The role of minimally invasive surgery in the treatment of HCC
Abstract
Liver surgery is the first-line treatment for hepatocellular carcinoma (HCC). Minimally invasive liver resection (MILS) has become an attractive option thanks to reduced intraoperative blood losses, shortened length of hospital stay, and similar oncological outcomes when compared to open liver resection. Nonetheless, the safety of MILS is still debated in challenging situations, such as in cirrhotic patients, difficult tumor locations, multiple or large tumors, and repeat resection. The aim of this review is to discuss current indications of laparoscopic liver resection for HCC treatment in the light of its outcomes, focusing on technical aspects of minimally invasive anatomic liver resection and state of the art of MILS in challenging situations.
Keywords
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third among cancer-related deaths worldwide[1]. It usually develops from chronic liver disease, and its treatment is complex because of the underlying liver condition. Surgery for HCC should be oncologically radical and simultaneously able to preserve as much parenchyma as possible, because of the high recurrence rate and the risk of post-hepatectomy liver failure (PHLF)[2]. The recurrence rate is around 20% after liver transplantation (LT), which represents the current therapy of choice, and 70% after liver resection[1,3]. Due to organ shortages, LT is currently reserved for patients who are not candidates for resections because of uncompensated cirrhosis, multiple bad prognostic factors on pathological examination after resection, and the patients with recurrent HCC[4]. Accordingly, liver resection can be considered the first-line treatment for HCC even in patients with compensated cirrhosis[5].
Minimally invasive liver resection (MILS) has slowly developed, even though the first report of laparoscopic liver resection (LLR) dates back to 1991[6]. Although some concerns about laparoscopic surgery had already been clarified for other surgical subspecialties, MILS has several peculiarities, including the risk for massive bleeding during the parenchymal transection, the technical complexity, the high anatomical variability, the oncological concerns about resection margins, and the consideration of cirrhotic patients as too fragile[7]. Therefore, the laparoscopic liver surgeon must have experience in both laparoscopy and open liver surgery.
Recently, many reports focusing on LLR have been published, even if mainly retrospective. Similarly, multicenter series including major hepatectomy and donor hepatectomy performed for living donor liver transplantation (LDLT) are increasing, indicating that MILS is also a viable option for both primary and secondary liver diseases[8,9]. Recommendations for implementation and adoption of LLR in HCC were proposed in 2008 by the first international consensus conference, held in Louisville[10]. This conference defined terminology about MILS (pure, hand-assisted laparoscopy, and hybrid techniques), even if with some concerns about too rapid dissemination of MILS in the absence of well-structured training. A further consensus conference was then held in Morioka in 2014, where the experts suggested carrying out a prospective reporting registry for better transparency, looking for a difficulty scoring system for a better patient selection, and creating a formal structure of education[11]. Finally, the Southampton Consensus guidelines (2018) were proposed as clinical practice guidelines to guide the development and the wide adoption of laparoscopic liver surgery[12].
In this review, we summarize state of the art about indications and limitations of LLR for HCC, focusing on anatomic liver resection strategies as well as strategies for overcoming old challenges.
OUTCOMES OF MILS FOR HCC TREATMENT
Differently from colorectal liver metastases, there are no prospective randomized comparative studies between LLR and open liver resection (OLR). However, a propensity score matching (PSM) study on 389 patients undergoing LLR until 2013 in a high-volume center showed a significantly shorter length of hospital stay (8 vs. 10 days, P ≤ 0.001) and a lower complication rate in the LLR (12.5% vs. 20.4%, P = 0.042). The one, three, and five year overall survivals (OS) were 91.6%, 87.5%, and 76.4% after LLR and 93.1%, 87.8%, and 73.2% after OLR (P = 0.944). Similarly, the one, three, and five year disease-free survivals (DFS) were 69.7%, 52%, and 44.2 and 74.7%, 49.5%, and 41.2%, respectively (P = 0.944)[13]. A consequent PSM multicenter study enrolling 665 consecutive patients from eight European high-volume centers showed surprising results also in elderly patients (> 70 years), with a lower rate of Clavien–Dindo grade III and IV complications after LLR (6% vs. 20%, P = 0.04)[14]. These results encouraged further PSM studies, showing that the laparoscopic approach is the only independent factor in reducing the complication rate in minor liver resections for HCC[15]. At the same time, the first wide metanalysis confirmed that LLR for HCC treatment is associated with reduced blood loss, transfusion rate, postoperative ascites, liver failure, and hospital stay with comparable recurrence rates, disease-free margins, and operation time[16,17]. However, it should be recognized that the biggest part of such good results come from single-center retrospective studies, and thus they may have selection bias as well as other limitations.
