Safety and efficacy of DEM-TACE performed with drug-eluting microspheres smaller than 300 μm in patients with HCC and TIPS
Abstract
Aim: Safety and efficacy evidence of drug-eluting-microspheres trans-arterial chemoembolization (DEM-TACE) in patients with hepatocellular carcinoma (HCC) and trans-jugular intrahepatic portosystemic shunt (TIPS) is lacking. The aim of this retrospective study was to report the safety and efficacy of DEM-TACE procedures performed with microspheres smaller than 300 μm in patients with HCC and TIPS in a high-volume transplant center.
Methods: Embolization was standardized by initiating DEM-TACE with microspheres smaller than 100 μm, and if stasis was not achieved, adjunctive embolization with 100-300 or 200 μm microspheres was administered. With regards to efficacy, the oncological response was evaluated and categorized according to mRECIST criteria at 1, 3-6, 9-12, and 15-18 months. Reporting the safety profile, detailed laboratory analysis was performed before, at 36-48 h, and 30-60 days after the procedure. Adverse events (AEs) were recorded; post-embolic syndrome was defined as the onset of fever/nausea/pain after the procedure. Late onset hepatobiliary complications were evaluated by follow-up imaging with computed tomography or magnetic resonance (CT/MR).
Results: From December 2007 to November 2020, 17 HCC patients (25 HCC nodules) with patent TIPS underwent 20 DEM-TACE. Embolization was performed only with microspheres smaller than 100 μm in 3/20 DEM-TACE (15%); adjunctive embolization with 100-300 or 200 μm microspheres was required in 17/20 DEM-TACE (85%). Reported early AEs were post-embolic syndrome (9/20; 45%) all of grade 1-2, late AEs were asymptomatic acute liver bile duct injury (2/20; 10%), and in one case we observed hepatic abscess (1/20; 5%) resulting in death due to sepsis. With regards to efficacy, the oncological response was evaluated and categorized according to mRECIST criteria. Complete response (CR) at 1, 3-6, 9-12, and 15-18 months was 52%, 50%, 50%, and 50%, respectively. Objective response (CR + partial response) at 1, 3-6, 9-12, and 15-18 months was 95%, 71%, 70%, and 50%, respectively.
Conclusion: DEM-TACE with drug-eluting-microspheres smaller than 300 μm can be performed in appropriately selected patients with TIPS.
Graphical Abstract
Keywords
INTRODUCTION
Trans-arterial chemoembolization (TACE) is the first-line treatment for intermediate or early-stage disease patients, according to the Barcelona Clinic Liver Cancer (BCLC), not eligible for curative therapies [surgical or ablative ones such as radiofrequency (RF) and microwave (MW)]. Although TACE is mainly performed for palliative reasons, in transplant centers, it can represent a possible option to maintain a patient on the waiting list (bridging) or to downstage (downstaging) a patient not eligible for a liver transplant[1].
These patients are often affected by concomitant portal hypertension due to their hepatic cirrhosis, and they may show related complications (variceal bleeding and refractory ascites). For this reason, during their disease, some of them can be potential candidates for trans-jugular intrahepatic portosystemic shunt (TIPS) placement [2].
While TACE induces ischemia of neoplastic area by selective arterial embolization, TIPS induces hepatic portal flow diversion, which normally supplies hepatic arterial occlusion. Therefore, TACE is now considered a relative contraindication in patients with TIPS.[3]
The literature regarding the feasibility of TACE in hepatocellular carcinoma (HCC) patients who previously underwent TIPS procedures is limited. Only some previous reports[4] dealing with c-TACE (Lipiodol Ultra Fluid, Guerbet, Roissy, France®) reported mild oncological results according to Kuo et al.[4] and Padia et al.[5] [complete response (CR) and overall response (OR) were 30%/50% and 14%/29%, respectively], while, regarding the safety profile, grade 3 or 4 severe adverse events rates within one month were high (36%) according to Wang et al.[6], all characterized by reversible liver function changes and post-embolization syndrome.
The innovation of the drug-eluting embolic platform has led to the progressive shrinkage of microspheres diameter due to the hypothetic advantage of a better oncological response.[7]
Furthermore, concern for the potential onset of hepatobiliary complication, in particular when doing drug-eluting-microspheres TACE (DEM-TACE) with smaller caliber microspheres, has been risen, demonstrating a major incidence and severity of hepatobiliary complications[8] in patients treated with microspheres < 100 μm compared to larger caliber microspheres. In particular, according to Odisio et al.[8], clinically symptomatic adverse events rates (AEs) were high (67.4%), but all were of grade 1 or 2 constituting post-embolization syndrome, and asymptomatic liver biliary injury occurred in 29.7% of cases with a mean time of 71 days. The most commonly encountered were biloma/liver infarct, biliary dilatation, and portal vein thrombosis or narrowing.
