Neoadjuvant chemotherapy alone or combined with trans-arterial therapies for downstaging unresectable intrahepatic cholangiocarcinoma to surgical resection: a narrative review
Abstract
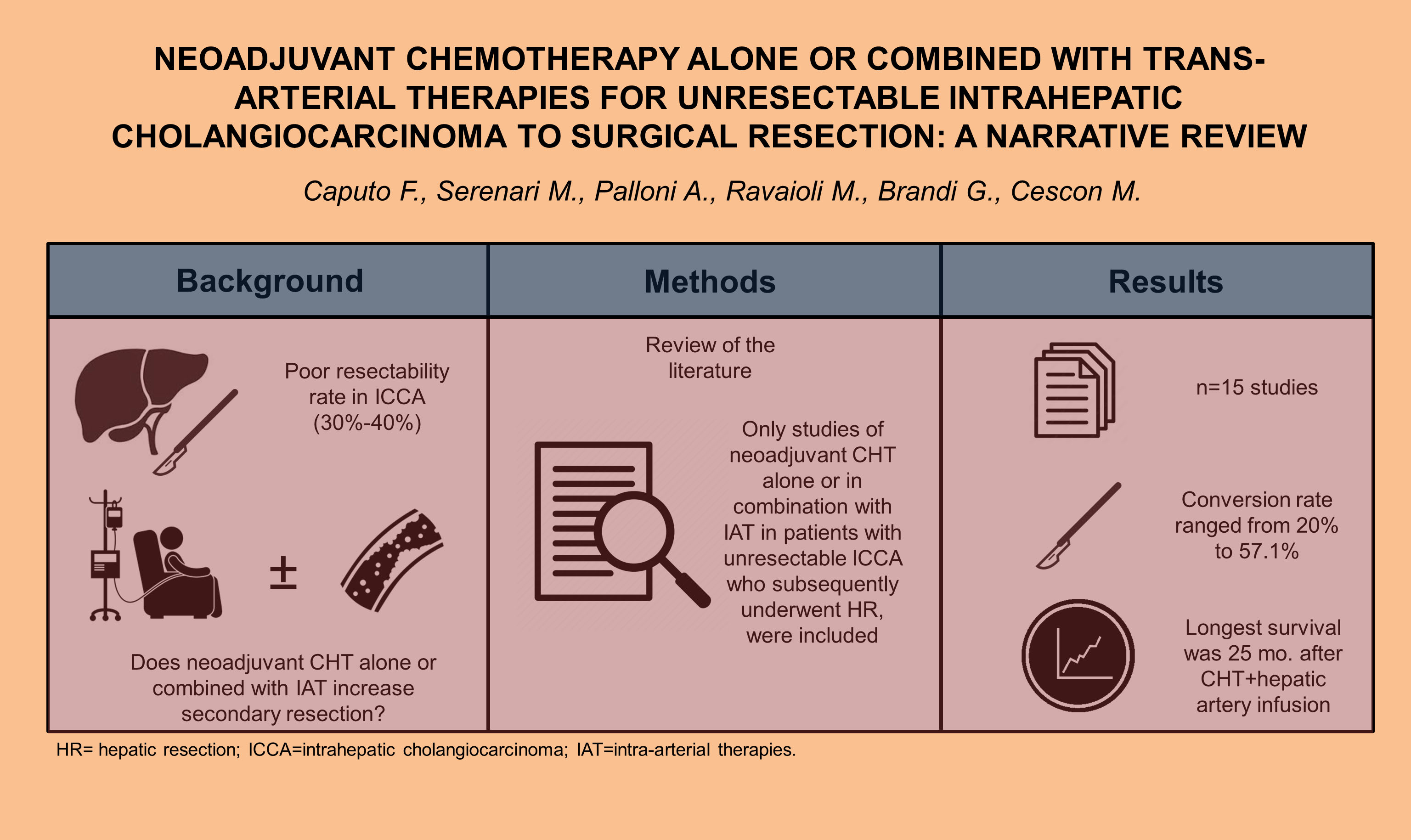
Intrahepatic cholangiocarcinoma (ICCA) incidence has been rising in the last few decades. Currently, hepatic resection is the only curative treatment for ICCA, and there is a lack of evidence supporting the use of preoperative treatment. A narrative review was conducted to analyze the available literature published on the role of neoadjuvant chemotherapy (CHT), either alone or combined with intra-arterial therapies (IAT), for downstaging unresectable ICCA to surgical resection. Most of the studies included in this review showed that secondary resection was associated with improvement in overall survival. In particular, studies analyzing CHT alone reported the highest conversion rate ranging from 20% to 57.1%, confirming that systemic treatment may yield the best results, and therefore, it should always be included as part of the neoadjuvant protocol. Among all the IATs, the longest overall survival reported was after CHT plus hepatic artery infusion, 25 months. Downsizing neoadjuvant multimodal approach, including combined systemic therapy with IAT, might improve the long-term outcomes of unresectable patients and expand surgical indications. However, randomized controlled trials are necessary to confirm their effectiveness.
