Chemoembolization with Degradable Starch Microspheres (DSM-TACE): expanding indications in HCC multidisciplinary tumor board
Abstract
The role of transarterial chemoembolization (TACE) in hepatocellular carcinoma (HCC) management has changed over the last twenty years. There has been a trend towards an overall decline in TACE procedures, but with a more aggressive approach, repeating multiple TACE sessions in case of tumor response. The survival of treated patients was prolonged because of better patient selection and advancements in TACE techniques aimed at preserving liver function. At present, TACE is approved by the International Guidelines also outside of the BCLC intermediate stage after evaluation of a multidisciplinary tumor board (MDTB), permitting a customized treatment for every patient. An alternative therapeutic strategy is represented by hepatic chemoembolization with Degradable Starch Microspheres (DSM-TACE), which is based on the chemotherapeutic effect rather than on the ischemic damage to the liver tumor, requiring multiple cycles of treatment. The higher safety profile of DSM-TACE has broadened the indications to patients waiting for liver transplantation (with bridging or downstaging intention), at high risk of liver failure and ineligible for systemic therapies. This review summarises the scientific publications supporting the use of DSM-TACE and illustrates its indications depending on the disease stage from the Interventional Radiologist’s perspective.
Keywords
INTRODUCTION
Transarterial chemoembolization (TACE) is a well-established treatment for hepatocellular carcinoma (HCC) with a strong grade of recommendation (1A) and a high level of evidence (1iiA)[1]. The traditional indications for TACE are limited to patients with multinodular HCC in the intermediate stage, preserved liver function (Child-Pugh class A or B7) and good performance status (ECOG 0-1), whereas contraindications are represented by decompensated liver cirrhosis (refractory ascites, jaundice, and encephalopathy), by portal vein thrombosis or hepatofugal flow and by extrahepatic metastases. TACE has demonstrated advantages in combination with percutaneous thermal ablation (RFA, MWA) with curative intent to control hepatic disease in patients waiting for liver transplantation (bridging) and for downstaging in patients outside the Milan criteria for liver transplantation, but is not recommended as a preoperative neoadjuvant treatment before surgical resection in the early stage or in combination with external ablation radiotherapy in the intermediate stage, nor in association with systemic agents for palliation in the advanced stage[2].
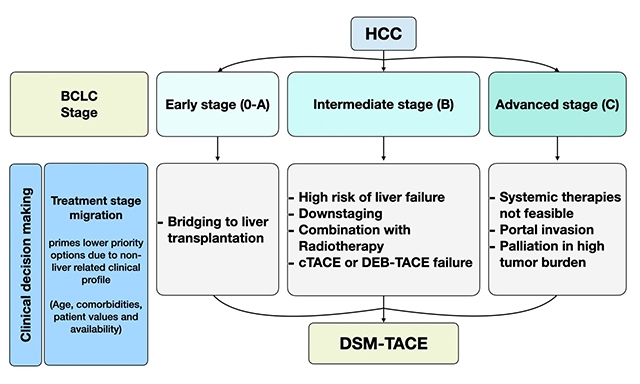
Over the last decades, there have been significant improvements in HCC management, leading the Barcelona Clinic Liver Cancer (BCLC) Group to publish recently the “2022 update” of the treatment strategy[3], which extends the indications both of locoregional and systemic therapies and recognizes the fundamental role in clinical decision making of the multidisciplinary tumor board (MDTB), composed of local experts in various disciplines, involving gastroenterologists, hepatobiliary surgeons, medical oncologists, radiation oncologists, nuclear medicine physicians, diagnostic, and interventional radiologists. The treatment stage migration (TSM) in the new BCLC algorithm is aimed at optimizing individualized treatments because it accounts for the stage of the disease, patient’s characteristics, and the accessibility of therapeutic options and technical skills in every single center. Where MDTB is available, the treatment decisions are usually based on its consensus, which may not fully adhere to the guidelines and allow a more flexible approach with a proven favourable impact on the progression-free survival (PFS) and the overall survival (OS) rate of HCC patients[4-6].
As interventional radiologists (IRs), we have noticed a trend towards a decline in the use of transarterial therapies that may result in liver function deterioration or worsening of portal hypertension. At the same time, in selected cases, a more aggressive approach is performed, repeating multiple TACE sessions for disease control[7]. This change in “real life” HCC management is due in part to the technical developments that have improved TACE procedures in terms of both efficacy and safety: the use of smaller microcatheters for superselective embolization in conventional TACE (cTACE) according to the Japanese school[8], as well as the introduction of Drug-eluting beads transarterial chemoembolization (DEB-TACE) in the early 2000s, used mainly in Western countries because of a benefit over cTACE in terms of inferior systemic side effects and post-embolic syndrome[9].
DSM-TACE, which focuses on the chemotherapeutic effect rather than the ischemic damage to the liver tumor, has shown an almost non-inferior efficacy in comparison with cTACE or DEB-TACE, but is better tolerated by the patients, particularly when non-selective (lobar) catheterization and repetitive treatments are performed. Despite being less “evidence-based” than other TACE techniques, or liver dominant disease. It may represent a second-line therapeutic option for HCC with diffuse multinodular pattern or high tumor burden, in patients awaiting for liver transplantation or at high risk of liver failure, in case of recurrence after cTACE or DSM-TACE or in combination with local ablative therapies such as radiotherapy and even when systemic therapies are not feasible in the advanced stage due to comorbidities.
To our knowledge, this article represents the first comprehensive review of literature about DSM-TACE, which has been recently included in the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) Guidelines for transarterial chemoembolization of HCC as an alternative to DEB-TACE and cTACE[10]. The topics of DSM-TACE’s development, mechanism of action, advantages and limitations in comparison with other chemoembolization agents are addressed, highlighting the concept of repeatable enhanced liver chemotherapy. We illustrate the change of TACE indications endorsed by the 2022 BCLC TSM strategy[3] and discuss the role of DSM-TACE alone and combined with other therapies in the multidisciplinary HCC management.
RATIONALE FOR DEGRADABLE STARCH MICROSPHERES USE
The research of an ideal temporary embolic agent, like gelatine sponge embolization (cTACE), has a long history, beginning more than 40 years ago. Degradable Starch Microspheres (DSMs) were initially studied in animal models in Scandinavia[11] in the late 1970s. In Japan, Yamada et al. introduced the transarterial treatment of HCC with an emulsion of chemotherapeutic agent and lipiodol followed by cTACE[12]. The first clinical trial[13] investigating the role of DSMs in humans, using Spherex® (Kabi Pharmacia, Lund, Sweden) mixed with cytotoxic drugs, called the procedure Transarterial Chemoocclusion (TACO) and demonstrated significantly greater tumour response for patients with either hepatocellular carcinoma or metastatic liver cancer in comparison with intra-arterial infusion of chemotherapeutic agents. Indeed, TACO was originally developed to enhance the effect of local chemotherapy without inducing the ischemic effects typical of embolization[14], as recently demonstrated in an animal model, showing complete reperfusion of the hepatic artery 30 mins after DSMs administration without damage to the liver parenchyma[15]. The therapeutic index of the chemotherapeutic agent in DSM-TACE is markedly improved in comparison with simple intra-arterial infusion[16]: the tumor concentration increases more than 10-15 times depending on the drug used, there is an inversion of the tumor/liver concentration ratio with relative sparing of liver parenchyma and the systemic exposure to the drug is reduced due to higher local drug uptake by the tumor[17].
EmboCept S® (PharmaCept GmbH, Berlin, Germany), currently the only available product for DSM-TACE, received market authorization in 2010 and was included in CIRSE guidelines for TACE in 2021[10]. EmboCept S consists of a suspension in saline solution of partially hydrolyzed starch molecules (called Amilomer) with a diameter of about 50 µm (45 ± 7), a size suitable for reaching the intratumoral arterioles, limiting the risk of pulmonary embolism. 99mTc labelled macroaggregated albumin (99mTc-MAA) nuclear scintigraphy demonstrated that particles smaller than 35 μm selectively injected in the hepatic artery, such as Yttrium-90-loaded microspheres used for transarterial radioembolization (TARE), can pass through the HCC artero-venous shunts and reach the lungs[18,19]. The problem of shunting to pulmonary vessels with TARE is due to the small size of embolic agents used for radioembolization (diameter 20-35 microns), while DSMs have a diameter of about 50 microns. The dangerous anastomoses with extrahepatic arteries should be identified and occluded in every case both before TACE and TARE. DSMs carry a lower risk of embolic complications in non-target territories because of their short half-life. After injection in the hepatic artery, the starch microspheres occlude the precapillary tumor network and undergo rapid and complete degradation by serum α-amylase (half-life 35 mins): the duration of arterial occlusion by DSMs is limited to a maximum of 80-90 mins[20,21]. Embocept S is thus a very short-term embolic agent, not causing significant ischemic or even cytotoxic liver damage on its own: it is usually administered mixed with chemotherapeutic agents for DSM-TACE to decrease the blood flow and minimize the washout of cytotoxic drugs from the HCC nodules. Some authors have used EmboCept S also as a temporary embolic agent alone, instead of absorbable gelatine sponge particles, to reach stasis of flow in the hepatic artery following cTACE[22-24].