In this light, further recent research focus has been posed on DEM-TACE hepatobiliary complication onset[8]. In particular, de Baere et al.[9] reported adverse events rates of 71% of grade 1-2 and 13.4% of grade > 3, all related to post-embolization syndrome; in addition, hepatobiliary toxicities had an occurrence of 15.5%, for which the strongest predictor was previous locoregional treatments.
However, up to date, studies concerning DEM-TACE in HCC patients with TIPS are limited, and there is no evidence on the safety of DEM-TACE (especially considering smaller than 300 μm microspheres) in patients with TIPS.
Thus, the aim of this study was to evaluate the adverse effects, long-term survival, and clinical outcomes of patients with TIPS undergoing DEM-TACE using the Modified Response Evaluation Criteria in Solid Tumors (mRECIST).
METHODS
Inclusion criteria were evaluated by a multidisciplinary board (composed of a transplant surgeon, an interventional radiologist, a body radiologist, and a hepatologist) and included Child-Pugh score up to B8 and BCLC stage up to B. All patients of this study were diagnosed with typical hypervascular HCCs according to the American Association for the Study of Liver Disease guidelines[10] by multidetector computed tomography (CT) and/or magnetic resonance (MR) imaging. Due to anatomic or disease-related reasons (total disease burden), all patients were considered ineligible for curative treatments (surgical interventions or percutaneous ablative procedures). Preoperative imaging also confirmed TIPS patency before trans-arterial chemoembolization in all patients. All TIPS procedures were performed within the five years prior to DEM-TACE.
Exclusion criteria were as follows: Child-Pugh score above B8 and C, BCLC stage C, previous anti-neoangiogenic systemic treatment, platelet count < 50,000/mm3, total bilirubin level > 3 mg/dL, and portal vein thrombosis. Patients without imaging before or after DEM-TACE or those eligible for surgical intervention, liver transplantation, or other liver-directed therapies to the target nodules before post-TACE follow-up imaging study were excluded.
DEE Transcatheter Arterial Chemoembolization Technique
All DEM-TACE procedures were standardized and performed by two interventional radiologists with 15 and 12 years of experience, respectively.
All procedures were performed by femoral access. A 4 F catheter was positioned in the common or proper hepatic artery to evaluate the presence of extrahepatic feeders. A detailed study of hepatic tumor feeders was performed by digital subtraction angiography (posteroanterior and right anterior oblique 25) and adjunctive dual-phase cone-beam CT since our first usage in March 2014. After detecting the nodule’s feeders, microcatheterizations were performed by a 2.7 F microcatheter (Progreat®; Terumo Europe NV, Leuven, Belgium) coaxially within the 4 F catheter, positioned as close as technically feasible to the tumor. Microparticles were previously loaded with 50 mg doxorubicin per syringe filled with 2 mL of embolic material (Lifepearl Terumo® microspheres 100 ± 25 μm/200 ± 50 μm, performed in 5/20 procedures since our first usage in May 2015, or M1 DC Beads Boston Scientific® microspheres 70-150 μm/300-500 μm, performed in 15/20 procedures since our first usage on December 2007) diluted with contrast material (350 mmol/mL) to reach 20 mL. The embolization protocol started first with smaller microparticles, immediately followed by larger ones. The embolization technical endpoint was achieving contrast media stasis after 10 heartbeats; if stasis was obtained with only smaller microparticles, the adjunctive larger microparticles were not administered.[11]
The total dose of microparticles in milliliters and chemotherapeutic drugs in milligrams was recorded. In particular, smaller microparticles were only administered in 6/20 DEM-TACE procedures, while both types were sequentially administered in 14/20 DEM-TACE procedures. Repeated embolization sessions were performed during follow-up if necessary and only in partial response (PR) cases when it was established as more feasible than other procedures such as percutaneous ablation of a residual nodule.[12]
Follow-up imaging timelines included multidetector CT and MR imaging within one month before planned treatment and multidetector CT and MR imaging follow-up at 1, 3-6, 9-12, and 15-18 months after the chemoembolization procedures; those results were evaluated according to mRECIST[13].
In the case of retreatments performed with other modalities (e.g., RF ablation, degradable starch microspheres trans-arterial chemoembolization, radioembolization, anti-neoangiogenic systemic treatment, and liver transplantation), the patients were excluded from this study.
Study Endpoints
The primary endpoints of this study were to assess the oncological outcomes by evaluating tumor response at 1, 3-6, 9-12, and 15-18 months and safety profile during the follow-up timeline, with regards to early onset of complications by liver function tests at the baseline before the procedure, 36-48 h after the procedure, and at 30-60 days and late onset of potential hepatobiliary complications by follow-up multidetector CT or MR imaging. The post-treatment assessment for the oncological response was performed by two radiologists with 20 years of experience in liver body imaging.