Keywords
INTRODUCTION
Cholangiocarcinoma may arise anywhere along the biliary tree. According to the American Joint Committee on Cancer (AJCC) staging system, intrahepatic cholangiocarcinoma (ICCA), perihilar cholangiocarcinoma, and distal cholangiocarcinoma differ in tumor characteristics; consequently, they require different treatment approaches. In particular, ICCA arises from the intrahepatic bile ducts. Its incidence has been rising in the last decades and it is currently the second most common primary liver tumor after hepatocellular carcinoma[1]. This is partially explained by an increase in risk factors such as chronic hepatitis, primary sclerosing cholangitis, choledocolithiasis, metabolic syndrome, and obesity[2]. The treatment of patients with ICCA often represents a challenging issue for hepato-pancreato-biliary surgeons. Currently, the only curative treatment for ICCA is hepatic resection (HR), but approximately only one-third of patients with ICCA present with a tumor susceptible to HR, and even in resectable cases, 5-year overall survival (OS) ranges from 20% to 35%[3]. The major determinants of resectability are the extent of the tumor within the biliary tree, the amount of hepatic parenchyma involved, vascular invasion, and the presence of metastatic disease. Within the M1 stage, some authors have suggested classifying patients with liver metastases only as a separate group, which could be helpful in guiding treatment decisions, enabling the utilization of liver-directed therapies and locoregional strategies[4]. Although extended resections (e.g., with associated vascular and/or extrahepatic bile duct resection) can be feasible, clinicopathological factors such as tumor size, lymph node metastasis and surgical margin status have an important prognostic role after surgery. Therefore, the limited survival benefit sometimes does not justify the considerably increased perioperative surgical risk. Moreover, a significant proportion of patients develop very early recurrence after HR[5]. Identifying effective prognostic biomarkers that might be related to the progression of ICCA is of great interest: for example, some authors have analyzed the correlation of calcium-binding protein S100A4 and Matrix metalloproteinase-9, considering them as potentially useful markers for prognosis ICCA[6]. Additionally, the identification of distinct patient subgroups harboring unique molecular alterations with corresponding targeted therapies such as isocitrate dehydrogenase-1 (IDH1) mutations or fibroblast growth factor receptor-2 fusions (FGFR2) is changing the treatment paradigm[7]. However, to date, there is neither evidence supporting the use of these biomarkers nor of neoadjuvant systemic chemotherapy (CHT) over upfront resection in patients with resectable ICCA[8], even though the rationale for preoperative treatments to decrease such a high recurrence rate is strong, especially considering the high incidence of R1 resections and the significant surgical morbidity often impairing the possibility of adjuvant therapies[9,10]. On the contrary, systemic CHT alone or combined with intra-arterial therapies (IAT) has been adopted in unresectable diseases, showing a possible conversion to surgery in case of adequate response after treatments.
The scope of this review is, therefore, to assess the role of neoadjuvant CHT alone or in combination with IAT in patients with unresectable ICCA who subsequently underwent hepatic resection (HR).
