Role of locoregional therapies in the management of patients with hepatocellular carcinoma
Abstract
Hepatocellular carcinoma (HCC) was the sixth most common cancer and the third cause of cancer-related deaths worldwide in 2020. Liver resection and transplantation remain the cornerstone for patients with early-stage disease and represent the only option for potential cure in HCC. However, fewer than 10% of patients are considered suitable for surgery at the time of diagnosis. Locoregional therapies, defined as minimally invasive image-guided liver tumour-directed procedures, are integral to in the management of HCC. This review discusses the role of locoregional therapies in HCC management in the emergence of immune and systemic treatments.
Keywords
INTRODUCTION
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third cause of cancer-related deaths, accounting for 8.3% of all cancer-related deaths worldwide in 2020[1]. Liver resection and transplantation remain the cornerstone for patients with early-stage disease and represent the only option for potential cure in HCC. However, due to tumour multi-nodularity, tumour location precluding curative resection and/or insufficient liver reserve due to underlying chronic liver disease, the reported resectability rate is low[2]. A recent international multicentre comparative study of 8656 patients with HCC showed that < 10% of patients satisfied preoperative criteria for resection[3]. Further, recurrence following liver resection is commonplace and strongly associated with worse overall survival[4,5], with recurrence rate up to 50% and 70% at two and five years, respectively[6,7]. Liver transplantation provides an alternative and arguably ideal treatment option for patients with unresectable HCC or those with HCC with a background of end-stage liver disease, as it addresses the underlying chronic liver disease and/or cirrhosis, as well as eliminates the cancer and any undetectable premalignant lesions[8]. However, few HCC patients meet the accepted transplant criteria, and the limited number of transplant centres together with the chronic shortage of donor organs, whether cadaveric or living-related, makes liver transplantation an unrealistic option for many patients. Hence, locoregional therapies have been developed, and globally it is estimated that up to 60% of HCC patients might receive locoregional therapies in their lifetime[9-13].
Various clinical staging systems stratify HCC patients based on disease extension, liver function and performance status and help guide therapeutic strategies. Examples include the Okuda system, Cancer of the Liver Italian Cancer score, Hong Kong Liver Cancer staging system and the Barcelona Clinic Liver Cancer (BCLC) classification system[14,15]. The BCLC treatment algorithm [Figure 1] has been widely adopted in the West, and it is endorsed by international societies including the European Association for the Study of the Liver (EASL) and the American Association for the Study of the Liver (AASLD)[11,12]. The Asia Pacific Association for the Study of the Liver (APASL) considers the selection criteria for liver resection set out by the BCLC system to be too strict and unsuitable for the Asia Pacific region[16]. Nonetheless, all three international societies recognise that locoregional therapies are integral to the management of HCC patients who have unresectable disease or those who are deemed unfit for surgery. Locoregional therapies can be performed with curative, downstaging, bridging, debulking or palliative intent[17]. Curative therapy is treatment aiming to eliminate HCC, achieve complete remission and prevent recurrence. Downstaging therapy is defined as treatment aimed at reducing tumour burden from beyond to within the accepted transplant criteria to facilitate enlistment for transplantation, whereas bridging therapy indicates treatment to control tumour growth to keep patients within the accepted transplant criteria and to prevent dropout caused by tumour progression during the waitlist period. Debulking therapy is treatment intended to prolong survival in patients with advanced unresectable HCC; however, data supporting the concept of debulking HCCs are scarce, which limits its implementation in routine clinical practice. Palliative therapy refers to treatment with the goal of alleviating symptoms and improving quality of life regardless of other outcomes such as survival benefit.
Figure 1. Treatment strategy in the management of HCC. BCLC: Barcelona Clinic Liver Cancer; CA: Cryoablation; DEB-TACE: Drug-eluting beads transarterial chemoembolization; MWA: Microwave ablation; PEI: Percutaneous ethanol injection; RFA: Radiofrequency ablation; TACE: Transarterial chemoembolization; TAE: Transarterial embolization.