The tumor response was assessed according to mRECIST classification[13] which defines CR as loss of any intra-nodular hypervascular solid tissue in all target lesions, PR as reduction >30% in the sum of diameters compared with naïve target lesions, stable disease (SD) as any cases not included in PR criteria or when the target lesion remains stable, and progressive disease (PD) as an increase of at least 20% in the sum of the diameters compared with the smallest sum of the diameters of treated target lesions. Objective response is defined as CR + PR rate, while disease control (DC) is defined as CR + PR + SD rate.
Regarding the safety profile of DEM-TACE, the following liver function tests were performed: alanine aminotransferase, aspartate aminotransferase, serum total and conjugated bilirubin, albumin dosage, alkaline phosphatase, and g-glutamyltransferase. Prothrombin time (international normalized ratio) and blood cell counts were also assessed.
AEs were recorded, and their degree was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, Version 6[14]. Post-embolic syndrome (PES) was defined as the onset of fever, nausea, and/or pain after the procedure, and a visual analog scale score > 6 (VAS score) was always evaluated before discharge. Late onset hepatobiliary complications were evaluated by follow-up multidetector CT or MR imaging and were considered as biliary duct dilatation or biloma formation onset after treatment without increases of bilirubin level, fever, or cholangitis[15].
Statistical Analysis
Continuous variables were expressed as mean ± SD. A linear regression using the Cox-Snell and Nagerlkerke models was performed to test the predictability of each dataset (etiology, laboratory analysis, hepatic status, epirubicin administration protocol, and embolization score) to predict the CR and DC at 1, 3-6, 9-12, and 15-18 months and the occurrence of early and/or late onset complications. Only p-values < 0.05 were considered statistically significant on the univariate test analysis. Survival curves were evaluated by Kaplan-Meier analyses.
Statistical analysis was performed using MedCalc 8.0 software (MedCalc Software bvba, Ostend, Belgium).
RESULTS
Between December 2007 and November 2020, 17 patients (mean age 58 ± 11.8 years; range, 34-77 years; 15 men and 2 women) with 25 hepatocellular carcinoma (HCC) nodules diagnosed by accepted radiological features and evidence of liver cirrhosis with previous TIPS (Gore Viatorr Endoprostheses, W.L. Gore & Associates, Inc, Flagstaff, Arizona®) were treated[14,16]. These patients (25 tumors) underwent 20 chemoembolization sessions (DEM-TACE); three multifocal HCC patients underwent the DEM-TACE procedure twice.
TIPS procedure indications were: 4/17 (23%) for primary prophylaxis variceal bleeding, 8/17 (47%) for secondary prophylaxis variceal bleeding, 2/17 (12%) for rescue, and 3/17 (18%) for refractory ascites. Clinical and demographic characteristics are summarized in Table 1.
Clinical and demographic characteristics of population
| Characteristics | Value |
| No.patients | 17 |
| No.transcatheter arterial chemioembolizations performed | 20 |
| No.tumors Nodules per patients 1 2 3 | 25 12/17 (70%) 2/17 (12%) 3/17 (18%) |
| Tumor dimensions Maximun diameter, mm, mean (range) Minimum diameter, mm, mean (range) | 26,6 (13 - 64) 23 (13 - 56) |
| Age, y, mean SD (range) | 58 12 |
| Sex, male/female | 15 / 2 |
| Child-Pugh score, n (%) A B | 10/17 (59%) 7/17 (41%) |
| Etiology, n (%) HCV HBV Alcohol-related cirrhosis Cryptogenetic cirrhosis Non-alcoholic steatohepatitis Mixed | 6/17 (35%) 3/17 (18%) 3/17 (18%) 1/17 (6%) 1/17 (6%) 3/17 (18%) |
| MELD < 15 ≥ 15 | 13/17 (76%) 4/17 (24%) |
| MELDNa < 15 ≥ 15 | 12/17 (70%) 5/17 (30%) |
| Monofocal/multifocal disease, n (%) | 7 (41%) / 10 (59%) |
| Monolobar/multilobar disease, n (%) | 13 (77%) / 4 (23%) |
| AFP serum level < 7 µg/L 7 - 200 µg/L ≥ 200 µg/L | 9/17 (53%) 5/17 (29%) 3/17 (18%) |
| TIPS Indication Variceal bleeding primary prevention Variceal bleeding secondary prevention Rescue Refractory ascites TIPS-TACE median time, days (range) | 4/17 (23%) 8/17 (47%) 2/17 (12%) 3/17 (18%) 944,6 (78 - 4074) |
| TACE Indication Downstaging Bridging Palliative | 2 / 20 (10%) 15 / 20 (75%) 3 / 20 (15%) |
| OLT Candidates Transplanted | 15 / 17 (88%) 6 / 17 (35%) |
Clinical Response
There were 17 HCC patients with 25 tumors at pre-procedural baseline time.