Systemic chemotherapy
Downsizing CHT and subsequent HR has already been reported as an effective approach in colorectal liver metastases and pancreatic cancer[11-13]. In ICCA, the introduction of a combination CHT with gemcitabine (GEM) and cisplatin (CIS) has significantly improved survival rates in patients with unresectable biliary tract cancer (BTC)[14]. Therefore, it seems reasonable to downsize an initially unresectable localized ICCA with CHT, followed by surgery in case of adequate radiological response[14-16]. Nevertheless, most of the evidence is based on retrospective studies, while randomized trials are still lacking. Kato et al. published two retrospective studies where the authors evaluated the effect of downsizing CHT in patients with initially unresectable BTC[14,15] [Table 1]. In both studies, unresectability was defined if HR could not be achieved even by an aggressive surgical approach (e.g., vascular resection). In their first series, CHT with GEM alone was provided to 22 patients with initially unresectable BTC, including 7 patients with ICCA. Secondary HR was performed in 4 of 7 (57.1%) patients with ICCA who had a partial response or at least stable disease to CHT. All patients who underwent surgery were discharged from hospital with nil perioperative mortality. No macroscopic residual tumor was found in any of the patients who underwent HR. Patients who underwent surgery after downsizing CHT survived significantly longer than those who did not (2-year OS 45% vs. 19%, P = 0.0032)[14]. In 2010, a very promising phase III clinical trial (ABC-02) of combination therapy using GEM and CIS was published, with survival rates reported up to 11.7 months, compared with 8.3 months in the GEM-only group[17]. According to this study, the same group of Kato et al. used a combination therapy to treat unresectable BTC[15]. The combination consisted of GEM (1,000 mg/m2) plus CIS (25 mg/m2) administered for 2 weeks. To compare these results, the authors used historical controls receiving downsizing CHT with GEM alone[14]. Of the total 39 BTC patients, 25 were diagnosed with ICCA. Reduction in tumor size, including the disappearance of vascular invasion, was found in 10 out of 39 patients (25.6%), resulting in HR in all of these patients. The average interval from the induction of CHT to HR was 5.9 ± 3.3 months. At the end of the study, no significant differences were demonstrated in terms of OS between downsizing CHT using GEM vs. the combination of GEM plus CIS. Interestingly, those who underwent HR following GEM plus CIS showed better pathological responses than those who received GEM only. The rate of secondary resection seemed to be lower in combined CHT (25.6% vs. 37.5%). Nevertheless, the median OS in patients with HR following downsizing CHT and those receiving CHT alone without HR was 17.9 and 12.4 months, respectively (P = 0.0378), whereas 2-year OS was 32% and 0%, respectively[15]. A similar finding was reported by Le Roy et al., where median OS was 3 years in patients with locally advanced ICCA who had CHT followed by resection compared with 11 months in patients treated with CHT alone (P < 0.001)[18]. Neoadjuvant CHT led to a conversion surgery in 53% of patients initially considered to have a locally advanced disease (without extrahepatic metastases and/or distant lymphadenopathy). In particular, in this study, OS and RFS rates after secondary resection treated with neoadjuvant CHT were similar to those obtained after resection in patients with resectable ICCA.
Selected studies on neoadjuvant systemic chemotherapy
| Authors | Year of enrollment | Type of studies | N°pts | Protocol | Response (%) | Resectability (%) | Survival rates |
| Kato et al. | 2004-2010 | Single-center retrospective study | 22 (ICCA = 7) | GEM | PR: n = 2 (28.6) SD: n = 4 (57.1) PD: n = 1 (14.3) | n = 4/7 (57.1) | Median OS: 19.3 mo 5-yr OS: 40% |
| Kato et al.[15] | 2011-2014 | Single-center prospective observational study | 39 (ICCA = 25) | GEM + CIS | PR: n = 9 (23.1) SD: n = 21 (53.8) PD: n = 9 (23.1) | n = 5/25 (20) | Median OS: 17.9 mo |
| Le Roy et al.[18] | 2010-2013 | Single-center retrospective study | 74 | GEM + OX (n = 44) GEM + other GEM (n = 3) Other (n = 23) | PR: n = 18 (24) SD: n = 33 (45) PD: n = 23 (31) | n = 39 (53) | Median OS: 18 mo 3- and 5-yr OS: 27 and 21% |
Hepatic arterial infusion
To improve the outcomes in patients with unresectable disease, some authors tested a combination therapy of systemic CHT and hepatic arterial infusion (HAI)[19]. HAI consists of a continuous infusion typically of floxuridine into the hepatic arterial circulation that is administered through a surgically implanted pump. Konstantinidis et al. showed that the response of the patients who received HAI and CHT was better than CHT alone; furthermore, the combination of HAI and CHT was associated with improved OS (30.8 vs. 18.4 months, P < 0.001)[19] [Table 2]. Eight patients who initially presented with unresectable disease confined to the liver showed a sufficient radiological response to undergo complete resection and had a median OS of 37 months. Several years later, still, the Memorial Sloan-Kettering group reported in a recent phase 2 clinical trial a similar median OS of 25 months, using a combined approach of HAI plus CHT (GEM and oxaliplatin)[20].