Locoregional therapies are defined as minimally invasive image-guided liver tumour-directed procedures that can be categorised into ablative therapy, transcatheter therapy and radiation therapy[18]. Current evidence for the indication and efficacy of specific locoregional therapy is primarily based on retrospective reports as there are few prospective clinical trials, most of which only have best supportive therapy as the control arm. Due to the many permutations and variations in the treatment of different stages of HCC, an AASLD panel of experts on trial design in HCC has published a set of consensus guidelines to help standardise future trial design and provide recommendations on the target population, stratification and randomisation, study endpoints and control and intervention arms for each stage of HCC[19]. This review discusses the role of locoregional therapies in HCC management in the emergence of immune and systemic treatments [Figure 2].
Figure 2. Locoregional therapies in the management of HCC. BCLC: Barcelona Clinic Liver Cancer. (A) Radiofrequency ablation generates frictional heat using high frequency alternating current around an active electrode resulting in tissue hyperthermia. (B) Microwave ablation employs electromagnetic energy using an antenna to deliver thermal energy-induced cellular injury. (C) Cryoablation delivers subfreezing temperature via a cryoprobe induced by high-pressure argon gas and the repetitive freeze-thaw cycle leads to tumour necrosis. (D) Irreversible electroporation delivers high-voltage, low-energy direct current pulses to induce irreversible disruption of cell membrane integrity. (E) Transarterial embolization disrupts tumour blood supply resulting in tumour ischaemia/hypoxia. (F) Transarterial chemoembolization delivers high-dose chemotherapy and lipiodol followed by arterial embolization to promote tumour ischaemia/hypoxia. (G) Drug-eluting bead chemoembolization delivers embolic microspheres (beads) loaded with a chemotherapeutic agent providing local, sustained tumour drug delivery combined with tumour ischaemia/hypoxia. (H) Radioembolization delivers Yttrium-90 microspheres providing local, high-dose tumour radiation. (I) Stereotactic body radiotherapy is a form of external beam radiotherapy that accurately delivers high-dose radiation to the target tumour while limiting radiation dose to adjacent non-target liver.
ABLATIVE THERAPIES
Background
Ablative therapies for HCC include percutaneous ethanol injection (PEI), radiofrequency ablation (RFA), microwave ablation (MWA), cryoablation, irreversible electroporation (IRE), laser-induced interstitial thermotherapy (LITT) and high-intensity focused ultrasound (HIFU). Apart from PEI, which is a chemical ablation procedure, the objective of thermal ablation is to induce coagulative tumour necrosis using extremes of temperature (> 60 C and < 40 C)[18]. Thermal ablation ablates both tumoral and peri-tumoral tissue with the aim of inducing an adequate margin (usually 5-10 mm) around the tumour to eliminate surrounding undetected microsatellites. These cancer microenvironments are frequently found in HCCs > 2-2.5 cm, which might account for suboptimal efficacy of ablation over this size threshold[20].
PEI was the first ablative technique used in clinical practice for treating early-stage HCC[17], where absolute alcohol is injected into the tumour. Ethanol diffuses into cells causing cellular dehydration and protein denaturation, followed by fibrosis and small-vessel thrombosis, thereby inducing coagulative necrosis of the tumour[21]. With PEI, tumour targeting may be challenging as the treated tumour margins are less distinctly defined[22] and, as such, prolonged treatment and multiple sessions may be necessary to induce complete tumour necrosis[23,24].
RFA generates frictional heat using high frequency alternating current around an active electrode, with grounding pads to close the electric circuit[25]. The high temperature generated boils, vaporises, necroses and chars the tissue[26]. The resultant eschar increases tissue impedance and limits energy transmission to adjacent cells, hence decreasing RFA efficacy towards the peripheries of the ablation zone. Another limitation of RFA is the heat-sink effect that may lead to incomplete ablation for perivascular tumours due to convection cooling into large vessels[27,28]. For tumours close to bile ducts, RFA might cause biliary complications, namely biliary stricture and biloma.