Gelatine sponge particles, being a temporary embolic agent that promotes thrombus formation, have some disadvantages compared with DSMs: (a) recanalization time is longer and unpredictable, lasting from a few days to some weeks[20] (vs. 30-60 mins of DSMs), leading to parenchymal ischemia; (b) the material provokes foreign body reaction with chronic vessel wall inflammation and fibrosis[25], resulting in incomplete arterial patency which precludes repeated interventions (vs. non-immunogenic DSMs allowing complete revascularization and retreatment)[9]; and (c) particles are irregular, not calibrated, mostly larger than 300-500 micron[26] with a distribution peak of 1,000 micron (vs. homogeneous 50 microns of DSMs), therefore occluding extratumoral arteries (vs. intratumoral arterioles). The mean arterial occlusion time ranges from 5-12 weeks in cTACE using gelatin sponge particles applied after chemoembolization to reach the “stop flow”[27], but repeated procedures usually lead to occlusion of the hepatic artery. Therefore, according to EASL guidelines, cTACE in the same hepatic branches should be repeated only after a time interval of almost 3 months, also because more intense regimens, such as cTACE every 2 months, might induce liver failure in an unacceptable proportion of patients. On the contrary, DSM-TACE is usually repeated at a 4-6 weeks interval up to three times (one treatment cycle) before the tumor response imaging assessment, when a scheduled strategy is adopted[27-31]. Transarterial Lipiodol-only chemoembolization, without gelatine sponge particles, was tried in high-risk patients (Child-Pugh B-C, main portal vein occlusion, bilirubin ≥ 3 mL/dL) in order to reduce harm, but showed a lower efficacy in comparison with both cTACE[32] and DSM-TACE[33].
Lipiodol Ultra-Fluid® (Guerbet, Roissy, France) is an oily contrast agent with drug-carrying, tumor-seeking and transient embolic properties. Following selective injection into the hepatic artery, it is selectively taken up and retained into the HCC nodules for several weeks to over a year, due to hypervascularization and the absence of Kupffer cells, which are responsible for its clearance in the normal liver sinusoids within 4 weeks. The “water in oil” emulsion of Lipiodol and chemotherapeutic agents (with a 2-3:1 ratio) is proven to be more stable, results in a slower delivery of the cytotoxic drug into the liver tumor and increases flow stasis in tumoral feeders, reducing the systemic toxicity in comparison with the “oil in water” emulsion[2,34]. The need for standardization of cTACE protocol has been advocated, because the variability in every step of the procedure - from the choice of the chemotherapeutic drug to the embolization endpoint - limits the possibility of comparison with previous studies[35]. Intratumoral accumulation of Lipiodol, as detected on a CT scan 1 month after the TACE procedure, has prognostic implications, being associated with increased drug uptake and improved overall survival[36]. Long-lasting Lipiodol deposits have the disadvantage of interfering with CT imaging evaluation of residual HCC viability, therefore making contrast-enhanced MR preferable during follow-up. Lipiodol droplets (smaller than 35 microns) have the unique “plastic” property of adapting to vessel dimension, reaching even the very small intratumoral capillary network and passing through the peribiliary capillary plexus into the distal portal branches, thus allowing transient blocking of the venous inflow and increasing the local ischemia due to a “dual embolization” effect. The possibility of pulmonary embolism is limited but must be taken into account, especially in HCC larger than 10 cm (that tend to have shunts with the hepatic vein due to vascular invasion), when the amount of administered Lipiodol is more than 15-20 mL and in case of HCC embolization through the inferior phrenic artery, potentially connected with the inferior branch of the pulmonary artery through dangerous anastomoses. In these situations, lipiodol pneumonitis[37], similar to post-traumatic fat embolism, may lead to acute respiratory distress syndrome (ARDS).
HCC is known to be insensitive to various systemic cytotoxic drugs: no intravenous single-drug or multiple-drugs therapy has been shown to be more effective than single-agent doxorubicin, which does not bring a survival benefit over supportive care[38]. On the contrary, chemoembolization was demonstrated to improve the survival of patients because of the combined effect of higher drug concentration and tumour ischemia[39]. The chemotherapy agents most frequently used for TACE of HCC are Doxorubicin, Cisplatin, Epirubicin, Mitoxantrone and Mitomycin C, mainly in monotherapy (75%), whereas double or triple therapy is used less frequently[39]. Drug eluting beads (DEBs) have an ionic charge to bind the chemotherapy agents to obtain a sustained local drug release, but result in permanent arterial occlusion. The amount of the drug that is actually released is variable and related to the size and the structure of the different microspheres available in the market: according to an in vitro study, the eluted drug is only 30% at 1 week[40], but in vivo models revealed a higher elution rate of up to 43% at 1 month and 90% at 3 months[41]. The starch microspheres in DSM-TACE do not interact with the chemotherapeutic agent or the endothelium, causing only temporary blood flow obstruction that allows the persistence of higher chemotherapy concentration at the level of the intratumoral vessels, increasing drug uptake by the tumor cells and reducing systemic exposure. DSM-TACE indeed represents an enhanced evolution of transarterial chemoinfusion (TACI), which was a highly concentrated and selective local hepatic chemotherapy procedure, devoid of Lipiodol injection, aimed at minimizing the risk of ischemic complications to the liver[42,43]. TACI was abandoned after the demonstration of a statistically significant inferior tumor response rate and median overall survival in HCC patients with portal vein thrombosis treated with TACI in comparison with selective cTACE[44]. Nowadays, selective intra-arterial chemotherapy alone or Lipiodolization only is not recommended by EASL Guidelines for HCC management (evidence 2A).
Another potential advantage of DSM-TACE over cTACE is its ability to minimize the expression of vascular endothelial growth factor (VEGF) induced by the ischemia-reperfusion mechanism in the residual tumor cells. VEGF plays a key role in neoangiogenesis and tumor growth, and in recent years has become one of the main targets of oncologic treatments based on specific anti-VEGF systemic drugs such as Bevacizumab. Schico et al. measured the plasma VEGF levels 24 h and 1 month after prolonged or permanent hepatic artery occlusion comparing the different embolic agents, with expected values of 90-120 mins for DSM-TACE, of 4-12 weeks with cTACE and irreversible occlusion in DEB-TACE[45]. An elevation of VEGF plasma levels was found with all the three TACE techniques at 24 h and persisted unchanged at one month, but the response was stronger and statistically significant after cTACE, probably because of a higher reperfusion rate after local ischemia and concomitant occlusion of both hepatic and portal inflow using Lipodol. The short-term hypoxia induced by DSM-TACE is therefore unable to completely prevent VEGF overexpression, although the response is lower than cTACE. Unexpectedly, DEB-TACE caused only a moderate VEGF levels elevation, like DSM-TACE: one hypothesis of the authors is that complete arterial occlusion avoided rapid reperfusion of the vital tumor, necessary to elicit VEGF up-regulation by the residual viable HCC. Up-regulation of VEGF plasma levels after TACE was shown to be a negative prognostic factor not only for tumor response rate but also for progression-free survival[46], being associated with a higher incidence of local tumor recurrence and distant metastases. Furthermore, it may be responsible for a more aggressive HCC behaviour leading to infiltrative or metastatic change and for the development of collateral tumor feeders, inducing TACE resistance[47].
SAFETY AND EFFICACY OF DSM-TACE FOR HCC
The standard technique of DSM-TACE for HCC, in line with the manufacturer’s recommendations, consists of slow hepatic injection, under fluoroscopic guidance through a coaxially positioned microcatheter, of the first 4 mL of EmboCept S (450 mg/7.5 mL) mixed with 6 mL of non-ionic contrast medium and Doxorubicin at a dose of 50 mg/m2 adjusted for body surface (even though many centers prefer to administer a fixed dose of 50 mg of Doxorubicin, less than prescribed, to reduce systemic toxicity) diluted in 5-10 mL of saline solution. Then the residual 3.5 mL of EmboCept S, suspended after agitation with an equivalent volume of contrast medium, is injected until complete flow stagnation is reached in the hepatic vessels feeding the tumor. To reach the target of stasis, defined as the persistence of contrast at the tip of the microcatheter for more than five heart beats without washout[48], when one vial of EmboCept S is not sufficient like in the case of large HCC lesions, the embolization may be completed with a second vial of DSMs or with Lipiodol UF to preserve long-term hepatic artery patency[49]. The combination of Lipiodol and DSM with different chemoembolization techniques showed promising results in some studies[23,24,33,50-52] in comparison with Lipiodol-only TACE (without gelatine sponge embolization at the end).