Selected studies on neoadjuvant systemic chemotherapy plus hepatic arterial infusion
| Authors | Year of enrollment | Type of study | N°pts | Protocol | Response | Resectability | Survival rates |
| Konstantinidis et al.[19] | 2000-2012 | Multicenter retrospective study | 104 (liver confined disease) | HAI floxuridine + CHT (mitomycin C or GEM): n = 78 (75%) CHT: n = 26 (25%) | NA | n = 8 (7.7) | Median OS: 20.1 mo |
| Cercek et al.[20] | 2013-2019 | Phase 2 Clinical Trial | 42 | HAI (floxuridine) + CHT (GEM + OX) | PR: n = 22 (58) | n = 4 (9.5) | Median OS: 25 mo (95%CI: 20.6-not reached) 1-yr OS: 89.5% |
Secondary resectability was 9.5% among patients with “sufficient” radiological response. The possibility of conversion to surgery increased by HAI compared to CHT alone was not demonstrated in any of the studies included in our review.
Trans-arterial radioembolization
Among all multimodal therapies, the combination of trans-arterial radioembolization (TARE) has been recently proposed as a promising downstaging method for initially unresectable ICCA. In most of the studies selected for the purpose of this review, CHT was administered prior to TARE, ranging from 23.5% to 79% of cases[21-23] [Table 3]. No comparisons have ever been made between patients who received CHT and those who did not receive CHT, except for one Italian multicenter study[22] where prior CHT did not change OS rates compared to naïve patients. Of note, the median OS was 12.4 months in the group with disease control after first-line CHT vs. 15 months in the CHT naïve group. Overall, the resectability rate was 3.7%, but differences between resection vs. non-resection groups were not taken into account.
Selected studies on neoadjuvant systemic chemotherapy plus radioembolization
| Authors | Year of enrollment | Type of study | N°pts | Protocol | Response (%) | Resectability (%) | Survival rates |
| Rayar et al.[16] | 2008-2013 | Single-center prospective study | 45 | Concomitant: n = 45 (100%) | NA | n = 8 (17.8) | Median OS: 15.6 mo |
| Buettner et al. | 2006-2017 | Multicenter retrospective study | 115 | Concomitant: n = 4 (3.5%) Prior CHT: n = 91 (79%) | PR: n = 7 (6.1) SD: n = 63 (54.8) PD: n = 26 (22.6) | n = 5 (4.3) | Median OS (from diagnosis): 29 mo Median OS (from TARE): 11 mo (95%CI: 8-13 mo) 1- and 3-yr OS: 44% and 4% Median PFS: 5 mo (95%CI: 3-7) |
| Bargellini et al.[22] | 2008-2017 | Multicenter retrospective study | 79 | Prior CHT: n = 19 (24%) | CR: n = 2 (10.5) PR: n = 7 (36.8) SD: n = 7 (36.8) PD: n = 3 (15.9) | n = 3 (3.7) | Median OS: 12.4 mo (95%CI: 6.9-17.3) |
| Helmberger et al.[23] | 2015-2017 | Multicenter prospective observational study | 120 | Concomitant: n = 11 (9.2%) Prior CHT: n = 73 (60.8%) | NA | n = 4 (3.3) | Median OS: 14.7 mo (95%CI: 10.9-17.9) |
| Edeline et al. | 2013-2016 | Phase 2 Clinical Trial | 41 | Concomitant: n = 41 (100%) | NA | n = 9 (22) | 2-yr OS: 88.9% 2-yr PFS: 66.7% |
| Saxena et al. | 2004-2009 | Single-center prospective observational study | 25 | Prior CHT: n = 18 (72%) | PR: n = 6 (24) SD: n = 11 (48) PD: n = 5 (20) | n = 1 (4) | Median OS: 9.3 mo 1-, 2-, and 3-yr OS of 40%, 27%, and 13% |
| Swinburne et al.[35] | 2008-2015 | Single-center retrospective study | 29 | Prior CHT: n = 15 (51.7%) | CR: n = 0 (0) PR: n = 3 (11.5) SD: n = 16 (61.5) PD: n = 7 (26.9) | n = 1 (3.4) | Median OS: 9.1 mo (95%CI: 1.7-16.4) Median TTP: 5.6 mo (95%CI: 0-12.0) |
| Shaker et al. | 2006-2016 | Single-center retrospective study | 17 | Prior CHT: n = 5 (29.4%) | NA | n = 1 (5.9) | Median OS: 33.6 mo (95%CI: 4-64.8) 5-yr OS: 26.8% Median PFS: 4 mo (95%CI: 0-12) 1-yr PFS: 37.5% |