MWA employs electromagnetic energy using an antenna to deliver thermal energy-induced cellular injury without the need for grounding pads. MWA attains threshold temperature faster, achieves larger and more uniform ablation zones with better delineated borders and is less susceptible to convection cooling from adjacent vasculature[29]. The size of the ablation zone however is more difficult to predict in MWA compared to RFA[18]. A systematic review of 34 studies totalling 12,158 HCC patients treated with PEI, RFA and MWA demonstrated similar mortality and complication rates among the three techniques with an overall mortality rate of 0.16% and major complication rate of 3.29%[30]. Complications of these ablative therapies include pain, bleeding, infection, abscess, bile leak, liver failure, portal vein thrombosis, cardiac arrythmias and pneumothorax[17]. Tumour seeding has been reported in 0.5-3% of RFA[31] and MWA[32], and the risk of tumour seeding can be reduced by cauterisation of the needle trajectory upon withdrawal of the needle and by avoiding direct puncture of subcapsular lesions[17]. In addition, ablation to subcapsular tumours close to neighbouring hollow viscera might be complicated by thermal injury to adjacent bowel wall leading to bowel perforation, and such complications can be circumvented by the infusion of artificial ascites[33].
Cryoablation delivers subfreezing temperature to the tumour via a penetrating vacuum cryoprobe induced by high-pressure argon gas and the repetitive freeze-thaw cycle leads to tumour necrosis. Intra-and extracellular ice crystal formation during the freezing process causes irreversible physical damage by cellular compression, membrane rupture and protein denaturation[34,35]. Cytotoxic free radicals are then released during the thawing process and consequent microvascular thrombosis and post-thaw ischaemia and infarction lead to cell death[36,37]. The use of cryotherapy for HCC has decreased since the advent of RFA and MWA as the complication rate associated with cryotherapy has been reported to be as high as 40%[2]. These include intraoperative hypothermia, bleeding caused by cracking of the liver, bile leak, abscess and “cryoshock”, a phenomenon of multiorgan failure and severe coagulopathy without evidence of sepsis[2].
IRE is a non-thermal ablation modality that delivers high-voltage, low energy direct current pulses to the target lesion. These bursts of current permeabilise the cell membrane by disrupting cellular homeostasis and forming “nanopores”, thereby inducing apoptosis of the target tumour cells[38]. The mechanism of cell death with IRE is predominantly non-thermal and hence is not compromised by the heat-sink effect. However, IRE is limited by the need of general anaesthesia and cardiac gating to prevent potentially serious cardiac arrhythmias[39]. LITT involves insertion of 1-4 fibres into the tumour, whereby neodymium-doped yttrium aluminium garnet and diode lasers penetrate tumour tissue and induce heat-induced cellular injury and coagulative necrosis[40]. Similar to RFA, LITT is limited by the heat-sink effect with treatment failure occurring at tumour periphery and in the region of highest tumour blood flow[41,42]. Currently, LITT is marginally used in clinical practice. Finally, HIFU uses either acoustic lenses or curved piezoelectric transducers to focus beams of low-frequency ultrasound (0.8-1.6 MHz) on target tissue generating an acoustic intensity sufficient to induce cellular damage[43]. Cell death can be obtained either by localised thermic effect which results in coagulative necrosis or, at higher acoustic intensities, by inertial cavitation whereby the frictional heat generated by oscillating microbubbles causes implosion with resultant tissue damage[44]. IRE, LITT and HIFU are all relatively novel ablative technologies and the overall experience with these techniques is limited.
Indications and efficacy
RFA is the most adopted technique for local ablation with complete response achieved in 70%-90% of cases after one or two sessions [45-48]. Cohort studies have shown that initial complete response was associated with significantly improved overall survival (P = 0.006)[49]. The overall survival after RFA ranges 40%-68% at 5 years and 27%-32% at 10 years[48,50-52] with the median overall survival of 60 months[53]. The crux of adequate treatment response with RFA is size of the tumour, with better response observed in tumours 2 cm and reduced response in tumours ≥ 2 cm[46,47,49,54].