DSM-TACE is a lobar treatment in the majority of procedures since the indication is multifocal HCC in patients ineligible for surgical resection or other local ablative techniques[30,31,53,54]. Despite the level of catheterization not being selective as in DEB-TACE or even superselective as in cTACE, the use of a microcatheter is essential to achieve flow-directed chemoembolization, to preserve hepatic artery patency avoiding dissections and to prevent reflux in non-target vessels (such as the cystic artery, which may be responsible for cholecystitis). If the patient has bilobar HCC involvement, usually the liver lobe with a higher tumor burden is treated first, then the other lobe is treated 2 weeks apart to avoid liver failure. However, an interval of 4-6 weeks between DSM-TACE sessions in the same liver lobe is recommended when multiple treatments are planned before imaging assessment, depending on the therapeutic objective. Such a different approach makes the DSM-TACE procedure more standardizable and less demanding for Interventional Radiologists than cTACE and DEB-TACE, better tolerated by patients who must repeat the treatment multiple times and easier to evaluate for the Diagnostic Radiologists responsible for the assessment of tumor viability at follow-up contrast-enhanced CT/MR imaging, usually 1 month after the treatment.
Literature about TACE for HCC with DSMs, as summarised in Table 1, reports variable results in terms of efficacy with objective response (OR), including partial response (PR) and complete response (CR), ranging from 26%[14] to 93%[55]. This is due in part to different inclusion criteria employed in the studies: the first trials included only HCC patients in the advanced BCLC stage (C) for palliation and after failure/ineligibility of all the other treatments[14,27,28,50-52]. In 2010, Yamasaki et al. were the first to compare three groups of patients (Lipiodol-only TACE , TACE with DSMs and TACE with Lipiodol + DSM) also in the BCLC intermediate stage, with more recent studies including even patients in the BCLC early stage[33,56,49,54,55,57]. The TACE regimen is another important factor of data heterogeneity: in the case of a “scheduled” strategy, the best tumor response was evaluated usually after a cycle of three treatment sessions, repeated every 4 weeks[23,49,24]. On the contrary, when DSM-TACE was performed only once or repeated “on-demand”, the best tumor response was registered after each procedure[18,54,58-60]. Finally, DSMs were not only mixed with chemotherapeutic agents as previously described, but also applied as embolic agent alone before (to protect normal liver parenchyma)[52], during (to increase drug concentration into the tumor)[50-51] or after (to achieve flow stasis)[23,33,24] cTACE.
TACE with DSMs studies for HCC treatment
| Author (year), category, and study design | TACE regimen and n° of cases | Tumor response and patients outcome | Adverse events and liver toxicity |
| Carr BI[27] (1997), case series Phase II Trial in advanced stage HCC with palliative intention | DSM + Doxorubicin and Cisplatin 35 pts treated every 4-6 weeks until disease progression | OR: 63% (CR: 6%, PR: 57%) OS: 45.7% at 1 year, 17.1% at 2 years | 1 death, 1 pancreatitis, 2 dyspnoea, 2 hypotension, 2 hepatitis, 4 hepatic artery thrombosis |
| Furuse J[28] (2003), case series Pilot Study in advanced stage HCC (DSM only followed by DSM + chemotherapy 4 weeks later) | DSM + Epirubicin 17 pts treated every 4-6 weeks until disease progression | OR: 52.9% (CR: 11.8%, PR: 41.2%) Median PFS: 9 months Median OS: 21.7 months | Pain, nausea/vomiting, fever and leukopenia were common. Grade III/IV liver toxicity in 4 pts. No deaths |
| Dettmer A[50] (2006), combination therapy Prospective Study in advanced stage HCC of PEI alone vs. DSM-TACE combined with PEI | DSM + Cisplatin and Doxorubicin mixed with Lipiodol 101 pts received PEI, only 37 pts in DSM-TACE + PEI arm Total of 67 TACE (mean 1.81/pt) | TACE + PEI achieved better survival than PEI alone 2/37 received liver transplant OS: 90% at 1 year, 52% at 2 years, 43% at 3 years | TACE has lower complications rate than PEI 2 reversible leukopenia, 1 pancytopenia, 2 reversible liver failure |
| Kirchhoff TD[14] (2006), case series Phase II Trial in advanced stage HCC comparing TACO with TACI (chemio alone infusion) | DSM + Cisplatin and Doxorubicin Maximum of 6 DSM-TACE repeated monthly until progression 35 TACO vs. 35 TACI | OR TACO: 26% (CR 0%) OR TACI: 9% (CR 0%) PFS median: 32 vs. 27 weeks OS: 60 vs. 69 weeks | Grade 4 AE were rare and lower than cTACE Good patient tolerability No treatment mortality |
| Kirchhoff TD[51] (2007), case series Prospective Study in advanced HCC using DSM after Lipiodol TACE with “sandwich” technique | DSM + Cisplatin and Doxorubicin mixed with Lipiodol 47 pts (112 TACE, median 2.4 treatment per patient) | OR: 36% (CR 0%, PR 35%) OS: (median 26 months) 75% at 1 year, 59% at 2 years, 41% at 3 years | Thrombocytopenia/ Leukopenia Grade III + IV in 7.1 + 3.6% = 10.7% Major complications: 5.4% Mortality: 2.1 % |
| Murata S[52] (2008), case series Prospective Study in unresectable HCC pts after repeated TACE | cTACE after DSM embolization of the tumor-free liver parenchyma 19 pts (21 TACE) | OR: 62% (complete necrosis of the target lesion in 26% of cases) OS: 32.5% at 2 years | No liver dysfunction after TACE-DSM protocol used to protect non tumoral parenchyma |
| Yamasaki T[33] (2011), comparative study Prospective Trial comparing 3 groups of intermediate stage HCC pts undergoing repeated TACE (up to 6 courses) | Cisplatin mixed with - Lipiodol in 15 pts (23 TACE, mean 1.5/pt); - DSM in 15 pts (29 TACE, mean 1.9/pt); - Lipiodol followed by DSM in 15 pts (29 TACE, mean 1.9/pt) | OS: no survival difference in the 3 groups PFS: significantly better in the Lipiodol + DSM group OR: 40% in Lipiodol; 53% in DSM; 80% in Lipiodol + DSM | No severe AE in all groups Thrombocytopenia not frequent Elevated ALT frequent in the Lipiodol + DSM group Only 1 biloma in the Lipiodol + DSM group |
| Yamasaki T[22] (2012), case series Prospective Study in HCC pts (Child-Pugh A/B) treated with cTACE + DSM embolization | Cisplatin mixed with Lipiodol + followed by DSM 50 pts (88 TACE, mean 1.8/pt, range 1-8) | OR: 72% (CR 38%, PR 34%) OS: (median 32.6 months) 85% at 1 year, 67% at 2 years, 41% at 3 years, 41% at 4 years | Grade 3 or 4 thrombocytopenia in 30% pts Elevated ALT in 20% pts Biloma developed in 6% of pts No severe AE or deaths procedure related |
| Niessen C[59] (2014), comparative study Retrospective analysis comparing DSM-TACE vs. cTACE in intermediate stage HCC | Doxorubicin mixed with: - DSM in 34 pts (68 TACE) - Lipiodol + optional Gelfoam in 35 pts (101 TACE) | OR: 44.1% in DSM; 48.6% in Lipiodol OS (not significantly different): 15.5 months in DSM; 19.1 months in Lipiodol | No differences in complication rate and severity Higher liver toxicity (AST increase) 24 h after cTACE vs. DSM-TACE |
| Schicho A[53] (2017), case series Prospective Multicenter Observational Study in intermediate stage HCC | DSM with monotherapy of Doxorubicin, Cisplatin or Epirubicin in 50 pts (179 TACE, mean 3.58/pt) | OR: 44% (CR 2%, PR 42%) No data about OS | AE in 50% of pts Minor AE in 48% of pts; Severe AE in 2% No liver toxicity |
| Gruber-Rouh T[23] (2018), comparative study Retrospective analysis comparing cTACE with or without DSM (instead of gelatine sponge particles) in early or intermediate stage HCC | Mitomycin C mixed with: - Lipiodol in 51 pts - Lipiodol + DSM in 48 pts Total of 99 pts (667 TACE, mean 6.7; range 2-19 courses repeated every 4 weeks) | Bias: Recist 1.1 criteria No complete responses - Lipiodol: PR 21.6%, SD 62.7%, Mean Survival 25 months - Lipiodol + DSM: PR 29.2%, SD 45.8%, Mean Survival 28 months | 15%: post embolic syndrome (pain, nausea for 2-7 days) No major complications, all pts discharged on the day of treatment |