More interestingly, instead, it is the use of concomitant CHT and TARE. Rayar et al. analyzed 45 patients with single tumors localized within non-cirrhotic livers and without extrahepatic metastases[16]. TARE was administered a few weeks after the initiation of the CHT. The authors reported that CHT with TARE allowed for secondary resection in 8 out of 10 potentially resectable cases. However, no criteria for secondary resectability were reported. Nevertheless, extended hepatectomies were required in all cases, with cava replacement in 4 cases, leading to R0 resection in all patients. The median follow-up for these patients was 8.2 months after surgery. Two patients experienced extrahepatic recurrence of the disease.
More recently, Edeline et al. reported a phase 2 clinical trial including 41 patients with unresectable ICCA (either non-cirrhotic or cirrhotic with Child-Pugh < B8) submitted to concomitant CHT and TARE who had not received preoperative CHT or locoregional therapy[24]. The regimen was based on GEM plus CIS for a recommended number of 6 cycles with a decreased dose of GEM during concomitant CHT and TARE due to any potential toxic effects. Among them, 9 patients (22%) could be downstaged to surgical intervention, with 8 out of 9 achieving R0 after HR. After a median of 46 months after surgery, median RFS was not reached among patients who underwent resection, confirming that TARE combined with CHT may have a potential for curative treatment in patients otherwise considered for palliative treatment.
Trans-arterial Chemoembolization
Among all the studies analyzing the role of trans-arterial chemoembolization (TACE) in treating unresectable ICCA combined with CHT, only two studies reported the rate of conversion surgery [Table 4]. Aliberti et al. treated 127 patients with drug-eluting beads (DEB) or polyethylene glycol drug-eluting microspheres, both loaded with doxorubicin[25]. Only 4 out of 127 (3.1%) were submitted to secondary liver resection. Schiffman et al. reported instead a case series of 24 patients with unresectable ICCA who underwent DEB therapy. Concurrent CHT was administered in 8 cases (33.3%)[26]. Three patients (12.5%) were downstaged to resection and underwent subsequent hepatectomy with complete pathological response in all three patients. Median OS was 17 months, significantly longer compared to that reported in the literature for the sole CHT, as stated by the authors.
Selected studies on neoadjuvant systemic chemotherapy plus trans-arterial chemoembolization
| Authors | Year or enrollment | Type of study | N°pts | Protocol | Response | Resectability (%) | Survival rates |
| Aliberti et al. | 2000-2016 | Multicenter prospective observational study | 127 | DEBDOX (n = 109, 86%) or LIFDOX (n = 18, 14%) | PR: n = 19 (15) SD: n = 101 (80) PD: n = 7 (5%) | n = 4 (3.8) | NA |
| Schiffman et al.[26] | NA | Multicenter prospective observational study | 24 | Concomitant CHT n = 8 (30%) | CR: n = 1 (4) PR: n = 1 (4) SD: n = 20 (83) PD: n = 2 (8) | n = 3 (12.5) | Median OS:17.5 mo |
DISCUSSION
BTC is often diagnosed at advanced stages and has a poor outcome due to limited systemic options. In particular, ICCA is a rare tumor of the liver accounting for 3% of all gastrointestinal malignancies[27] and comprises approximately 20% of all cholangiocarcinomas[28]. Surgery remains the cornerstone of curative-intent therapy, although many patients present at diagnosis with advanced disease that is not amenable to resection. Advances in the understanding of the biologic behavior and available multimodality treatments for this aggressive tumor have progressed over the last decade[29]. This has led to increased use of neoadjuvant treatments.