Several randomised controlled trials (RCTs) have demonstrated the superiority of RFA to PEI in objective response rates and overall survival[24,55-57], as well as comparable survival rates with surgical resection in selected patients[58-61]. A meta-analysis of 4 RCTs including 574 patients comparing surgical resection with RFA in early HCC showed no statistical difference in all-cause mortality[62]. However, cancer-related mortality and recurrence were lower in the surgery group, while the RFA group had shorter hospital stay and lower adverse event rates[62]. An RCT comparing hepatectomy with RFA in 240 patients with recurrent HCC previously treated with curative resection showed that there was no statistical difference in overall or disease-free survival. Subgroup analyses revealed that although surgery carries significantly higher complication rate, it was associated with improved overall survival in HCCs > 3 cm and alpha fetoprotein (AFP) level > 200 ng/mL [61]. In time, the complication rate associated with surgery might decrease with growing numbers of resection performed by minimally invasive approach. In the meantime, given the available evidence, the AASLD, EASL and APASL guidelines all advocate RFA as the first-line therapy for solitary tumours < 2 cm. Both AASLD and EASL recommended RFA as an alternative to surgery in early-stage single tumour 3-4 cm or 2-3 tumours < 3 cm[8,11], whilst APASL recommends RFA as the first choice for local ablation as an alternative to surgery for resectable patients with Child-Pugh class A or B cirrhosis with 3 nodules all 3 cm[16].
Despite the theoretical advantage of MWA over RFA in its ability to achieve higher temperatures faster and being less prone to the heat-sink effect, several RCTs reported no difference between the two techniques in local tumour progression, treatment-related morbidity, overall and disease-free survivals[63-65]. Similarly, three meta-analyses comparing the two techniques showed similar efficacy, with a trend towards greater efficacy but higher complication rate in tumours > 3 cm treated with MWA compared with those treated with RFA[66-68]. Despite the low level of evidence to suggest superiority of MWA over RFA, MWA is widely adopted clinically to achieve comparable local control and survival in early HCC as acknowledged by the EASL guidelines[11]. Regarding other ablative techniques such as cryoablation[69], IRE, LITT and HIFU, current evidence is scarce and thus these techniques are not yet recommended as standard practice[18]. Selected randomised controlled trials of ablative therapies in the treatment of HCC are summarised in Table 1.
Randomised controlled trials of ablative therapies in HCC
| Author | Year | Patients | BCLC; Size | Experimental arms | Endpoints | Outcomes | |
| RFA vs. PEI | |||||||
| Lencioni et al.[55] | 2003 | 102 | A; 5 cm or three 3 cm | RFA (n = 52) vs. PEI (n = 50) | OS | (2 years) | 98% vs. 88%; HR: 0.20; 95%CI: 0.02-1.69; P = 0.13 |
| Lin et al.*[23] | 2005 | 187 | A; 3 cm | RFA (n = 62) vs. PEI (n = 62) | LR | (3 years) | 14% vs. 34%; HR: 0.35; 95%CI: 0.21-0.89; P = 0.01 |
| OS | (3 years) | 74% vs. 51%; HR: 0.42; 95%CI: 0.21-0.98; P = 0.03 | |||||
| Shiina et al.[24] | 2005 | 232 | A; 3 cm | RFA (n = 118) vs. PEI (n = 114) | OS | (4 years) | 74% vs. 57%; HR: 0.54; 95%CI: 0.33-0.89; P = 0.02 |
| Giorgio et al.[57] | 2011 | 285 | A; 3 cm | RFA (n = 128) vs. PEI (n = 143) | OS | (5 years) | 70% vs. 68%; HR: 0.81; 95%CI: 0.46-1.39; P = 0.45 |
| RFA vs. MWA | |||||||
| Yu et al.[63] | 2017 | 403 | A; 5 cm | RFA (n = 200) vs. MWA (n = 203) | LTP | (5 years) | 19.7% vs. 11.4%; P = 0.11 |
| Vietti Violi et al.[64] | 2018 | 152 | A; 4 cm | RFA (n = 76) vs. MWA (n = 76) | LTP | (2 years) | 12% vs. 6%; HR: 1.62; 95%CI: 0.66-3.94; P = 0.27 |
| RFA vs. Cryoablation | |||||||
| Wang et al.[69] | 2015 | 360 | A; 4 cm | RFA (n = 180) vs. CA (n = 180) | LTP | (3 years) | 11% vs. 7%; P = 0.043 |
| OS | (5 years) | 38% vs. 50%; P = 0.747 | |||||
TRANSCATHETER THERAPIES
Background
Intra-arterial therapies are centred on the principle of parenchymal arterialisation, sinusoidal capillarisation and the development of unpaired arteries that occur during HCC carcinogenesis[70], whereas normal hepatocytes receive dual blood supply predominantly from the portal vein. The objective of transarterial embolization is to selectively deprive arterial inflow to the tumour, which causes ischaemia-induced cellular membrane disruption resulting in ischaemic necrosis[71]. Although the arterial supply of the treated segment is compromised after embolization, a patent portal vein compensates hepatocyte perfusion, thereby decreasing injury to normal parenchyma[17]. Portal venous thrombosis or invasion therefore is a relative contraindication for transarterial embolization[72]. There are four main types of transarterial therapies: transarterial embolization (or “bland” embolization, TAE), transarterial chemoembolization (TACE), TACE using drug-eluting beads (DEB-TACE) and transarterial radioembolization (TARE).