| Orlacchio A[54] (2020), case series Single Center Prospective Study in intermediate stage HCC (33 pts in BCLC stage A, 84 in stage B, 20 in stage C) considering the best response to TACE | Doxorubicin mixed with DSM, repeated “on demand” 137 pts (267 TACE, range 1-5, mean 1.94/pt) | OR: 84.3% OS: (median 36 months) 81.3% at 1 year, 57.9% at 2 years, 34.9% at 3 years PFS median: 12 months 24 pts underwent LT | 73.7%: post embolic syndrome Major complications: 6.8% 1 procedure related death due to liver failure, 1 portal vein thrombosis with variceal bleeding, 2 abscesses, 4 cholecystitis |
| Ludwig JM[49] (2021), case series including case series of Iezzi R (2019), of Gross A (2020) and of Haubold J (2020) Multicenter European Retrospective Study in high tumor burden HCC ineligible for or failing other therapies considering the best response after a cycle of 3 TACE (range 1-6) | TACE sessions planned every 4 weeks DSM mixed with Doxorubicin in 75 pts, Epirubicin in 43 pts, Doxorubicin + MitomycinC in 3 pts 121 pts: 11 in BCLC stage A, 64 in stage B, 43 in stage C, 3 in stage D (558 TACE, range 2-12) - Lipiodol added at the end to achieve stasis in 91 procedures | mRecist criteria OR: 58% (CR: 13.5%) SD: 25.2% OS median: 15.5 months PFS median: 9.5 months (in pts with CR: 21.5 months) 6 pts underwent LT 1 pt resected after successful downstaging | Minor AE: Grade 1 in 15.8%, Grade 2 in 0.36%, Grade 3 in 0.9% Major AE: no deaths or permanent sequelae Liver toxicity: AST increases in 8% of cases No liver failure or biliary toxicity (bilomas, abscesses or PV thrombosis) |
| Vogl TJ[24] (2021), comparative study Prospective Trial to compare TACE using Lipiodol only with TACE adding DSM as embolic agent (instead of gelatine sponge particles) | 3 TACE cycle scheduled at 4 weeks interval Mitomycin C + Lipiodol + DSM injected after cTACE 54 pts: 26 TACE Lipiodol only, 28 TACE Lipiodol + DSM | mRecist criteria OR: 7.7% in Lipiodol only, 35.7% in Lipiodol + DSM OS median: 33.4 months in Lipiodol only, 32.5 months in Lipiodol + DSM | Minor AE: Grade 1 in 14.8%, Grade 2 in 3.3% No differences in complications between the two groups No major AE |
| Auer TA[60] (2021), comparative study Retrospective Single Center Study comparing DSM-TACE with TARE in HCC without portal vein invasion | Doxorubicin mixed with DSM 36 pts: 18 TARE group, 18 DSM-TACE group (performed ≥ 3 times in 11 pts and ≤ 3 times in 7 pts), | OS median: 9.5 months in both arms, but increases to 11 months if DSM-TACE was made ≥ 3 times PFS: 6 months in SIRT-TARE, 4 months in DSM-TACE | No differences in toxicity profiles except for nausea and vomiting, more frequent in the TARE group |
| Minici R[58] (2021), combination therapy Retrospective Analysis in intermediate HCC pts with Child-Pugh Score of 8-9 using DSM-TACE for Downstaging before liver transplantation (LT) | Doxorubicin DSM-TACE performed “on demand” 50 pts outside of Milan criteria for LT 142 DSM-TACE (mean 2.84/pt, range 1-6) | OR: 68% (CR 12%, PR 56%) PFS: 37% at 1 year, 12% at 2 years OS: 81.4% at 1 year, 50% at 2 years Downstaging in 6 pts (12%) | AE in 44% of pts Serious AE in 4% of pts (2 cases of cholecystitis) No deaths procedure related |
| Minici R[56] (2021), combination therapy Retrospective Analysis in early stage HCC pts eligible for LT using DSM-TACE for bridging | DSM-TACE “on demand” 54 pts waiting for LT in Child-Pugh class B 154 DSM-TACE (mean 2.85) | OR: 70.4% (CR 11.1%, PR 59.3%) OS: 92% at 1 year 18 pts (33%): successful LT | AE grade 1 and 2 in 31.5% Serious AE grade 3 in 3.7% (2 cases of cholecystitis). No increase in drop-out rate |
| Yildiz I[55] (2022), comparative study Retrospective Single Center Study comparing DSM-TACE with DEB-TACE (300-500 µm) in unresectable HCC pts | Doxorubicin as chemotherapy 54 pts: 29 DSM-TACE group + 25 DEB-TACE group Performed only single treatments Evaluation with mRecist Criteria | OR in DEB: 84% (CR 0%, PR 84%) OR in DSM: 93.1% (CR 27.6%, PR 65.5%) Greater reduction of AFP levels in DSM-TACE No difference in survival or recurrence rate | Not available |
| Mohr I[57] (2022), comparative study Retrospective Study comparing DSM-TACE with cTACE and with DEB-TACE in 148 HCC pts (61 in the bridging to LT group and 87 in the palliative group) | Overall 492 TACE in 148 pts (348 DEB-TACE, 60 cTACE, 84 DSM-TACE) 334 TACE in 87 pts in the palliative setting 158 TACE in 61 pts in the bridging to LT group | OR in the palliative group: DEB-TACE 74%, cTACE 71.4%, DSM-TACE 81% No difference between DEB and DSM-TACE in tumor response In the bridging group 42/61 pts underwent LT (67%) | cTACE used more frequently in the bridging group, but resulted in more AE, ALT elevation and prolonged hospitalization No differences in systemic toxicity No abscesses in DSM-TACE (vs. DEB-TACE 3%; cTACE 3.7%) |
Tumor objective response (OR) after DSM-TACE is considered almost noninferior, especially if repeated
Figure 1. HCC with vascular invasion: 67 years-old female with HCV viral cirrhosis, Child A6, MELD 9. (A-C) CT scan in arterial (A), portal (B) and delayed (C) phases shows ill defined HCC (diameter about 4 cm) in segment VIII with infiltrative growth and portal vein branch tumor thrombosis. (D) Selective right lobe DSA before the first DSM TACE demonstrating multiple small hypervascular areas. (E) Angiography after 4 weeks before the second DSM-TACE treatment shows good tumor response to chemotherapy with contrast blush disappearance and patency of the hepatic artery with irregularities of the distal branches due to chemical vasculitis. (F) Follow-up CT at 5 years: complete tumor response without recurrence in the arterial phase.
A survival benefit following DSM-TACE was demonstrated in the largest multicenter European series[49] even in advanced BCLC C stage patients (median OS = 12.7 months), similar to the reported effect of systemic therapy with Sorafenib (median OS = 10.7 months), in comparison with placebo and best supportive care (median OS = 7.9 months)[64]. However, the theoretical advantage of reduced tumor recurrence rate after DSM-TACE due to inferior VEGF expression has never been proven in terms of progression-free survival. Gross et al. demonstrated increased median OS only in the responders group (29.2 months vs. 9.5 months for nonresponders)[29]. In the study of Orlacchio et al., the time to progression (TTP) was significantly increased at 12 months only in the subgroups of patients with more than 50% HCC necrosis after the first procedure[54]. The initial objective response after TACE has already been proven to be a predictor of good outcomes also for DEB-TACE[65] and c-TACE[66]. In their study involving Child-Pugh B 8-9 patients with intermediate-stage HCC, Minici et al. underlined that also sustained response duration ≥ 6 months after downstaging with DSM-TACE (repeated “on demand” until the viable tumor was recognized on contrast-enhanced CT/MR) is associated with a significant increase in overall survival (35 months vs. 16.5 months)[58].
The safety profile of DSM-TACE is considered better than that of cTACE[57] in terms of fewer adverse events (fever, abdominal pain, and nausea/vomit), shorter hospitalization period due to post-embolization syndrome and inferior laboratory transaminase elevation within 24 h from the procedure. Transient transaminemia, usually considered a sign of liver toxicity, has been recently demonstrated to be a positive prognostic factor of tumor response after superselective cTACE[67]. The side effects of DSM-TACE due to chemotherapeutic drug systemic exposure (alopecia, leukopenia/thrombocytopenia, and mucositis) are reduced in comparison with cTACE and similar to DEB-TACE[23,28,49,57-59], which has an incidence of 11.8% according to the PRECISION V study[68]. Cardiomyopathy due to cumulative doses of anthracyclines may represent an issue if multiple DSM-TACE sessions are planned. Patients should thus undergo preliminary cardiologic investigations to ensure that heat dysfunction is not a contraindication and that the overall chemotherapy dose must not exceed the toxicity threshold (450 mg/m2 for Doxorubicin). In general, lobar DSM-TACE is demonstrably safer and better tolerated than cTACE, represents a cost-effective alternative to TARE in case of portal vein neoplastic thrombosis and may be performed when DEB-TACE is contraindicated[31,49,60] such as in Child-Pugh class B8/B9/C or after hemihepatectomy [Figure 2].