In this narrative review, we aimed to select all the studies reporting the rates of resectability after systemic therapy combined or not with IAT. Most of the studies showed that secondary resection was associated with improvement in OS. These results indicate that neoadjuvant CHT may have the potential to expand the surgical indication and represent a real opportunity for patients with initially unresectable ICCA. In particular, studies analyzing CHT alone reported the highest conversion rate ranging from 20% to 57.1%[14,15], confirming that systemic treatment may provide the best results and therefore should always be included as a part of the neoadjuvant protocol. Interestingly, the longest OS reported was that after CHT plus HAI (25 months). Moreover, when HR was achieved, OS was even longer and comparable to patients with resectable disease[18]. On the contrary, multimodal treatments including systemic therapy and TARE or TACE provided inferior results. Only the study by Edeline et al., where concomitant CHT was administered together with TARE, reported a 2-year OS of 88%, confirming once again the importance of systemic therapy[24]. However, it must be said that survival rates in most of the studies with prior CHT were calculated from the time of TARE/TACE and not from the start of CHT, limiting any definitive conclusion. For instance, Buettner et al. calculated OS from the time of diagnosis, which was 29 months, compared to 11 months when it was instead calculated from the TARE treatment[21,22,30]. Certainly, the role of concomitant CHT combined with TARE is appealing, but the concerns with cumulative toxicity have limited such a kind of approach[30]. Nevertheless, the possibility of a higher conversion rate to surgery using a combination therapy rather than CHT alone was not clearly demonstrated in any of the studies included in our review. There were too few studies on the use of TACE as downstaging therapy to surgery to be commented on appropriately. However, secondary resectability and survival rates were similar to those reported for TARE.
Opening issues still include both the absence of definitive criteria regarding the duration of re-evaluation after downsizing CHT, and clear criteria regarding the ideal timing for surgery. The design and validation of effective CHT protocols may also require the identification of appropriate biomarkers to guide its use and refine radiological criteria that seem to be not enough to effectively evaluate the response after neoadjuvant therapy[31]. In the future, interesting data about the potential role of downstaging to surgery may derive from the application of newly approved targeted therapies (FGFR2 or IDH1 inhibitors) to ICCA patients with specific genomic alterations (FGFR2 rearrangement or IDH1 mutations, respectively)[32,33]. Similarly, immunotherapy is playing an increasing role in the treatment of ICCA[7,34], but to our knowledge, there are currently no studies that have investigated the neoadjuvant role of targeted therapy or immunotherapy in patients with advanced ICCA who were initially unresectable and subsequently underwent liver resection.
In conclusion, downsizing CHT may have the potential to increase surgical indications and the possibility of improved survival. A multimodal approach including combined systemic therapy with IAT might improve long-term outcomes of unresectable patients and convert locally advanced cancer into resectable disease with an improved margin-negative resection rate. However, most studies in the literature are limited by their retrospective nature and randomized controlled trials are awaited to confirm their effectiveness. Immunotherapy, along with CHT and/or targeted therapy, represent promising strategies; however, the lack of validated predictive biomarkers to determine which patients would benefit from it remains a crucial challenge.
DECLARATIONS
Authors’ contributionsMade substantial contributions to conception of the paper, reviewed the literature and drafted the manuscript; equally contributed to the work: Caputo F, Serenari M
Reviewed the literature: Palloni A, Ravaioli M
Supervised the work, reviewed the manuscript, provided technical support and expert guidance: Brandi G, Cescon M
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. Singal AK, Vauthey JN, Grady JJ, Stroehlein JR. Intra-hepatic cholangiocarcinoma-frequency and demographic patterns: thirty-year data from the M.D. Anderson Cancer Center. J Cancer Res Clin Oncol 2011;137:1071-8.