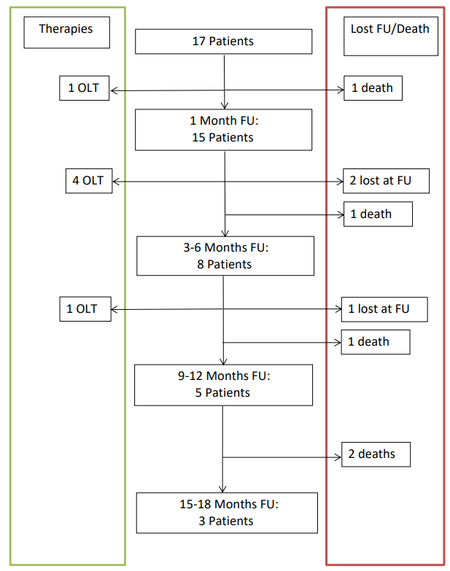
At one month, there were 15 patients with 21 tumors as we had one death and one orthotopic liver transplant (OLT). CR and OR rates were 52% (11/21) and 95% (20/21), respectively.
At 3-6 months, there were 8 patients with 14 tumors as we had one death, two lost to follow-up, and four underwent OLT. CR and OR rates were 50% (7/14) and 71% (10/14), respectively.
At 9-12 months, there were 5 patients with 10 tumors as we had one death, one lost to follow-up, and one underwent OLT (this patient underwent DEM-TACE twice). CR and OR rates were 50% (5/10) and 70% (7/10), respectively.
At 15-18 months, there were 3 patients with 6 tumors as we had two deaths. CR and OR rates were both 50% (3/6).
Follow-up details are summarized in Figure 1 and Table 2.
Follow-up mRECIST analysis
| Tumor response | 1 Month | 3-6 Months | 9-12 Months | 15-18 Months |
| CR | 12/21 (52) | 4/14 (50) | 5/10 (50) | 3/6 (50) |
| PR | 9/21 (43) | 3/14 (21) | 2/10 (20) | 0/6 (0) |
| OR (CR+PR) | 20/21 (95) | 11/14 (71) | 7/10 (73) | 3/6 (50) |
| SD | 1/21 (5) | 1/14 (7) | 2/10 (20) | 1/6 (17) |
| DC (OR+SD) | 21/21 (100) | 11/14 (79) | 9/10 (90) | 4/6 (67) |
| PD | 0/21 (0) | 3/14 (21) | 1/10 (10) | 2/6 (33) |
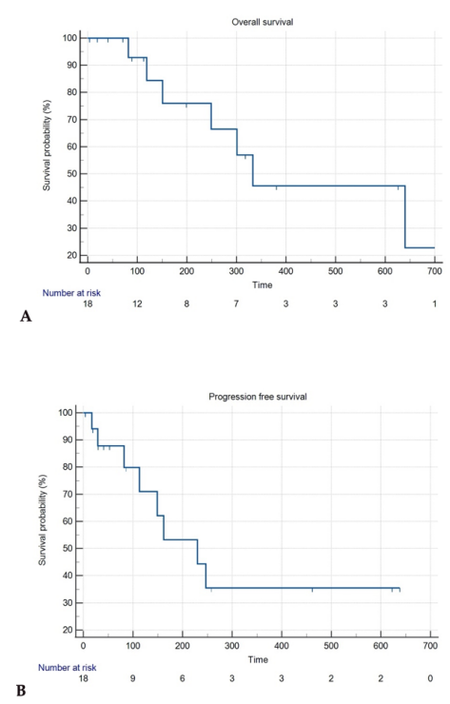
The overall survival (OS)and the progression-free survival are shown in Figure 2A and B.
Figure 2. A: Death liver related Kaplan-Meier curve; B: progression-free survival Kaplan-Meier curve
Finally, during follow-up, 6/17 patients affected by nine hepatic HCC nodules underwent OLT (35%) according to Milan criteria discussed in a multidisciplinary tumor board with a median time of 98.6 days (4-198).