Figure 2. DSM-TACE for bridging to liver transplantation. 36 years-old female with HBV/HDV related hepatic cirrhosis, Child B9, MELD 18, complicated by portal hypertension. (A) Arterial phase CT shows a HCC nodule in segment II (diameter 3.5 cm) and an hemangioma in segment VIII. (B) Follow up CT after surgical left lobectomy shows multinodular HCC recurrence in the right liver lobe. (C) Late phase angiography before DSM-TACE of the whole residual liver confirms some small hypervascular nodules next to the hemangioma. The patient underwent successful liver transplantation two months later.
Biliary toxicity has usually been associated with DEB-TACE due to ischemic damage of the peribiliary capillary plexus, especially when using small diameter microspheres (< 100 μm), with an incidence of nearly 30%[69]. In the acute stage, bile duct necrosis followed by rupture may lead to biloma formation, which may become infected and require abscess drainage. The risk of abscess formation after TACE is higher among patients with bilioenteric anastomoses[70]. In the chronic stage, bile ducts may develop strictures and dilations even 3-6 months after the last TACE, and there may be gradual portal vein obliteration with parenchymal atrophy and deterioration of liver function that occur gradually over time[71]. The risk of biliary toxicity is increased in case of repeted TACE precedures at a short distance (4 weeks) or when increased chemotherapy drug dose are administered and in because the hypertrophy of peribiliary arterioles in fibrotic liver represents a protective factor[69]. Biliary toxicity was seldom reported and just as an early complication[22,28,33,54] after DSM-TACE, considered less aggressive than DEB-TACE, causing only temporary arterial occlusion. However, in our experience, it is not rare to find bile duct injury changes [Figure 3] manifesting in the late phase, probably due to chemical arteritis of the peribiliary capillary plexus induced by high chemotherapy drugs concentration. The finding of small cystic biliary dilatations, bilomas and portal vein branches narrowing at follow-up imaging should not be overlooked after TACE, even if asymptomatic[71], and must prompt the IRs to avoid TACE repetition, in order to limit further parenchymal cirrhosis and serious complications such as liver abscess formation and portal vein thrombosis[72].
Figure 3. DSM-TACE and biliary toxicity. 58 years-old male with viral/alcoholic hepatic cirrhosis, Child B7, MELD 11, portal hypertension. Multinodular HCC relapse after surgical resection of a single nodule of 3 cm in segment III. (A) Selective hepatic DSA before DSM-TACE shows multiple hypervascular small nodules in both lobes. (B) Good response after 2 cycles of DSM-TACE: the HCC nodules are no more visible in the preliminary DSA performed before the third session. (C) CT control at 6 months after the 3rd DSM-TACE revealed delayed formation of small cystic bilomas. (D) MR Cholangiography one year later showed progression of intrahepatic bile duct dilatation in the right lobe and formation of a larger biloma, despite interruption of TACE repetition.
INDICATIONS OF TACE IN MDTB-SELECTED HCC PATIENTS
Multidisciplinary care of patients involving physicians of various disciplines in a scheduled meeting was recognized for the first time in the United Kingdom in the early 2000s as part of national guidelines for breast cancer[73]. Since then, many benefits for the patients discussed in MDTB have been demonstrated not only in terms of improved likelihood of treatment and overall survival, but also in terms of cost-effectiveness and patient satisfaction[74]. In particular, the referral of HCC cases to a MDTB proved important to save time for diagnosis, avoiding unnecessary examinations and liver biopsy, since CT or MR revision by an expert Radiologist often leads to changes in imaging interpretation that affects treatment recommendations[75]. The decision making process based on MDTB consensus in HCC patients is the result of a complex analysis that has to take into account many factors including the clinical profile of the patient (liver function, performance status, and personal preferences), the tumor pathological features (number and size, location, type of growth, vascular invasion, nodal extension, and extrahepatic spread) and the local expertise in the different therapeutic options. The success of the multidisciplinary management of HCC in the last few years has changed the “stage hierarchy” strategy based on rigid treatment algorithms, urging International Scientific Societies to introduce the concept of “treatment stage migration” and the model of “therapeutic alternative options” for every disease stage in the European Association for the Study of Liver (EASL) and American Association for the Study of Liver Diseases (AASLD) updated guidelines, respectively, which provide a better fit for the modern individualized approach of precision medicine[76].
The adherence of MDTB treatment decisions to guidelines recommendations has been shown to be suboptimal mainly in the case of advanced age, severe comorbidities and strategic tumor localization[77], but also in relation to differences in the availability of the therapeutic options in every center[78]. The overall rate of guidelines violations may reach 40%[77] and, interestingly, the adherence rate drops with the increasing disease stages to less than 50% in the intermediate and advanced stages, according to an Italian study[79]. Our MDTB, for example, is “non-compliant” with guidelines offering stereotactic body radiotherapy (SBRT) or proton beam therapy (PT)[80] for local HCC ablation in case of contraindications to surgical resection or thermal ablation. SBRT is mentioned in the AASLD guidelines as second-line therapy only for early disease stage, whereas in the EASL Guidelines, TARE is considered an alternative option for a single lesion with a diameter ≤ 8 cm. TARE is not available in our center, but DSM-TACE alone [Figure 1] has been proposed as a valid alternative to TARE in case of advanced-stage disease with portal vein branch thrombosis due to vascular invasion. There is a potential oncologic advantage in using DSM-TACE combined with radiotherapy instead of DEB-TACE: permanent embolization with DEBs may enhance HCC radio-resistance because of chronic tumor hypoxia, which is recognized as a limiting factor for the efficacy of radiotherapy[81].
The combination of transarterial chemoembolization with other therapeutic options is still under research, with no clear indication yet in the current guidelines as many studies report encouraging but discordant results[2,82]. The use of TACE as a neoadjuvant treatment before liver resection is debated, because it may be useful to eradicate intrahepatic metastases and prevent tumor cell dissemination during surgery [Figure 4], but carries the risks of liver function impairment, delay in surgery and perihepatic adhesions rendering liver resection more difficult[83,84]. A meta-analysis[85] showed that the overall survival after liver resection was worse in the case of preoperative TACE, but the subgroup with complete tumor response to TACE had a benefit in disease-free survival after surgery because of reduced tumor recurrence rate. Although DSM-TACE has not yet been tested for this neoadjuvant indication, the significantly better patient outcome was obtained with hepatic artery infusion (HAI)[86], which is based on the same principle of chemotherapeutic action.TACE has been successfully applied before thermal ablation to increase the HCC necrotic area in HCC nodules based on the ischemic effect of embolization[2,87], but also in combination with external radiotherapy, proving effective in terms of increased tumor response and patient outcome due to the synergistic effect of chemo-radiotherapy[88]. Finally, the combination of TACE and Sorafenib, belonging to the tyrosine kinase inhibitors class (TKIs), while expected to inhibit VEGF overexpression, is actually not recommended because numerous trials have failed to prove a significant advantage. In the TACTICS trial[89], administering the systemic chemotherapy 3 weeks before TACE, a benefit was found only in terms of progression-free survival. In “TACE refractory” patients with disease progression after two cycles or contraindications such as biliary toxicity, switching to systemic therapies should be made as soon as possible[90] since novel molecular target drugs have been approved for advanced-stage HCC, mainly the immune checkpoint inhibitors (ICIs) atezolizumab plus bevacizumab, which demonstrated increased tumor response rate and better overall survival[91].
Figure 4. Preoperative neoadjuvant TACE: 53 years-old female with HCV-related cirrhosis, Child-Pugh A6, MELD 9, portal hypertension. (A) Coronal CT MIP reconstruction in the arterial phase shows a single hypervascular HCC nodule (diameter 5.2 cm) in the segment VII. (B) At the end of selective DSMTACE followed by Lipiodol UF injection in the feeding vessel, to reach stasis of flow, the accumulation of iodized oil in the HCC is evident. (C) MR control 1 month after TACE: in the coronal arterial phase MPR nearly complete tumor necrosis was found, except for a subtle rim of enhancement due to residual viable tumor tissue at the periphery. (D) Follow-up CT in the coronal plane 5 years after surgical resection: no signs of HCC recurrence are visible in the arterial phase.