2. Banales JM, Marin JJG, Lamarca A, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol 2020;17:557-88.
3. Zhang XF, Beal EW, Bagante F, et al. Early versus late recurrence of intrahepatic cholangiocarcinoma after resection with curative intent. Br J Surg 2018;105:848-56.
4. Lamarca A, Santos-Laso A, Utpatel K, et al. Group: on behalf of the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Liver metastases of intrahepatic cholangiocarcinoma: implications for an updated staging system. Hepatology 2021;73:2311-25.
5. Tsilimigras DI, Sahara K, Wu L, et al. Very early recurrence after liver resection for intrahepatic cholangiocarcinoma: considering alternative treatment approaches. JAMA Surg 2020;155:823-31.
6. Tian X, Wang Q, Li Y, et al. The expression of S100A4 protein in human intrahepatic cholangiocarcinoma: clinicopathologic significance and prognostic value. Pathol Oncol Res 2015;21:195-201.
8. Machairas N, Lang H, Jayant K, Raptis DA, Sotiropoulos GC. Intrahepatic cholangiocarcinoma: limitations for resectability, current surgical concepts and future perspectives. Eur J Surg Oncol 2020;46:740-6.
9. de Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol 2011;29:3140-5.
10. Hyder O, Hatzaras I, Sotiropoulos GC, et al. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery 2013;153:811-8.
11. Adam R, Delvart V, Pascal G, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg 2004;240:644-57; discussion 657.
12. Turrini O, Viret F, Moureau-Zabotto L, et al. Neoadjuvant chemoradiation and pancreaticoduodenectomy for initially locally advanced head pancreatic adenocarcinoma. Eur J Surg Oncol 2009;35:1306-11.
13. Bismuth H, Adam R, Lévi F, et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg 1996;224:509-20; discussion 520.
14. Kato A, Shimizu H, Ohtsuka M, et al. Surgical resection after downsizing chemotherapy for initially unresectable locally advanced biliary tract cancer: a retrospective single-center study. Ann Surg Oncol 2013;20:318-24.
15. Kato A, Shimizu H, Ohtsuka M, et al. Downsizing chemotherapy for initially unresectable locally advanced biliary tract cancer patients treated with gemcitabine plus cisplatin combination therapy followed by radical surgery. Ann Surg Oncol 2015;22 Suppl 3:S1093-9.
16. Rayar M, Sulpice L, Edeline J, et al. Intra-arterial yttrium-90 radioembolization combined with systemic chemotherapy is a promising method for downstaging unresectable huge intrahepatic cholangiocarcinoma to surgical treatment. Ann Surg Oncol 2015;22:3102-8.
17. Valle J, Wasan H, Palmer DH, et al. ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81.
18. Le Roy B, Gelli M, Pittau G, et al. Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Br J Surg 2018;105:839-47.
19. Konstantinidis IT, Groot Koerkamp B, Do RK, et al. Unresectable intrahepatic cholangiocarcinoma: systemic plus hepatic arterial infusion chemotherapy is associated with longer survival in comparison with systemic chemotherapy alone. Cancer 2016;122:758-65.
20. Cercek A, Jarnagin WR. Locoregional therapy plus systemic chemotherapy in unresectable intrahepatic cholangiocarcinoma-reply. JAMA Oncol 2020;6:935-6.
21. Buettner S, Braat AJAT, Margonis GA, et al. Yttrium-90 radioembolization in intrahepatic cholangiocarcinoma: a multicenter retrospective analysis. J Vasc Interv Radiol 2020;31:1035-1043.e2.
22. Bargellini I, Mosconi C, Pizzi G, et al. Yttrium-90 radioembolization in unresectable intrahepatic cholangiocarcinoma: results of a multicenter retrospective study. Cardiovasc Inter Rad 2020;43:1305-14.
23. Helmberger T, Golfieri R, Pech M, et al. On behalf of the CIRT Steering Committee; On behalf of the CIRT Principal Investigators. Clinical application of trans-arterial radioembolization in hepatic malignancies in Europe: first results from the prospective multicentre observational study CIRSE registry for SIR-Spheres therapy (CIRT). Cardiovasc Inter Rad 2021;44:21-35.
24. Edeline J, Touchefeu Y, Guiu B, et al. Radioembolization plus chemotherapy for first-line treatment of locally advanced intrahepatic cholangiocarcinoma: a phase 2 clinical trial. JAMA Oncol 2020;6:51-9.