Thanks to the aforementioned results, a stepwise adoption of LLR for HCC is now recommended by western guidelines in each high-volume HPB center[12]. Similarly, the last Asia-Pacific expert consensus on LLR for HCC confirmed that MILS can be safely carried out. However, it is underlined that major resections and posterosuperior (PS) resections should be performed only in centers of excellence[18]. A center of excellence should have a multidisciplinary liver team that includes surgeons, hepatologists, radiologists, oncologists, anesthesiologists, and specialized nurses. At least 30 laparoscopic liver resections per year must be performed, with surgeons who must have performed at least 100 laparoscopic liver resections on their own over their career and at least 20 cases per year. The initial experience should follow a stepwise approach, from minor liver resection in anterolateral segments to major liver resections. Such a gradual approach seems to follow a slow learning curve for LLR, with 60 procedures having been proposed as necessary to achieve proficiency with minor liver resections[19,20] and further 50 procedures needed to reach the ability to perform major LLR[21].
MINIMALLY INVASIVE ANATOMIC LIVER RESECTIONS
When dealing with HCC, oncological issues have to be combined with tumor biology and technical considerations about the surgical anatomy of the liver. The concept of anatomical resection (AR) was introduced by Makuuchi in 1985, according to which the tumor-free margin does not depend only on the distance from the tumor[22,23]. AR aims to remove the entire hepatic parenchymal tissue supplied by the portal venous system draining the lesion[24], based on the concept that HCC invades the portal branches, spreading tumor cells into the portal flow and then forming satellite nodules[25]. After an initial phase of widespread adoption of AR, especially in Asia, the first large comparative studies did not reveal survival advantages. In particular, Marubashi et al. compared 1102 patients, showing how disease-free survival (DFS) and overall survival (OS) rates were not significantly different after a PSM analysis (P = 0.704 and
One of the most important additional aspects of liver surgery is that unintentional damage to a segment’s inflow or outflow can result in ischemia of the tissue supplied by the damaged vessel. Thus, a remnant liver ischemia (RLI) can easily result from NAR, and this condition was shown to be associated with worse surgical and oncological outcomes. In a study including 328 patients, RLI was associated with more complications and longer hospital stay, as well as an impaired OS (OR: 6.98; CI: 4.27-11.43; P < 0.001) and DFS (OR: 5.15; CI: 3.62-7.35; P < 0.001)[29]. Furthermore, selective clamping is also important from the hemodynamic point of view because it avoids splanchnic stasis and fluid replacement[30].
Since advanced surgical techniques and improvements in imaging technologies have facilitated surgical approaches involving AR of individual hepatic segments, the widespread correct adoption of MIALR could lead to better outcomes in HCC patients[31].
Glissonean approach for MIALR
Since the AR is the removal of a portion of hepatic tissue with its vascular inflow, the technical approaches to the portal system must be well mastered by the liver surgeon.
Couinaud described three main approaches to the inflow system at the hepatic hilus: the classical intrafascial, the extrafascial, and the extrafascial-transfissural approaches[32]. The two latter techniques are considered the Glissonean approach according to the majority of authors[33]. This term was firstly used by Takasaki et al.in 1986, referring to a right sectionectomy when dealing with the so-called extrafascial technique[34]. However, this procedure was previously described by Tien-Yu Lin in 1960 and Ton That Tung et al. in 1963, as also reported by Bismuth[35-37].
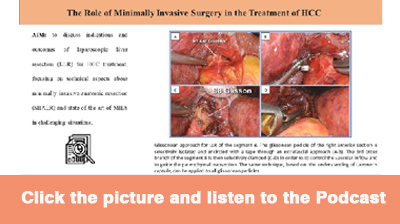
The Glissonean pedicle approach is well available in MILS and provides a better understanding of the surgical anatomy, especially for sectionectomies, segmentectomies, and smaller anatomical liver resections. The Glissonean pedicle can be easily dissected from liver parenchyma, leading to isolating and encircling the first, second, and third branches of Glissonean pedicles [Figure 1], reaching the concept of resection of the “cone unit”, defined as the area of the liver parenchyma fed by the tertiary branches, representing the smallest anatomically resectable part[34]. In particular, one of the biggest advantages of the minimally invasive approach is the magnification and the 3D vision, together with the small and precise movements, which make it easier to apply this approach also for third Glissonean branches. Standardization of the technique for MIALR is important, and the Glissonean approach itself is the best way to perform a standardized and effective MIALR.
Figure 1. Glissonean approach for LLR of segment 8. The Glissonean pedicle of the right anterior section is selectively isolated and encircled with a tape through an extrafascial approach (A,B). The third-order branch of segment 8 is then selectively clamped (C,D) to control the vascular inflow and guide the parenchymal transection. The same technique can be applied to all Glissonean pedicles.