The role of TACE in HCC management has changed over the last two decades, resulting in its use even outside of the BCLC intermediate stage (B). The overall number of TACE procedures has declined over time, but the survival of treated patients was prolonged, plausibly thanks to a better patient selection aimed at avoiding liver function deterioration, as recently reported by a study on the Italian Liver Cancer (ITA.LI.CA) database[7]. Other important improvements are represented by the technical evolution of TACE, including selective catheterization, the introduction of DEBs and the repetition of procedures to increase the response rate. A recent study conducted in a European tertiary care center[57] reported that out of 492 TACE procedures (78% for palliation, 32% for bridging) performed in ten years (2008-2017), 348 were DEB-TACE, 60 cTACE, and 84 DSM TACE. In our Interventional Radiology service in a secondary care public hospital, the number of DSM-TACE performed over the last ten years was even higher than that of other TACE techniques (n = 172 DSM-TACE vs. 138 other TACE, including 106 DEB-TACE, 29 cTACE, and 3 TAE).
Considering that DSM-TACE was introduced in our service in 2014, it has radically changed our daily practice, preferring lobar or less selective but repeatable treatments over selective, more aggressive DEB-TACE, usually resulting in irreversible hepatic HCC feeders occlusion with the development of collaterals (mostly from phrenic, internal mammary, and omental arteries) difficult to catheterize. Another important factor for the preference of DSM TACE was the positive feedback obtained by the hepatologists of the MDTB in terms of safety and tumor response, with even better results than DEB-TACE for small HCC nodules [Figure 1]. In our series of 81 patients treated with DSM-TACE, 21 had multifocal recurrence after previous treatment with cTACE or DSM TACE. One limitation of our experience is represented by the use of larger DEBs (200-400 µm) in our center due to concerns that smaller microspheres would cause biliary toxicity.
At present, TACE is considered the first-line therapeutic option in patients unfit for surgery or local ablative therapies, in agreement with the “treatment stage migration” strategy approved by the 2022 update of BCLC algorithm[92]. Superselective or ultraselective cTACE still remains the “gold standard”[8] for radical treatment of small HCC nodules with a diameter ≤ 3 cm (that still have a residual portal supply) in case of limited liver disease (≤ 3-4 HCC nodules), but it is often not applicable in real-life HCC management because of higher tumor burden, diffuse multifocal disease or poor liver function. Since its introduction in the early 2000s, DEB-TACE has progressively replaced cTACE for the majority of traditional indications in Western countries, although the superiority of DEB-TACE over cTACE was never demonstrated in terms of patient survival, tumor response and safety[2]. The preference of IRs for DEB-TACE may be explained with reference to better patient tolerance patients, ease of monitoring in the follow-up CT imaging and reduced harm to the liver, except for the biliary toxicity. DSM-TACE can be considered a lighter and diluted over time alternative to DEB-TACE, based on the oncologic principles of chemotherapy rather than on the ischemic effect of embolization. The cycles of DSM-TACE may be repeated on demand multiple times to prevent drop-out of patients on the waiting list for liver transplantation [Figure 4], with the downstaging purpose of meeting the Milan criteria, in combination with other local ablative therapies such as Radiation Therapy, for palliation in fragile patients to delay tumor progression and macrovascular invasion, even in case of portal vein thrombosis.
CONCLUSION
Lobar or less selective DSM-TACE may represent an effective alternative to selective cTACE and DEB-TACE in the following stages of HCC. In the early BCLC stage (0-A), DSM-TACE can be used for bridging to liver transplantation in order to avoid hepatic artery occlusion and VEGF overexpression, which may accelerate disease progression. In the intermediate BCLC stage (B), DSM-TACE is feasible in patients at high risk of liver failure (Child-Pugh B 8-9 or C, bilirubin ≥ 2 mg/dL, ascites requiring diuretic treatment) when DEB-TACE is contraindicated, for downstaging to meet the Milan liver transplantation criteria, in combination with external radiotherapy to increase tumor response and as second-line therapy after cTACE or DEB-TACE failure. In the advanced BCLC stage (C), DSM-TACE represents a second line option for palliation of HCC patients with liver-only or dominant disease who are ineligible for systemic therapies in case of portal invasion or high tumor burden. The decision is left to the discretion of the local MDTB after a case-based discussion of the therapeutic options.
DECLARATIONS
Authors’ contributionsMade substantial contributions to study conception and design, data analysis and interpretation: Rozzanigo U, Gatti F, Luppi G, Costa L, Petralia B, Pravadelli C, Maioli I, Frisinghelli M, Fersino S, Riccardo B,
Not applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declare that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182-236.
2. Raoul JL, Forner A, Bolondi L, Cheung TT, Kloeckner R, de Baere T. Updated use of TACE for hepatocellular carcinoma treatment: how and when to use it based on clinical evidence. Cancer Treat Rev 2019;72:28-36.
3. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol 2022;76:681-93.
4. Charriere B, Muscari F, Maulat C, et al. Outcomes of patients with hepatocellular carcinoma are determined in multidisciplinary team meetings. J Surg Oncol 2017;115:330-6.
5. Serper M, Taddei T, Mehta R, et al. Association of provider specialty and multidisciplinary care with hepatocellular carcinoma treatment and mortality. Gastroenterology 2017;152:1954-64.
6. Matsumoto MM, Mouli S, Saxena P, et al. Comparing real world, personalized, multidisciplinary tumor board recommendations with BCLC algorithm: 321-patient analysis. Cardiovasc Intervent Radiol 2021;44:1070-80.
7. Pelizzaro F, Haxhi S, Penzo B, et al. Transarterial chemoembolization for hepatocellular carcinoma in clinical practice: temporal trends and survival outcomes of an iterative treatment. Front Oncol 2022;12:822507.
8. Kudo M, Han KH, Ye SL, et al. A changing paradigm for the treatment of intermediate-stage hepatocellular carcinoma: asia-pacific primary liver cancer expert consensus statements. Liver Cancer 2020;9:245-60.
10. Lucatelli P, Burrel M, Guiu B, de Rubeis G, van Delden O, Helmberger T. CIRSE standards of practice on hepatic transarterial chemoembolisation. Cardiovasc Intervent Radiol 2021;44:1851-67.
11. Forsberg JO. Transient blood flow reduction induced by intra-arterial injection of degradable starch microspheres. Experiments on rats. Acta Chirurgica Scandinavica 1978;144:275-281.
12. Yamada R, Nakatsuka H, Nakamura K, et al. Super-selective arterial embolization in unresectable hepatomas (author’s transl). Nihon Igaku Hoshasen Gakkai zasshi 1979;39:540-3.
13. Taguchi T. Chemo-occlusion for the treatment of liver cancer. A new technique using degradable starch microspheres. Clin Pharmacokinet 1994;26:275-91.
14. Kirchhoff TD, Rudolph KL, Layer G, et al. Chemoocclusion
15. Pieper CC, Meyer C, Vollmar B, Hauenstein K, Schild HH, Wilhelm KE. Temporary arterial embolization of liver parenchyma with degradable starch microspheres (EmboCept®S) in a swine model. Cardiovasc Intervent Radiol 2015;38:435-41.
16. Ebert M, Ebert J, Berger G. Intravital microscopic research of microembolization with degradable starch microspheres. J Drug Deliv 2013;2013:242060.
17. Håkansson L, Håkansson A, Morales O, Thorelius L, Warfving T. Spherex (degradable starch microspheres) chemo-occlusion-enhancement of tumor drug concentration and therapeutic efficacy: an overview. Semin Oncol 1997;24:S6-100.
18. Cappelli A, Pettinato C, Golfieri R. Transarterial radioembolization using yttrium-90 microspheres in the treatment of hepatocellular carcinoma: a review on clinical utility and developments. J Hepatocell Carcinoma 2014;1:163-82.
19. Liapi E, Geschwind JF. Intra-arterial therapies for hepatocellular carcinoma: where do we stand? Ann Surg Oncol 2010;17:1234-46.
20. Aronsen KF, Hellekant C, Holmberg J, Rothman U, Teder H. Controlled blocking of hepatic artery flow with enzymatically degradable microspheres combined with oncolytic drugs. Eur Surg Res 1979;11:99-106.
21. Wiggermann P, Wohlgemuth WA, Heibl M, et al. Dynamic evaluation and quantification of microvascularization during degradable starch microspheres transarterial Chemoembolisation (DSM-TACE) of HCC lesions using contrast enhanced ultrasound (CEUS): a feasibility study. Clin Hemorheol Microcirc 2013;53:337-48.