25. Aliberti C, Carandina R, Sarti D, et al. Chemoembolization with drug-eluting microspheres loaded with doxorubicin for the treatment of cholangiocarcinoma. Anticancer Res 2017;37:1859-63.
26. Schiffman SC, Metzger T, Dubel G, et al. Precision hepatic arterial irinotecan therapy in the treatment of unresectable intrahepatic cholangiocellular carcinoma: optimal tolerance and prolonged overall survival. Ann Surg Oncol 2011;18:431-8.
27. Beal EW, Tumin D, Moris D, et al. Cohort contributions to trends in the incidence and mortality of intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr 2018;7:270-6.
28. Akateh C, Ejaz AM, Pawlik TM, Cloyd JM. Neoadjuvant treatment strategies for intrahepatic cholangiocarcinoma. World J Hepatol 2020;12:693-708.
29. Amini N, Ejaz A, Spolverato G, Kim Y, Herman JM, Pawlik TM. Temporal trends in liver-directed therapy of patients with intrahepatic cholangiocarcinoma in the United States: a population-based analysis. J Surg Oncol 2014;110:163-70.
30. Saxena A, Bester L, Chua TC, Chu FC, Morris DL. Yttrium-90 radiotherapy for unresectable intrahepatic cholangiocarcinoma: a preliminary assessment of this novel treatment option. Ann Surg Oncol 2010;17:484-91.
31. Serenari M, Cappelli A, Cucchetti A, et al. Deceased donor liver transplantation after radioembolization for hepatocellular carcinoma and portal vein tumoral thrombosis: a pilot study. Liver Transpl 2021;27:1758-66.
32. Abou-Alfa GK, Sahai V, Hollebecque A, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol 2020;21:671-84.
33. Zhu AX, Macarulla T, Javle MM, et al. Final overall survival efficacy results of ivosidenib for patients with advanced cholangiocarcinoma with idh1 mutation: the phase 3 randomized clinical ClarIDHy trial. JAMA Oncol 2021;7:1669-77.
34. Oh DY, Lee KH, Lee DW, et al. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: an open-label, single-centre, phase 2 study. Lancet Gastroenterol Hepatol 2022;7:522-32.
35. Swinburne NC, Biederman DM, Besa C, et al. Radioembolization for unresectable intrahepatic cholangiocarcinoma: review of safety, response evaluation criteria in solid tumors 1.1 imaging response and survival. Cancer Biother Radiopharm 2017;32:161-8.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Caputo F, Serenari M, Palloni A, Ravaioli M, Brandi G, Cescon M. Neoadjuvant chemotherapy alone or combined with trans-arterial therapies for downstaging unresectable intrahepatic cholangiocarcinoma to surgical resection: a narrative review. Hepatoma Res 2023;9:31. http://dx.doi.org/10.20517/2394-5079.2022.71
AMA Style
Caputo F, Serenari M, Palloni A, Ravaioli M, Brandi G, Cescon M. Neoadjuvant chemotherapy alone or combined with trans-arterial therapies for downstaging unresectable intrahepatic cholangiocarcinoma to surgical resection: a narrative review. Hepatoma Research. 2023; 9: 31. http://dx.doi.org/10.20517/2394-5079.2022.71
Chicago/Turabian Style
Caputo, Francesca, Matteo Serenari, Andrea Palloni, Matteo Ravaioli, Giovanni Brandi, Matteo Cescon. 2023. "Neoadjuvant chemotherapy alone or combined with trans-arterial therapies for downstaging unresectable intrahepatic cholangiocarcinoma to surgical resection: a narrative review" Hepatoma Research. 9: 31. http://dx.doi.org/10.20517/2394-5079.2022.71
ACS Style
Caputo, F.; Serenari M.; Palloni A.; Ravaioli M.; Brandi G.; Cescon M. Neoadjuvant chemotherapy alone or combined with trans-arterial therapies for downstaging unresectable intrahepatic cholangiocarcinoma to surgical resection: a narrative review. Hepatoma. Res. 2023, 9, 31. http://dx.doi.org/10.20517/2394-5079.2022.71
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 6 clicks
Cite This Article 6 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.