Recently the study group of precision anatomy for minimally invasive hepato-biliary-pancreatic surgery (PAM-HBP), composed of the major worldwide experts in MILS, investigated and confirmed the safety and feasibility of MIALR, and also concluded several advantages of the Glissonean approach compared to the conventional hilar approach[38,39].
In our experience, the Glissonean approach is a simple and versatile application procedure to carry out MIALR, including for difficult resections such as posterior segments[40]. However, if the tumor has close relationships to the portal pedicle at the hilus, the surgeon should be able to change from the extrafascial approach to the intrafascial approach or the transfissural one to carry out the AR and tailor the technique to the single case.
Fundamental concepts about liver anatomy for MIALR
Surgical anatomy is still the true basis of LLR, especially for MIALR. Clear knowledge of Laennec’s capsule anatomy is necessary and serves as a guide for surgical dissection and the standardization of the surgical procedure. The lack of a clear anatomical understanding represents the main obstacle to the standardization of the surgical technique of the Glissonean pedicle approach.
Each liver segment consists of 6-8 cone units. In limited liver resections, the respective tertiary branches must be isolated selectively, according to the number of cone units to be resected. Thanks to the anatomical separation of the Walaeus sheath, which encircles the portal triads, and the liver Laennec’s capsule, at the hepatic hilus, vascular pedicles can be safely approached extrahepatically[41,42].
The schema of a novel comprehensive surgical anatomy of the liver was described in detail by Sugioka et al., who identified four anatomical landmarks and six gates[42]. According to his description, Laennec’s capsule covers not only the entire surface of the hepatic parenchyma beneath the serosa but also the Glissonean pedicle surface. Consequently, a gap exists between the Glissonean pedicle (the plate system) and Laennec’s capsule that allows isolating the extrahepatic Glissonean pedicle without parenchymal destruction. In this context, the plate system and the cystic plate represent the best landmarks for extrahepatic dissection. The Glissonean pedicles can be dissected only at the gaps between Laennec’s capsule and the Glissonean pedicle, which represent the 6 “gates”, as described elsewhere[42].
Thus, this novel anatomy gives a theoretical background for the standardization of the systematic extrahepatic Glissonean pedicle isolation[42].
Indocyanine green fluorescence
Indocyanine green (ICG) fluorescence can help the surgeon to perform MIALR thanks to the possibility of obtaining an enhancement of the transection line, given the absence of a clear demarcation of the hepatic segments during parenchymal transection. To perform a formal AR with the Glissonean approach, according to the technique described by Takasaki et al., a negative contrast delineation (counterstaining) can be achieved through an intravenous systemic ICG injection[34,42] [Figure 2]. Firstly, the Glissonean pedicle of the segment is isolated and selectively closed. Then, the contrast is administered intravenously, obtaining a green fluorescence of the remnant liver and a negative enhancement of the transection line. Alternatively, ICG can be injected directly into the secondary/tertiary order portal after its surgical dissection to obtain positive counterstaining. However, the positive staining technique can be technically more demanding because of the possibility of extra vasal spillover[43].
Figure 2. The Glissonean approach for Laparoscopic right posterior sectionectomy. ICG fluorescence is intravenously injected at the dose of 0.025 mg/kg, after the selective control of the right posterior Glissonean pedicle, to obtain a negative counter-staining of the transection plane.
Another advantage of ICG during LLR is given by the possibility of enhancing the tumoral lesion by intraoperative ICG fluorescence imaging. A dose of 0.5 mg/kg, administrated about three days prior to surgery, allows detecting small lesions and can provide a real-time demarcation of liver tumor[44]. Even if false-positive nodules can be observed in cirrhotic livers, Tanaka et al. reported a false-positive rate of only 1%, with 99% of lesions identified and an overall sensitivity of 96%[45]. Tumors located deeper than 10 mm from the liver surface are unfortunately difficult to recognize: 10 mm is considered the maximum limit of depth for tumor detection by ICG fluorescence.
OPEN ISSUES
Cirrhotic liver
HCC develops on a chronic and cirrhotic liver in approximately 80%-90% of cases[46]. This was initially the main obstacle to MILS because of the frailty of the patients for developing hepatic decompensation and failure, as well as low platelets and impaired coagulation. Later, several studies reported the safety of LLR in cirrhotic patients: a recent metanalysis on eleven studies comprising 1618 patients indicated a 16%-26% reduction in the hazard ratio of death for patients with HCC and cirrhosis undergoing LLR when compared to OLR, as well as reduced blood loss, reduced major complications, and shorter length of hospital stay[47].
Furthermore, when dealing with cirrhotic patients, it is important to consider not only the oncological and perioperative outcomes but also the surgical stress. An important reported advantage of LLR in cirrhotic patients is the lower incidence of PHLF and ascites. It seems to be related to a reduced interruption of portosystemic shunts, together with lower electrolyte imbalances due to minimal exposure of the abdominal content to the air[48]. Furthermore, the reduced invasiveness of laparoscopy minimizes liver manipulation, preserves collateral vessels, and preserves the abdominal musculature[49].