22. Yamasaki T, Saeki I, Harima Y, et al. Effect of transcatheter arterial infusion chemotherapy using iodized oil and degradable starch microspheres for hepatocellular carcinoma. J Gastroenterol 2012;47:715-22.
23. Gruber-Rouh T, Schmitt C, Naguib NNN, et al. Transarterial chemoembolization (TACE) using mitomycin and lipiodol with or without degradable starch microspheres for hepatocellular carcinoma: comparative study. BMC Cancer 2018;18:188.
24. Vogl TJ, Langenbach MC, Hammerstingl R, Albrecht MH, Chatterjee AR, Gruber-Rouh T. Evaluation of two different transarterial chemoembolization protocols using Lipiodol and degradable starch microspheres in therapy of hepatocellular carcinoma: a prospective trial. Hepatol Int 2021;15:685-94.
25. Maeda N, Verret V, Moine L, et al. Targeting and recanalization after embolization with calibrated resorbable microspheres versus hand-cut gelatin sponge particles in a porcine kidney model. J Vasc Interv Radiol 2013;24:1391-8.
26. Katsumori T, Kasahara T. The size of gelatin sponge particles: differences with preparation method. Cardiovasc Intervent Radiol 2006;29:1077-83.
27. Carr BI, Zajko A, Bron K, Orons P, Sammon J, Baron R. Phase II study of Spherex (degradable starch microspheres) injected into the hepatic artery in conjunction with doxorubicin and cisplatin in the treatment of advanced-stage hepatocellular carcinoma: interim analysis. Semin Oncol 1997;24:S6-97.
28. Furuse J, Ishii H, Satake M, et al. Pilot study of transcatheter arterial chemoembolization with degradable starch microspheres in patients with hepatocellular carcinoma. Am J Clin Oncol 2003;26:159-64.
29. Gross A, Albrecht T. Transarterial chemoembolisation (TACE) with degradable starch microspheres (DSM) and anthracycline in patients with locally extensive hepatocellular carcinoma (HCC): safety and efficacy. Cardiovasc Intervent Radiol 2020;43:402-10.
30. Haubold J, Reinboldt MP, Wetter A, et al. DSM-TACE of HCC: evaluation of tumor response in patients ineligible for other systemic or loco-regional therapies. Rofo 2020;192:862-9.
31. Iezzi R, Pompili M, Rinninella E, et al. HepatoCatt Study Group. TACE with degradable starch microspheres (DSM-TACE) as second-line treatment in HCC patients dismissing or ineligible for sorafenib. Eur Radiol 2019;29:1285-92.
32. Takayasu K, Arii S, Ikai I, et al. Liver Cancer Study Group of Japan. Overall survival after transarterial lipiodol infusion chemotherapy with or without embolization for unresectable hepatocellular carcinoma: propensity score analysis. AJR Am J Roentgenol 2010;194:830-7.
33. Yamasaki T, Hamabe S, Saeki I, et al. A novel transcatheter arterial infusion chemotherapy using iodized oil and degradable starch microspheres for hepatocellular carcinoma: a prospective randomized trial. J Gastroenterol 2011;46:359-66.
34. Idée JM, Guiu B. Use of Lipiodol as a drug-delivery system for transcatheter arterial chemoembolization of hepatocellular carcinoma: a review. Crit Rev Oncol Hematol 2013;88:530-49.
35. Renzulli M, Peta G, Vasuri F, et al. Standardization of conventional chemoembolization for hepatocellular carcinoma. Ann Hepatol 2021;22:100278.
36. Kim DY, Ryu HJ, Choi JY, et al. Radiological response predicts survival following transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma. Aliment Pharmacol Ther 2012;35:1343-50.
37. Kim S, Kim HY, Lee SL, Ku YM, Won YD, Kim CW. Lipiodol pneumonitis following transcatheter arterial chemoembolization for hepatocellular carcinoma. J Liver Cancer 2020;20:60-6.
38. Burroughs A, Hochhauser D, Meyer T. Systemic treatment and liver transplantation for hepatocellular carcinoma: two ends of the therapeutic spectrum. Lancet Oncol 2004;5:409-18.
39. Marelli L, Stigliano R, Triantos C, et al. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? Cardiovasc Intervent Radiol 2007;30:6-25.
40. Jordan O, Denys A, De Baere T, Boulens N, Doelker E. Comparative study of chemoembolization loadable beads:
41. Namur J, Wassef M, Millot JM, Lewis AL, Manfait M, Laurent A. Drug-eluting beads for liver embolization: concentration of doxorubicin in tissue and in beads in a pig model. J Vasc Interv Radiol 2010;21:259-67.
42. Ha BY, Ahmed A, Sze DY, et al. Long-term survival of patients with unresectable hepatocellular carcinoma treated with transcatheter arterial chemoinfusion. Aliment Pharmacol Ther 2007;26:839-46.
43. Daniels JR, Wallman M. Subselective intra-arterial chemotherapy infusion in the treatment of hepatocellular carcinoma. Semin Oncol 2010;37:83-8.
44. Kim JH, Yoon HK, Kim SY, et al. Transcatheter arterial chemoembolization
45. Schicho A, Hellerbrand C, Krüger K, et al. Impact of different embolic agents for transarterial chemoembolization (TACE) procedures on systemic vascular endothelial growth factor (VEGF) levels. J Clin Transl Hepatol 2016;4:288-92.
46. Sergio A, Cristofori C, Cardin R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol 2008;103:914-21.
47. Chegai F, Orlacchio A, Merolla S, Monti S, Mannelli L. Intermediate hepatocellular carcinoma: the role of transarterial therapy. Hepat Oncol 2015;2:399-408.
48. Brown KT, Do RK, Gonen M, et al. Randomized trial of hepatic artery embolization for hepatocellular carcinoma using doxorubicin-eluting microspheres compared with embolization with microspheres alone. J Clin Oncol 2016;34:2046-53.
49. Ludwig JM, Iezzi R, Theysohn JM, Albrecht T, Posa A, Gross A. European multicenter study on degradable starch microsphere TACE: the digestible way to conquer HCC in patients with high tumor burden. Cancers (Basel) 2021;13:5122.
50. Dettmer A, Kirchhoff TD, Gebel M, et al. Combination of repeated single-session percutaneous ethanol injection and transarterial chemoembolisation compared to repeated single-session percutaneous ethanol injection in patients with non-resectable hepatocellular carcinoma. World J Gastroenterol 2006;12:3707-15.
51. Kirchhoff TD, Bleck JS, Dettmer A, et al. Transarterial chemoembolization using degradable starch microspheres and iodized oil in the treatment of advanced hepatocellular carcinoma: evaluation of tumor response, toxicity, and survival. Hepatobiliary Pancreat Dis Int 2007;6:259-66.
52. Murata S, Tajima H, Ichikawa K, et al. Oily chemoembolization combined with degradable starch microspheres for HCC with cirrhosis. Hepatogastroenterology 2008;55:1041-6.
53. Schicho A, Pereira PL, Haimerl M, et al. Transarterial chemoembolization (TACE) with degradable starch microspheres (DSM) in hepatocellular carcinoma (HCC): multi-center results on safety and efficacy. Oncotarget 2017;8:72613-20.
54. Orlacchio A, Chegai F, Roma S, Merolla S, Bosa A, Francioso S. Degradable starch microspheres transarterial chemoembolization (DSMs-TACE) in patients with unresectable hepatocellular carcinoma (HCC): long-term results from a single-center 137-patient cohort prospective study. Radiol Med 2020;125:98-106.
55. Yildiz I, Deniz S, Ozer A, Caliskan K. Trans-arterial chemoembolization with 50 μm degradable starch microspheres versus
56. Minici R, Ammendola M, Manti F, et al. Safety and efficacy of degradable starch microspheres transcatheter arterial chemoembolization as a bridging therapy in patients with early stage hepatocellular carcinoma and child-pugh stage B eligible for liver transplant. Front Pharmacol 2021;12:634084.
57. Mohr I, Vogeler M, Pfeiffenberger J, et al. Clinical effects and safety of different transarterial chemoembolization methods for bridging and palliative treatments in hepatocellular carcinoma. J Cancer Res Clin Oncol 2022;148:3163-74.
58. Minici R, Ammendola M, Manti F, et al. Safety and efficacy of degradable starch microspheres transcatheter arterial chemoembolization (DSM-TACE) in the downstaging of intermediate-stage hepatocellular carcinoma (HCC) in patients with a child-pugh score of 8-9. Front Pharmacol 2021;12:634087.
59. Niessen C, Unterpaintner E, Goessmann H, et al. Degradable starch microspheres versus ethiodol and doxorubicin in transarterial chemoembolization of hepatocellular carcinoma. J Vasc Interv Radiol 2014;25:240-7.