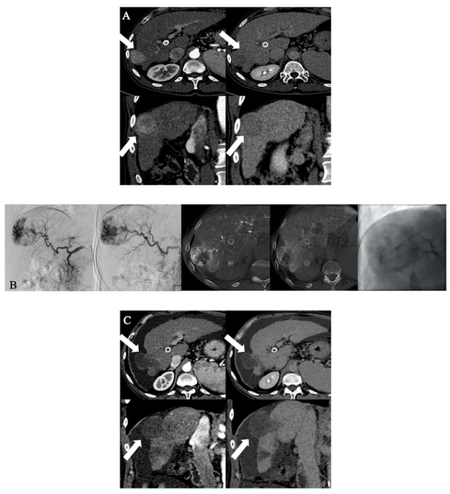
By evaluating the selectivity of the DEM-TACE procedure, an “only-tumor” embolization (score 0) was obtained for 4 tumors (4/25 16%); a superselective embolization (score 1) was obtained for 5 tumors (5/25 20%); segmental embolization (score 2) was achieved for 16 tumors (16/25 64%) [Figure 3A-C].
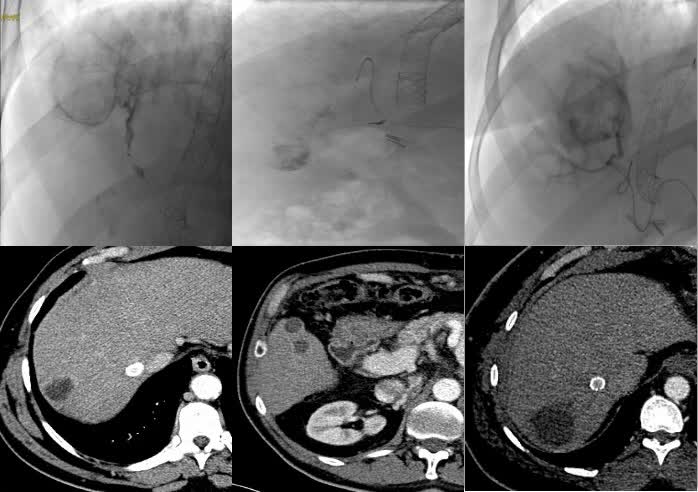
Figure 3. A: 58-year-old man with hepatitis C virus-related cirrhosis (Barcelona Clinic Liver Cancer Stage A; Child- Pugh Score B7) with a 4334 mm VII segment subcapsular HCC nodule. A: Multiphasic CT demonstrates typical wash-in (arrows) in the arterial phase and late wash-out at the delayed phase. B:Superselective catheterization was achieved (score 1) and 100% of 100 μm microspheres were administered. C: Follow-up CT at 12 months after the procedure demonstrates no residual vital tissue (arrows) categorizing the patient as having CR.
Safety Evaluation
The laboratory tests were compared before, at 36-48 h, and 30-60 days after the procedure. No statistically significant variations in laboratory tests were found.
Among clinical mild early adverse events (eAEs), PES occurred in 9 of 20 treatments (45%), consisting of fever onset in four patients with nausea and pain within 24 h. All mild eAEs were observed within the first 24 h after treatment and were treated by medical therapy alone. No prolonged hospitalization was required in any case.
Among late AEs, asymptomatic liver bile duct injuries were observed after two transcatheter arterial chemoembolizations (10.5%) during follow-up exams in both cases. The median visual analog scale score was 4. Among severe AEs, in one case (5%), we observed the occurrence of hepatic abscess resulting in the patient’s death.
During the follow-up time, we recorded five deaths with a median time of 166.8 days (19-333 days).
Except for one case, all deaths were not correlated to procedures’ adverse events; in particular, two were caused by digestive bleeding due to gastric varices, while the other two deaths were related to acute abdominal bleeding due to HCC rupture in peritoneum.
In all AEs, we obthe occtained the resolution of symptoms with only medical therapy administrated within the first 24 h after treatment without any prolonged hospitalizations.
Predictive Model Linear Regression Analysis
The independent variables tested did not show any significant trend to predict oncological outcome (CR, SD, PR, PD, and DC at 1, 3-6, 9-12, and 15-18 months) or early and late onset adverse events occurrence (post-embolic syndrome, asymptomatic biliary injuries, or hepatic abscess).
DISCUSSION
This study demonstrates that DEM-TACE performed with microspheres (< 300 μm) in HCC patients with patent TIPS is technically feasible, with comparable oncological response to those reported in the literature for conventional TACE (c-TACE). However, our data are still too limited to definitively confirm the safety of this procedure in these patients.
A patent TIPS diverges the portal venous flow into the systemic circulation, altering hepatic portal venous perfusion, thus limiting its potential to supply the area around the site of TACE treatment. Consequently, in HCC patients with TIPS, TACE is usually considered a relative contraindication[17], due to the potential increase of ischemic complications. Although TACE may be feasible in a selected cohort of patients[11,18], this procedure may be responsible for increased liver injury compared to similar patients without TIPS[9].