Finally, the advantages of LLR have also been reported in Child B cirrhotic patients, who still are not included in any recommendations for liver resection for HCC. An international multicenter propensity score-matched study involving high-volume centers showed reduced blood loss, morbidity, and major complications after the laparoscopic approach in this setting of patients[50].
Difficult positions
The feasibility of LLR for HCC in every segment of the liver has been reported for more than 20 years by experienced laparoscopic surgeons in high volume centers[51-53]. The technical difficulty of MILS is linked to a limited ability to explore deep and posterior regions of the liver, as well as the difficulty of performing the parenchymal transection and hemostasis at these transection planes. LLR of PS segments are considered major liver resections, according to the most recent international consensus[12], since they have shown significantly longer operative time, a longer length of hospital stay, higher rate of open conversion, and higher estimated blood loss when compared to antero-lateral resections[54]. LLR was initially reserved for superficial or left-sided lesions according to the first recommendations, followed by more and more studies reporting the technical feasibility of LLR for PS segments in experienced high-volume centers (IVa, VII, and VIII)[40,51,55,56].
Improved laparoscopic techniques, better visualization of the operative field using a flexible laparoscope, and routine use of a cavitron ultrasonic aspirator for transecting liver parenchyma have allowed reaching excellent outcomes for LLR for HCC located even in PS segments, resulting in reduced blood loss, fewer complications, and shorter postoperative hospital stay compared with OLR[57-59].
Similarly, encouraging results have been shown for laparoscopic caudate lobectomy, which has been considered as a challenging procedure even for an experienced surgeon. A recent study by Parikh et al. involving 21 caudate lobectomies showed similar operation time, estimated blood loss, complication rate, and hospital stay between laparoscopy and open group, as well as long-term oncological outcomes[60].
Giant and multiple tumors
As mentioned above, in the initial phase of MILS adoption, LLR was indicated only for small and superficial antero-lateral lesions due to both technical and oncological issues. Currently, most European and Asian guidelines recommend liver resections (LR) in the case of a resectable lesion regardless of its size[61-63]. Recently, Hong et al. published good long-term oncological outcomes from a nationwide cohort of 466 patients with large HCC, for patients with tumor diameter > 10 cm[64].
The question of feasibility depends primarily on whether the resection can be performed safely according to center experience. If technically feasible, the well-known advantages of MILS have been confirmed for giant tumors[65]. The safety of LLR for large malignant tumors had already been reported in 2015, even if with higher blood loss and a longer operative time[66]. From the technical point of view, the placement of trocars, the mobilization of the liver, and the possibility of an accidental tumor perforation by shear forces represent the main challenges. Similarly, the size of the tumor is taken into account in several difficulty scores, so tumors larger than 3 cm are considered of increased difficulty[67].
Regarding the number of tumors, a recent Japanese national series reported better results for Child A patients with multiple HCCs compared to radiofrequency or TACE in terms of OS, even if at the cost of greater morbidity[68]. Yin et al. published a randomized trial confirming that liver resections provided better OS for patients with multiple HCC outside of Milan Criteria than TACE[69], later confirmed by a propensity score matched analysis focusing on multiple HCC within Milan criteria[70]. Currently, the number of tumors is an independent risk factor for poor long-term outcomes. However, eastern countries do not consider the presence of multiple HCCs as a contraindication[62].
Repeat resections
As HCC develops on underlying chronic liver disease, there is a high risk for multifocal tumors and liver recurrence. Therefore, reports of repeat laparoscopic liver resection (RLLR) have increased, also based on the assumption that an LLR can reduce adhesions and allow a safer second surgical procedure[71]. Kanazawa et al. showed that the operation time for repeat LLR after previous LLR was significantly shorter than that after previous OLR[72].
RLLR was associated with less blood loss, fewer postoperative complications, and shorter hospital stay compared with repeat OLR in a series from Belli et al.[73]. The reason could be found in the use of a narrow scope with a magnified view, which can help dissection and avoid visceral injury, as well as minimal adhesiolysis. Similarly, Berardi et al. reported the feasibility of a laparoscopic approach for repeat resection also after LT, pushing the limits of MILS even further[74].
Recently, an international multi-institutional propensity score-based study of RLLR for HCC was recently published by Morise et al., showing less blood loss and shorter hospital stay for the laparoscopic group. Further prospective studies are needed to confirm these results[75].
