan S, et al. Neoadjuvant stereotactic body radiation therapy, capecitabine, and liver transplantation for unresectable hilar cholangiocarcinoma. Liver Transpl 2014;20:81-8.
56. Marchan EM, Landry JC. Neoadjuvant chemoradiation followed by orthotopic liver transplantation in cholangiocarcinomas: the emory experience. J Gastrointest Oncol 2016;7:248-54.
57. Zaborowski A, Heneghan HM, Fiore B, et al. Neoadjuvant Chemoradiotherapy and liver transplantation for unresectable hilar cholangiocarcinoma: the irish experience of the mayo protocol. Transplantation 2020;104:2097-104.
58. Dopazo C, Lladó L, Fondevila C, et al. Applicability and results of liver transplant combined with neoadjuvant chemo-radiotherapy in the treatment of unresectable hilar cholangiocarcinoma. Cir Esp (Engl Ed) 2021;99:190-9.
59. Ahmed O, Vachharajani N, Chang SH, et al. Single-center experience of liver transplantation for perihilar cholangiocarcinoma. HPB (Oxford) 2022;24:461-9.
60. Acher AW, Weber SM, Pawlik TM. Liver transplantation for perihilar cholangiocarcinoma: patient selection and outcomes. Expert Rev Gastroenterol Hepatol 2021;15:555-66.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Dopazo C, Charco R. Liver Transplantation for perihilar cholangiocarcinoma. Do we need to move forward?. Hepatoma Res 2023;9:10. http://dx.doi.org/10.20517/2394-5079.2022.75
AMA Style
Dopazo C, Charco R. Liver Transplantation for perihilar cholangiocarcinoma. Do we need to move forward?. Hepatoma Research. 2023; 9: 10. http://dx.doi.org/10.20517/2394-5079.2022.75
Chicago/Turabian Style
Dopazo, Cristina, Ramón Charco. 2023. "Liver Transplantation for perihilar cholangiocarcinoma. Do we need to move forward?" Hepatoma Research. 9: 10. http://dx.doi.org/10.20517/2394-5079.2022.75
ACS Style
Dopazo, C.; Charco R. Liver Transplantation for perihilar cholangiocarcinoma. Do we need to move forward?. Hepatoma. Res. 2023, 9, 10. http://dx.doi.org/10.20517/2394-5079.2022.75
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 4 clicks
Cite This Article 4 clicks














Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.