The literature regarding the feasibility of TACE in HCC patients with TIPS is limited to c-TACE (Lipiodol Ultra fluid, Guerbet, Roissy, France®)[19], and available evidence about DEM-TACE is lacking as the few studies are only about the usage of larger microspheres (> 100 μm) with short follow-up period[20,21]. Thus, there is a lack of evidence on the efficacy and safety of DEM-TACE performed with microspheres <100 µm. This corroborates and strengthens the importance of this study.
With regards to oncological response, CR and OR rates reported in previous studies of HCC patients without TIPS treated by DEM-TACE are comparable to those observed in our study (52% vs. 61% and 95% vs. 83%, respectively)[11,22,23,24]. However, the median OS and time to progression (TTP) of DEM-TACE in our study were 333 days (95%CI: 151-640) and 230 days (95%CI: 82-247), which are shorter than the outcomes of previous studies dealing with patients without TIPS (OS = 369-783 days, TTP = 345-759 days)[14,25,26]. This could be explained by the fact that our patient cohort had more advanced liver disease that required interventional management of underlying portal hypertension with TIPS placement, and liver function is one of the most important factors in determining the OS[27].
Furthermore, by comparing CR and OR rates obtained after c-TACE in HCC patients with TIPS to those of our series, it emerged that CR and OR at one month after DEM-TACE were better (52% vs. 30%, and 95% vs. 50%, respectively)[3,4]. Finally, even OS and TTP were longer than those reported by Kuo et al.[4] in the course of c-TACE (230 vs. 103 days).
These local effects and survival benefits may be due to the known improved efficacy of DEM-TACE, as the administration of the drug is maintained longer within the nodule at the concentration dose above the cytotoxic threshold.
Finally, during the follow-up time, 6/17 patients affected by nine hepatic nodules of HCC underwent OLT (35%); this demonstrates the efficacy of DEM-TACE procedures in patients with TIPS being able to bridge one-third of our patients to liver transplant.
With regards to the safety profile, the incidence of mild AEs such as PES was 45% (9/20), being all grade 1-2, and the rate of asymptomatic acute biliary injuries was 10% (2/20), which were all clinically insignificant.
In terms of AEs, we found that the safety of c-TACE in cirrhotic patients with TIPS showed contrasting results in the c-TACE literature. Kang et al. demonstrated that c-TACE is safe in patients with cirrhosis with TIPS[20]. Conversely, a more recent case-control study showed that the occurrence of AEs within 30 days was significantly higher in patients with TIPS than those without TIPS (70% vs. 36%)[4].
However, our reported incidence of grade 3-4 AEs after DEM-TACE is lower than those reported in the c-TACE literature. This could be explained by the fact that DEM-TACE is able to release microparticles to selectively spare the blood flow to normal liver tissues, allowing a deeper distal embolization of tumor feeding arteries[28]. As a consequence, some recent studies investigating DEM-TACE safety in cirrhotic patients with TIPS have shown comparable results to ours.[22,23]
Regarding death causes, in the c-TACE literature, the death rates correlated to the procedure (within one month) is low, while the long-term death causes occurring during follow-up are comparable[5]. In fact, two of our deaths were caused by digestive bleeding due to gastric varices that were not completely solved despite TIPS patency. In these two reported cases, it is worth underlining that no HVPG measurements were performed, so the potential correlation between the procedure itself and the occurrence of the complication cannot be totally excluded[29].
In our series, we observed one severe adverse event (hepatic abscess), finally resulting in patient death for sepsis. To better understand this occurrence, a detailed evaluation of the clinical background is mandatory.
First, our patient previously underwent percutaneous biliary drainage positioning for intrahepatic left biliary dilatation due to unknown stenosis. Cholangioscopy and brushing failed to demonstrate tumoral presence, thus permitting to classify the stenosis as benign. These maneuvers led to the colonization of the biliary tree. Finally, TIPS placement, as already extensively discussed above, determined increased ischemic risk. Moreover, contamination of the biliary system without administration of antibiotic prophylaxis may have led to an increased risk of hepatotoxicity. Lastly, we treated in the same session both HCC nodules located in two different hepatic segments, which, due to their dimensions (3 and 2.5 cm each), were treated with a relatively high embolization score (score 2). We believe that the concomitancy of all these conditions led to complication onset. Retrospectively, antibiotic prophylaxis would have prevented this complication, and we believe that prophylaxis is mandatory in these patients with theoretically increased risk of hepatotoxicity, which is actually not always deemed necessary in patients undergoing TACE.
Although in the present study it is not feasible to precisely compare our findings with the existing literature because of the differences among the patient population, our results show a superimposable safety compared with reported evidence with lipiodol (c-TACE) and DEB-TACE counterparts without TIPS.