Difficulty scores
To guide a stepwise progression in the adoption of LLR, several difficulty scores and classification systems have been described and validated as well to estimate the difficulty of a given operation preoperatively[76]. An ideal difficulty score should incorporate patient-, surgeon-, and tumor-related factors. Unfortunately, to date, the most widely used scores take into account only some aspects of the procedure and have been developed only trying to predict the postoperative outcomes.
An easy-to-use recommendation score for guiding minimally invasive surgeons at a high-volume center was proposed by Mosteanu et al., and it can be even more useful if combined with other scores in order to clarify the decision-making process on MILS[77]. According to this score, up to seven points are assigned based on tumor location and scheduled surgery. Then, five further elements are considered to determine the final score: tumor size, tumor number, relationships with vascular structures, liver function, and previous surgery. The highest possible score is 12, and with a score higher than 7, a minimally invasive approach is weakly recommended, or it should only be performed by very experienced surgeons[77].
As always, every surgical procedure must be individually discussed and tailored by a single surgeon, based on the skills and clinical experience, as well as the available instruments and the complexity of each case.
Robotic liver resection
Since the first series of robotic liver resections (RLR) were reported in 2003, more and more studies have been published, leading to the first international consensus statement on RLR in 2018[78,79]. RLR can ideally overcome some drawbacks of traditional LLR, such as inflexible fixation of the operating instruments and a large swing of the visual field leverage effect, thanks to an exceptional ability to articulate the instruments and a magnified three-dimensional vision, as well as to considerable ergonomic advantages[80,81].
Safe and effective RLR for tumors located in PS segment has also been reported by several authors[82,83]. In addition, Hu et al. reported how robotic hemi-hepatectomies for large tumors were associated with less intraoperative blood loss and a shorter operation time than LLR[65]. However, a recent meta-analysis including 487 RLR and 902 LLR confirmed the lower bleeding rates for RLR but a longer operation time than LLR[84]. There was no significant difference in hospital stay, conversion rate, R0 resection rate, and total complication rate between the two groups.
Therefore, the high cost of treatment and logistic and organizational aspects may be the biggest shortcomings in the further development of robotic surgery[85]. Furthermore, to date, there are still no randomized controlled trials comparing LLR and RLR.
CONCLUSION
With the advances in surgical techniques and perioperative management, indications for LLR in HCC patients have tremendously improved. The actual frontiers of MILS will probably be redefined by advanced techniques and increasing experiences. The whole process is based on a reassuring increasing quantity and quality of positive outcomes of LLR, as also assumed by all the most recent international recommendations, despite the absence of randomized controlled trials.
MIALR remains one of the cornerstones of HCC surgery, combining the ability to remove all the territory supplied by a portal branch and avoid RLI that can lead to worse outcomes. Anatomical knowledge is still considered the basis for a safe widespread adoption of MIALR for HCC treatment, together with a technical standardization following the Glissonean approach through a stepwise guided process. New technologies, such as ICG and RLR, can also be extremely helpful in this process.
It is fundamental to be aware of the specific limitations that still exist in HCC patients, such as LLR of difficult positions, repeat resections, and giant and multiple tumors, in order to overcome them and be an active part of this surgical technological revolution. Technical expertise and the learning curve should remain the key points of this process, as well as the correct selection of appropriate candidates.
Following these conditions, MILS will be considered the standard procedure in the management of HCC in all hepato-biliary centers.
DECLARATIONS
Authors’ contributionsManuscript conception and design: Cassese G, Han HS
Manuscript draft and revision: Cassese G, Han HS, Troisi R, Lee HW, Cho JY
Administrative, technical, and material support: Cho JY, Lee B, Lee HW
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021;7:6.
2. Cassese G, Han HS, Al Farai A, Guiu B, Troisi RI, Panaro F. Future remnant liver optimization: preoperative assessment, volume augmentation procedures and management of PVE failure. Minerva Surg 2022; doi: 10.23736/S2724-5691.22.09541-7.
3. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology 2018;68:723-50.
4. Tribillon E, Barbier L, Goumard C, et al. When should we propose liver transplant after resection of hepatocellular carcinoma? J Gastrointest Surg 2016;20:66-76; discussion 76.
5. Graf D, Vallböhmer D, Knoefel WT, et al. Multimodal treatment of hepatocellular carcinoma. Eur J Intern Med 2014;25:430-7.
6. Reich H, McGlynn F, DeCaprio J, Budin R. Laparoscopic excision of benign liver lesions. Obstet Gynecol 1991;78:956-8.
7. Cassese G, Han HS, Lee B, Lee HW, Cho JY, Troisi R. Leaping the boundaries in laparoscopic liver surgery for hepatocellular carcinoma. Cancers (Basel) 2022;14:2012.
8. Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg 2009;250:831-41.
9. Han HS, Cho JY, Kaneko H, et al. Expert panel statement on laparoscopic living donor hepatectomy. Dig Surg 2018;35:284-8.