60. Auer TA, Jonczyk M, Collettini F, et al. Trans-arterial chemoembolization with degradable starch microspheres (DSM-TACE) versus selective internal radiation therapy (SIRT) in multifocal hepatocellular carcinoma. Acta Radiol 2021;62:313-21.
61. Vincenzi B, Di Maio M, Silletta M, et al. Prognostic relevance of objective response according to EASL criteria and mRECIST criteria in hepatocellular carcinoma patients treated with loco-regional therapies: a literature-based meta-analysis. PLoS One 2015;10:e0133488.
62. Chang WC, Hsu HH, Chiu SH, et al. Transcatheter arterial chemoembolization with drug-eluting beads for the treatment of hepatocellular carcinoma: recommended selection for small-caliber (< 100 μm) beads. J Hepatocell Carcinoma 2021;8:937-49.
63. Lee IJ, Lee JH, Lee YB, et al. Effectiveness of drug-eluting bead transarterial chemoembolization versus conventional transarterial chemoembolization for small hepatocellular carcinoma in Child-Pugh class A patients. Ther Adv Med Oncol 2019;11:1758835919866072.
64. Llovet JM, Ricci S, Mazzaferro V, et al. SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90.
65. Kalva SP, Pectasides M, Yeddula K, Ganguli S, Blaszkowsky LS, Zhu AX. Factors affecting survival following chemoembolization with doxorubicin-eluting microspheres for inoperable hepatocellular carcinoma. J Vasc Interv Radiol 2013;24:257-65.
66. Zhang Y, Zhang M, Chen M, et al. Association of sustained response duration with survival after conventional transarterial chemoembolization in patients with hepatocellular carcinoma. JAMA Netw Open 2018;1:e183213.
67. Granito A, Facciorusso A, Sacco R, et al. TRANS-TACE: prognostic role of the transient hypertransaminasemia after conventional chemoembolization for hepatocellular carcinoma. J Pers Med 2021;11:1041.
68. Lammer J, Malagari K, Vogl T, et al. PRECISION V Investigators. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol 2010;33:41-52.
69. Guiu B, Deschamps F, Aho S, et al. Liver/biliary injuries following chemoembolisation of endocrine tumours and hepatocellular carcinoma: lipiodol
70. Woo S, Chung JW, Hur S, et al. Liver abscess after transarterial chemoembolization in patients with bilioenteric anastomosis: frequency and risk factors. AJR Am J Roentgenol 2013;200:1370-7.
71. Spina JC, Ulla M, Yeyati EL, et al. MDCT findings after hepatic chemoembolization with DC-beads: what the radiologist needs to know. Abdom Imaging 2013;38:778-84.
72. Dhamija E, Paul SB, Gamanagatti SR, Acharya SK. Biliary complications of arterial chemoembolization of hepatocellular carcinoma. Diagn Interv Imaging 2015;96:1169-75.
73. Salgia R, Mendiratta V. The Multidisciplinary Management of Hepatocellular Carcinoma. Clin Liver Dis (Hoboken) 2021;17:405-8.
74. Gadsden MM, Kaplan DE. Multidisciplinary approach to HCC management: how can this be done? Dig Dis Sci 2019;64:968-75.
75. Zhang Y, Weinreb JC, Czeyda-Pommersheim F, Taddei TH. Assessing the impact of referral on multidisciplinary tumor board outcomes in patients with hepatocellular carcinoma. J Am Coll Radiol 2020;17:1636-43.
76. Vitale A, Trevisani F, Farinati F, Cillo U. Treatment of hepatocellular carcinoma in the precision medicine era: from treatment stage migration to therapeutic hierarchy. Hepatology 2020;72:2206-18.
77. Leoni S, Piscaglia F, Serio I, et al. Adherence to AASLD guidelines for the treatment of hepatocellular carcinoma in clinical practice: experience of the Bologna Liver Oncology Group. Dig Liver Dis 2014;46:549-55.
78. Jogi S, Varanai R, Bantu SS, Manne A. Selecting the first line treatment in non-metastatic hepatocellular carcinoma - comparing clinical practice guidelines. Oncol Rev 2020;14:515.
79. Sangiovanni A, Triolo M, Iavarone M, et al. Multimodality treatment of hepatocellular carcinoma: How field practice complies with international recommendations. Liver Int 2018;38:1624-34.
80. Dionisi F, Brolese A, Siniscalchi B, et al. Clinical results of active scanning proton therapy for primary liver tumors. Tumori 2021;107:71-9.
81. Colliez F, Gallez B, Jordan BF. Assessing tumor oxygenation for predicting outcome in radiation oncology: a review of studies correlating tumor hypoxic status and outcome in the preclinical and clinical settings. Front Oncol 2017;7:10.
82. Kennedy AS, Sangro B. Nonsurgical treatment for localized hepatocellular carcinoma. Curr Oncol Rep 2014;16:373.
83. Akateh C, Black SM, Conteh L, et al. Neoadjuvant and adjuvant treatment strategies for hepatocellular carcinoma. World J Gastroenterol 2019;25:3704-21.
84. Lei J, Zhong J, Yan L, et al. Preoperative adjuvant transarterial chemoembolization cannot improve the long term outcome of radical therapies for hepatocellular carcinoma. Sci Rep 2017;7:41624.
85. Qi X, Liu L, Wang D, Li H, Su C, Guo X. Hepatic resection alone versus in combination with pre- and post-operative transarterial chemoembolization for the treatment of hepatocellular carcinoma: a systematic review and meta-analysis. Oncotarget 2015;6:36838-59.
86. Tsutsui R, Nagamatsu H, Itano O, et al. Neoadjuvant hepatic arterial infusion chemotherapy for resectable hepatocellular carcinomas. Hepatoma Res 2018;4:13.
87. Chen QW, Ying HF, Gao S, et al. Radiofrequency ablation plus chemoembolization versus radiofrequency ablation alone for hepatocellular carcinoma: a systematic review and meta-analysis. Clin Res Hepatol Gastroenterol 2016;40:309-14.
88. Jacob R, Turley F, Redden DT, et al. Adjuvant stereotactic body radiotherapy following transarterial chemoembolization in patients with non-resectable hepatocellular carcinoma tumours of ≥ 3 cm. HPB (Oxford) 2015;17:140-9.
89. Kudo M, Ueshima K, Ikeda M, et al. TACTICS study group. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut 2020;69:1492-501.
90. Ogasawara S, Ooka Y, Koroki K, et al. Switching to systemic therapy after locoregional treatment failure: definition and best timing. Clin Mol Hepatol 2020;26:155-62.
91. Vogel A, Martinelli E. ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org, ESMO Guidelines Committee. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann Oncol 2021;32:801-5.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Rozzanigo U, Gatti F, Luppi G, Costa L, Petralia B, Pravadelli C, Maioli I, Frisinghelli M, Fersino S, Berletti R, de Pretis G, Brolese A. Chemoembolization with Degradable Starch Microspheres (DSM-TACE): expanding indications in HCC multidisciplinary tumor board. Hepatoma Res 2023;9:14. http://dx.doi.org/10.20517/2394-5079.2022.66
AMA Style
Rozzanigo U, Gatti F, Luppi G, Costa L, Petralia B, Pravadelli C, Maioli I, Frisinghelli M, Fersino S, Berletti R, de Pretis G, Brolese A. Chemoembolization with Degradable Starch Microspheres (DSM-TACE): expanding indications in HCC multidisciplinary tumor board. Hepatoma Research. 2023; 9: 14. http://dx.doi.org/10.20517/2394-5079.2022.66
Chicago/Turabian Style
Rozzanigo, Umberto, Francesco Gatti, Giacomo Luppi, Lorenzo Costa, Benedetto Petralia, Cecilia Pravadelli, Ivana Maioli, Michela Frisinghelli, Sergio Fersino, Riccardo Berletti, Giovanni de Pretis, Alberto Brolese. 2023. "Chemoembolization with Degradable Starch Microspheres (DSM-TACE): expanding indications in HCC multidisciplinary tumor board" Hepatoma Research. 9: 14. http://dx.doi.org/10.20517/2394-5079.2022.66
ACS Style
Rozzanigo, U.; Gatti F.; Luppi G.; Costa L.; Petralia B.; Pravadelli C.; Maioli I.; Frisinghelli M.; Fersino S.; Berletti R.; de Pretis G.; Brolese A. Chemoembolization with Degradable Starch Microspheres (DSM-TACE): expanding indications in HCC multidisciplinary tumor board. Hepatoma. Res. 2023, 9, 14. http://dx.doi.org/10.20517/2394-5079.2022.66
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 18 clicks
Cite This Article 18 clicks















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.