This study has several limitations. First, our study is limited by its retrospective nature, and further prospective randomized controlled investigations are needed. Second, the enrolled sample size (17 patients with 25 treated tumors) is small, and it was a single-arm experience without control (non-TIPS patients). Third, regarding safety evaluation, HVPG measurements were not assessed after any DEM-TACE procedures, so we cannot exclude the possibility of worsening of portal hypertension after the procedure.
In conclusion, the results of our study indicate that DEM-TACE with drug-eluting-microspheres smaller than 300 μm can be performed in appropriately selected patients with TIPS; however, these data are not sufficient to assess the safety profile and should be confirmed with further and more detailed studies.
DECLARATIONS
Authors’ contributionsMade substantial contributions to conception and design of the study and performed data analysis and interpretation: Lucatelli P, Zilahi de Gyurgyokai S
Performed data collection: Forlino M, Parisse S, Ferri F
Performed statistical analysis: De Rubeis G
Assisted interventional procedures as well as provided administrative, technical, and material support: Rocco B, Ciaglia S, Zilahi de Gyurgyokai S
Confirmation of the draft and guarantee of integrity: Cantisani V, Catalano C
Availability of data and materialsAuthors declare that dataset supporting their findings will be provided blinded upon request.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateThe ethical institutional review boards of our center approved the study. Informed consent for the procedure was obtained from all participants included in the study. (RIF.CE: 5291)
Consent for publicationInformed consent for the anonymized publication of this series of patients was obtained from all participants included in the study.
Copyright© The Author(s) 2022.
REFERENCES
1. Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002;35:1164-71.
2. Dariushnia SR, Haskal ZJ, Midia M, et al. ; Society of Interventional Radiology Standards of Practice Committee. Quality Improvement Guidelines for Transjugular Intrahepatic Portosystemic Shunts. J Vasc Interv Radiol 2016;27:1-7.
3. Lucatelli P, Burrel M, Guiu B, et al. CIRSE standards of practice on hepatic transarterial chemoembolisation. Cardiovasc Intervent Radiol 2021;44:1851-67.
4. Kuo YC, Kohi MP, Naeger DM, et al. Efficacy of TACE in TIPS patients: comparison of treatment response to chemoembolization for hepatocellular carcinoma in patients with and without a transjugular intrahepatic portosystemic shunt. Cardiovasc Intervent Radiol 2013;36:1336-43.
5. Padia SA, Chewning RH, Kogut MJ, et al. Outcomes of locoregional tumor therapy for patients with hepatocellular carcinoma and transjugular intrahepatic portosystemic shunts. Cardiovasc Intervent Radiol 2015;38:913-21.
6. Wang Z, Zhang H, Zhao H, et al. Repeated transcatheter arterial chemoembolization is safe for hepatocellular carcinoma in cirrhotic patients with transjugular intrahepatic portosystemic shunt. Diagn Interv Radiol 2014;20:487-91.
7. Padia SA, Shivaram G, Bastawrous S, et al. Safety and efficacy of drug-eluting bead chemoembolization for hepatocellular carcinoma: comparison of small-versus medium-size particles. J Vasc Interv Radiol 2013;24:301-6.
8. Odisio BC, Ashton A, Yan Y, et al. Transarterial hepatic chemoembolization with 70-150 µm drug-eluting beads: assessment of clinical safety and liver toxicity profile. J Vasc Interv Radiol 2015;26:965-71.
9. de Baere T, Guiu B, Ronot M, et al. Real life prospective evaluation of new drug-eluting platform for chemoembolization of patients with hepatocellular carcinoma: paris registry. Cancers (Basel) 2020;12:3405.
10. Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2.
11. Lucatelli P, Argirò R, De Rubeis G, et al. Polyethylene glycol epirubicin-loaded transcatheter arterial chemoembolization procedures utilizing a combined approach with 100 and 200 μm microspheres: a promising alternative to current standards. J Vasc Interv Radiol 2019;30:305-13.
12. Facciorusso A, Licinio R, Muscatiello N, Di Leo A, Barone M. Transarterial chemoembolization: evidences from the literature and applications in hepatocellular carcinoma patients. World J Hepatol 2015;7:2009-19.
13. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52-60.
14. Lucatelli P, Argirò R, Ginanni Corradini S, et al. Comparison of image quality and diagnostic performance of cone-beam ct during drug-eluting embolic transarterial chemoembolization and multidetector CT in the detection of hepatocellular carcinoma. J Vasc Interv Radiol 2017;28:978-86.
15. Wang Z, Wang M, Duan F, Song P, Liu F. Bile duct injury after trans- catheter arterial chemoembolization: risk factors and clinical implications. Hepatogastroenterology 2014; 61:947-953.
16. Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part I. Development, growth, and spread: key pathologic and imaging aspects. Radiology 2014;272:635-54.
17. Liapi E, Geschwind JF. Transcatheter arterial chemoembolization for liver cancer: is it time to distinguish conventional from drug-eluting chemoembolization? Cardiovasc Intervent Radiol 2011;34:37-49.
18. Kang JW, Kim JH, Ko GY, et al. Transarterial chemoembolization for hepatocellular carcinoma after transjugular intrahepatic portosystemic shunt. Acta Radiol 2012;53:545-50.
19. Kohi MP, Fidelman N, Naeger DM, et al. Hepatotoxicity after transarterial chemoembolization and transjugular intrahepatic portosystemic shunt: do two rights make a wrong? J Vasc Interv Radiol 2013;24:68-73.
20. Fan W, Guo J, Zhu B, et al. Drug-eluting beads TACE is safe and non-inferior to conventional TACE in HCC patients with TIPS. Eur Radiol 2021;31:8291-301.
21. Chen X, Qiu ZK, Wang GB, et al. Effect of transjugular intrahepatic portosystemic shunt on transarterial chemoembolization for hepatocellular carcinoma: a systematic review and meta-analysis. Diagn Interv Radiol 2021;27:671-6.
22. Lammer J, Malagari K, Vogl T, et al. ; PRECISION V Investigators. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol 2010;33:41-52.
23. Grosso M, Vignali C, Quaretti P, et al. Transarterial chemoembolization for hepatocellular carcinoma with drug-eluting microspheres: preliminary results from an Italian multicentre study. Cardiovasc Intervent Radiol 2008;31:1141-9.
24. Malagari K, Chatzimichael K, Alexopoulou E, et al. Transarterial chemoembolization of unresectable hepatocellular carcinoma with drug eluting beads: results of an open-label study of 62 patients. Cardiovasc Intervent Radiol 2008;31:269-80.
25. Kloeckner R, Weinmann A, Prinz F, et al. Conventional transarterial chemoembolization versus drug-eluting bead transarterial chemoembolization for the treatment of hepatocellular carcinoma. BMC Cancer 2015;15:465.
26. Liu YS, Lin CY, Chuang MT, et al. Five-year outcome of conventional and drug-eluting transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. BMC Gastroenterol 2018;18:124.
27. Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182-236.
28. Varela M, Real MI, Burrel M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol 2007;46:474-81.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Lucatelli P, Zilahi De Gyurgyokai S, De Rubeis G, Argirò R, Ciaglia S, Rocco B, Ferri F, Parisse S, Forlino M, Cannavale A, Basilico F, Nardis PG, Corona M, Cantisani V, Catalano C. Safety and efficacy of DEM-TACE performed with drug-eluting microspheres smaller than 300 μm in patients with HCC and TIPS. Hepatoma Res 2022;8:18. http://dx.doi.org/10.20517/2394-5079.2021.143
AMA Style
Lucatelli P, Zilahi De Gyurgyokai S, De Rubeis G, Argirò R, Ciaglia S, Rocco B, Ferri F, Parisse S, Forlino M, Cannavale A, Basilico F, Nardis PG, Corona M, Cantisani V, Catalano C. Safety and efficacy of DEM-TACE performed with drug-eluting microspheres smaller than 300 μm in patients with HCC and TIPS. Hepatoma Research. 2022; 8: 18. http://dx.doi.org/10.20517/2394-5079.2021.143
Chicago/Turabian Style
Lucatelli, Pierleone, Simone Zilahi De Gyurgyokai, Gianluca De Rubeis, Renato Argirò, Simone Ciaglia, Bianca Rocco, Flaminia Ferri, Simona Parisse, Mariana Forlino, Alessandro Cannavale, Fabrizio Basilico, Pier Giorgio Nardis, Mario Corona, Vito Cantisani, Carlo Catalano. 2022. "Safety and efficacy of DEM-TACE performed with drug-eluting microspheres smaller than 300 μm in patients with HCC and TIPS" Hepatoma Research. 8: 18. http://dx.doi.org/10.20517/2394-5079.2021.143
ACS Style
Lucatelli, P.; Zilahi De Gyurgyokai S.; De Rubeis G.; Argirò R.; Ciaglia S.; Rocco B.; Ferri F.; Parisse S.; Forlino M.; Cannavale A.; Basilico F.; Nardis PG.; Corona M.; Cantisani V.; Catalano C. Safety and efficacy of DEM-TACE performed with drug-eluting microspheres smaller than 300 μm in patients with HCC and TIPS. Hepatoma. Res. 2022, 8, 18. http://dx.doi.org/10.20517/2394-5079.2021.143
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 20 clicks
Cite This Article 20 clicks










Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.