10. Buell JF, Cherqui D, Geller DA, et al. World Consensus Conference on Laparoscopic Surgery. The international position on laparoscopic liver surgery: the Louisville statement, 2008. Ann Surg 2009;250:825-30.
11. Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29.
12. Abu Hilal M, Aldrighetti L, Dagher I, et al. The southampton consensus guidelines for laparoscopic liver surgery: from indication to implementation. Ann Surg 2018;268:11-8.
13. Han HS, Shehta A, Ahn S, Yoon YS, Cho JY, Choi Y. Laparoscopic versus open liver resection for hepatocellular carcinoma: case-matched study with propensity score matching. J Hepatol 2015;63:643-50.
14. Delvecchio A, Conticchio M, Riccelli U, et al. Laparoscopic versus open liver resection for hepatocellular carcinoma in elderly patients: a propensity score matching analysis. HPB (Oxford) ;2021:S1365-182X(21)01691.
15. Sposito C, Battiston C, Facciorusso A, et al. Propensity score analysis of outcomes following laparoscopic or open liver resection for hepatocellular carcinoma. Br J Surg 2016;103:871-80.
16. Jiang B, Yan XF, Zhang JH. Meta-analysis of laparoscopic versus open liver resection for hepatocellular carcinoma. Hepatol Res 2018;48:635-63.
17. Xiong JJ, Altaf K, Javed MA, et al. Meta-analysis of laparoscopic vs open liver resection for hepatocellular carcinoma. World J Gastroenterol 2012;18:6657-68.
18. Cheung TT, Han HS, She WH, et al. The Asia Pacific consensus statement on laparoscopic liver resection for hepatocellular carcinoma: a report from the 7th Asia-Pacific primary liver cancer expert meeting held in Hong Kong. Liver Cancer 2018;7:28-39.
19. Tomassini F, Scuderi V, Colman R, Vivarelli M, Montalti R, Troisi RI. The single surgeon learning curve of laparoscopic liver resection: a continuous evolving process through stepwise difficulties. Medicine (Baltimore) 2016;95:e5138.
20. Vigano L, Laurent A, Tayar C, Tomatis M, Ponti A, Cherqui D. The learning curve in laparoscopic liver resection: improved feasibility and reproducibility. Ann Surg 2009;250:772-82.
21. van der Poel MJ, Besselink MG, Cipriani F, et al. Outcome and learning curve in 159 consecutive patients undergoing total laparoscopic hemihepatectomy. JAMA Surg 2016;151:923-8.
22. Makuuchi M, Hasegawa H, Yamazaki S. Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet 1985;161:346-350.
24. Pang YY. The Brisbane 2000 terminology of liver anatomy and resections. HPB 2000; 2:333-39. HPB (Oxford) 2002;4:99; author reply 99-100.
25. Okusaka T, Okada S, Ueno H, et al. Satellite lesions in patients with small hepatocellular carcinoma with reference to clinicopathologic features. Cancer 2002;95:1931-7.
26. Marubashi S, Gotoh K, Akita H, et al. Anatomical versus non-anatomical resection for hepatocellular carcinoma. Br J Surg 2015;102:776-84.
27. Tang H, Li B, Zhang H, Dong J, Lu W. Comparison of anatomical and nonanatomical hepatectomy for colorectal liver metastasis: a Meta-analysis of 5207 patients. Sci Rep 2016;6:32304.
28. Xu H, Liu F, Hao X, et al. Laparoscopically anatomical versus non-anatomical liver resection for large hepatocellular carcinoma. HPB (Oxford) 2020;22:136-43.
29. Cho JY, Han HS, Choi Y, et al. Association of remnant liver ischemia with early recurrence and poor survival after liver resection in patients with hepatocellular carcinoma. JAMA Surg 2017;152:386-92.
30. Giordano M, Lopez-Ben S, Codina-Barreras A, et al. Extra-Glissonian approach in liver resection. HPB (Oxford) 2010;12:94-100.
31. Makuuchi M, Imamura H, Sugawara Y, Takayama T. Progress in surgical treatment of hepatocellular carcinoma. Oncology 2002;62 Suppl 1:74-81.
32. Couinaud C. Surgical anatomy of the liver revisited. C. Couinaud; 1989.
33. Yamamoto M, Ariizumi SI. Glissonean pedicle approach in liver surgery. Ann Gastroenterol Surg 2018;2:124-8.
34. Takasaki K. Glissonean pedicle transection method for hepatic resection: a new concept of liver segmentation. J Hepatobiliary Pancreat Surg 1998;5:286-91.
35. Lin TY, Chem KM, Liu TK. Total right hepatic lobectomy for primary hepatoma. Surgery 1960;48:1048-60.
36. That Tung, Nguyen Duong Quang. A new technique for operating on the liver. The Lancet 1963;281:192-3.
38. Morimoto M, Tomassini F, Berardi G, et al. Study group of precision anatomy for minimally invasive hepato-biliary-pancreatic surgery (PAM-HBP surgery). Glissonean approach for hepatic inflow control in minimally invasive anatomic liver resection: a systematic review. J Hepatobiliary Pancreat Sci 2022;29:51-65.
39. Nakamura M, Wakabayashi G, Tsuchida A, Nagakawa Y. Study group of precision anatomy for minimally invasive hepato-biliary-pancreatic surgery (PAM-HBP surgery). Precision anatomy for minimally invasive hepatobiliary pancreatic surgery: PAM-HBP surgery project. J Hepatobiliary Pancreat Sci 2022;29:1-3.
40. Yoon YS, Han HS, Choi YS, et al. Total laparoscopic right posterior sectionectomy for hepatocellular carcinoma. J Laparoendosc Adv Surg Tech A 2006;16:274-7.
41. Shirata C, Kokudo T, Gillet M, et al. Reappraisal of Laennec’s capsule. Surg Oncol 2020;33:222-3.
42. Sugioka A, Kato Y, Tanahashi Y. Systematic extrahepatic Glissonean pedicle isolation for anatomical liver resection based on Laennec's capsule: proposal of a novel comprehensive surgical anatomy of the liver. J Hepatobiliary Pancreat Sci 2017;24:17-23.
43. Cassese G, Troisi RI. Indocyanine green applications in hepato-biliary surgery. Minerva Surg 2021;76:199-201.
44. Ishizawa T, Fukushima N, Shibahara J, et al. Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer 2009;115:2491-504.
45. Tanaka T, Takatsuki M, Hidaka M, et al. Is a fluorescence navigation system with indocyanine green effective enough to detect liver malignancies? J Hepatobiliary Pancreat Sci 2014;21:199-204.
46. Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 2004;127:S35-50.
47. Kabir T, Tan ZZ, Syn NL, et al. Laparoscopic versus open resection of hepatocellular carcinoma in patients with cirrhosis: meta-analysis. Br J Surg 2021;109:21-9.
48. Morise Z. Laparoscopic liver resection for the patients with hepatocellular carcinoma and chronic liver disease. Transl Gastroenterol Hepatol 2018;3:41.
49. Guro H, Cho JY, Han HS, Yoon YS, Choi Y, Periyasamy M. Current status of laparoscopic liver resection for hepatocellular carcinoma. Clin Mol Hepatol 2016;22:212-8.
50. Troisi RI, Berardi G, Morise Z, et al. Laparoscopic and open liver resection for hepatocellular carcinoma with Child-Pugh B cirrhosis: multicentre propensity score-matched study. Br J Surg 2021;108:196-204.
51. Cho JY, Han HS, Yoon YS, Shin SH. Feasibility of laparoscopic liver resection for tumors located in the posterosuperior segments of the liver, with a special reference to overcoming current limitations on tumor location. Surgery 2008;144:32-8.
52. Cherqui D, Husson E, Hammoud R, et al. Laparoscopic liver resections: a feasibility study in 30 patients. Ann Surg 2000;232:753-62.
53. Han HS, Cho JY, Yoon YS. Techniques for performing laparoscopic liver resection in various hepatic locations. J Hepatobiliary Pancreat Surg 2009;16:427-32.
54. Yoon YS, Han HS, Cho JY, Ahn KS. Total laparoscopic liver resection for hepatocellular carcinoma located in all segments of the liver. Surg Endosc 2010;24:1630-7.
55. Cho JY, Han HS, Yoon YS, Shin SH. Experiences of laparoscopic liver resection including lesions in the posterosuperior segments of the liver. Surg Endosc 2008;22:2344-9.
56. Jang JY, Han HS, Yoon YS, et al. Three-dimensional laparoscopic anatomical segment 8 liver resection with Glissonian approach. Ann Surg Oncol 2017;24:1606-9.
57. Xiao L, Xiang LJ, Li JW, Chen J, Fan YD, Zheng SG. Laparoscopic versus open liver resection for hepatocellular carcinoma in posterosuperior segments. Surg Endosc 2015;29:2994-3001.
58. Haber PK, Wabitsch S, Krenzien F, et al. Laparoscopic liver surgery in cirrhosis - addressing lesions in posterosuperior segments. Surg Oncol 2019;28:140-4.
59. Cho JY, Han HS, Yoon YS, Shin SH. Outcomes of laparoscopic liver resection for lesions located in the right side of the liver. Arch Surg 2009;144:25-9.
60. Parikh M, Han HS, Cho JY, D'Silva M. Laparoscopic isolated caudate lobe resection. Sci Rep 2021;11:4328.
61. Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182-236.
62. Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 2017;11:317-70.
63. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358-80.
64. Hong SK, Lee KW, Hong SY, et al. Efficacy of liver resection for single large hepatocellular carcinoma in Child-Pugh a cirrhosis: analysis of a nationwide cancer registry database. Front Oncol 2021;11:674603.
65. Hu M, Chen K, Zhang X, Li C, Song D, Liu R. Robotic, laparoscopic or open hemihepatectomy for giant liver haemangiomas over 10 cm in diameter. BMC Surg 2020;20:93.
66. Shelat VG, Cipriani F, Basseres T, et al. Pure laparoscopic liver resection for large malignant tumors: does size matter? Ann Surg Oncol 2015;22:1288-93.
67. Barron JO, Orabi D, Moro A, et al. Validation of the IWATE criteria as a laparoscopic liver resection difficulty score in a single North American cohort. Surg Endosc 2022;36:3601-9.
68. Fukami Y, Kaneoka Y, Maeda A, et al. Liver Cancer Study Group of Japan. Liver resection for multiple hepatocellular carcinomas: a Japanese nationwide survey. Ann Surg 2020;272:145-54.
69. Yin L, Li H, Li AJ, et al. Partial hepatectomy vs. Transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan criteria: a RCT. J Hepatol 2014;61:82-8.
70. Peng Y, Liu F, Xu H, Lan X, Wei Y, Li B. Outcomes of laparoscopic liver resection for patients with multiple hepatocellular carcinomas meeting the Milan criteria: a propensity score-matched analysis. J Laparoendosc Adv Surg Tech A 2019;29:1144-51.
71. Szomstein S, Lo Menzo E, Simpfendorfer C, Zundel N, Rosenthal RJ. Laparoscopic lysis of adhesions. World J Surg 2006;30:535-40.
72. Kanazawa A, Tsukamoto T, Shimizu S, et al. Laparoscopic liver resection for treating recurrent hepatocellular carcinoma. J Hepatobiliary Pancreat Sci 2013;20:512-7.
73. Belli G, Cioffi L, Fantini C, et al. Laparoscopic redo surgery for recurrent hepatocellular carcinoma in cirrhotic patients: feasibility, safety, and results. Surg Endosc 2009;23:1807-11.
74. Berardi G, Colasanti M, Ettorre GM. ASO author reflections: pushing the limits in laparoscopic liver surgery for hepatocellular carcinoma. Ann Surg Oncol 2022; doi: 10.1245/s10434-021-11322-1.
75. Morise Z, Aldrighetti L, Belli G, et al. ILLS-Tokyo Collaborator group. Laparoscopic repeat liver resection for hepatocellular carcinoma: a multicentre propensity score-based study. Br J Surg 2020;107:889-95.
76. Im C, Cho JY, Han HS, et al. Validation of difficulty scoring system for laparoscopic liver resection in patients who underwent laparoscopic left lateral sectionectomy. Surg Endosc 2017;31:430-6.
77. Mosteanu BI, Han HS, Cho JY, Lee B. When should we choose a laparoscopic approach? Surg Oncol 2020;34:208-11.
78. Giulianotti PC, Coratti A, Angelini M, et al. Robotics in general surgery: personal experience in a large community hospital. Arch Surg 2003;138:777-84.
79. Liu R, Wakabayashi G, Kim HJ, et al. International consensus statement on robotic hepatectomy surgery in 2018. World J Gastroenterol 2019;25:1432-44.
80. Milone L, Daskalaki D, Fernandes E, Damoli I, Giulianotti PC. State of the art in robotic hepatobiliary surgery. World J Surg 2013;37:2747-55.
81. Zhang C, Li N. Advances in minimally invasive surgery for hepatocellular carcinoma. HR 2020:2020.
82. Casciola L, Patriti A, Ceccarelli G, Bartoli A, Ceribelli C, Spaziani A. Robot-assisted parenchymal-sparing liver surgery including lesions located in the posterosuperior segments. Surg Endosc 2011;25:3815-24.
83. Zhao ZM, Yin ZZ, Meng Y, et al. Successful robotic radical resection of hepatic echinococcosis located in posterosuperior liver segments. World J Gastroenterol 2020;26:2831-8.
84. Hu Y, Guo K, Xu J, et al. Robotic versus laparoscopic hepatectomy for malignancy: a systematic review and meta-analysis. Asian J Surg 2021;44:615-28.
Cite This Article
How to Cite
Cassese G, Han HS, Lee B, Lee HW, Cho JY, Troisi RI. The role of minimally invasive surgery in the treatment of HCC. Hepatoma Res. 2022;8:26. http://dx.doi.org/10.20517/2394-5079.2022.14
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.

























Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.