TAE, TACE and DEB-TACE
TAE relies exclusively on occlusion of arterial supply to the tumour with embolic material such as gel foam or microparticles without the addition of chemotherapy or radiation[17]. Conventional TACE, on the other hand, involves direct injection of chemotherapeutic agents (either doxorubicin or cisplatin) mixed with lipiodol into the feeding hepatic artery branch followed by embolic material. Despite the common use of doxorubicin in TACE in the last three decades, there is no solid evidence demonstrating its superiority over other agents such as epirubicin, cisplatin or idarubicin[73]. Further, the rationale of chemotherapeutic agents is still debated as some studies suggested that bland embolization provides comparable survival and TACE may not be better than TAE[74-78]. Nonetheless, TACE has become the gold standard for the treatment of intermediate stage HCC, as discussed below. Recently, DEB-TACE has been adopted in many centres, and it involves the use of embolic microspheres (beads) loaded with chemotherapeutic agents. DEB-TACE has the advantage over conventional TACE in that it permits a sustained and controlled delivery of the therapeutic agent, increasing exposure time locally to the tumour while reducing systemic exposure [79], as pharmacokinetic studies have demonstrated that the plasma concentration of the chemotherapeutic agent after conventional TACE may approach that of systemic chemotherapy[80].
Transcatheter therapies are well tolerated with up to 50% of patients discharged on the first post-treatment day[81]. The most common side effect, experienced by up to 40% of patients, is post-embolization syndrome, which is a constellation of non-specific symptoms including nausea, fatigue, fever and abdominal pain and can last for up to 10 days post-treatment[82]. The severity and duration of post-embolization syndrome might be associated with the extent of ischaemia induced in normal parenchyma and the underlying liver function[83,84]. Other complications include liver decompensation, kidney injury, biliary injury, abscess formation, sepsis, gastrointestinal bleeding and embolization of non-target extrahepatic arterial supply, namely cystic artery to the gallbladder leading to ischaemic cholecystitis[85]. Rarer complications have been reported, including pulmonary embolization via undetected arteriovenous shunts within the tumour and/or small embolic particle size[86-88], as well as tumour lysis syndrome when rapid destruction of tumour cells can lead to renal insufficiency, metabolic disturbance, arrhythmias, seizures and a 30-day mortality of 1%[89,90].
TARE
In TARE, radioactive particles (commonly Yttrium-90 microspheres) are injected after selective cannulation of the hepatic artery branch. Yttrium-90 undergoes beta decay, thereby irradiating the tumour and causing cell death[91]. These beta particles have a short 2.4 mm tissue penetration and hence a high radiation dose is preferentially distributed in tumours compared to the adjacent healthy hepatocytes[92,93]. Pre-procedural angiographic mapping and planning are performed 1-2 weeks before to identify variant anatomy and intrahepatic portosystemic shunts, during which technetium-99m labelled macroaggregated albumin is injected into the hepatic artery followed by single-photon emission computed tomography[85]. Non-target vessels may be prophylactically embolised at pre-procedural angiography in order to prevent irradiation of non-target tissue at the time of treatment[94]. The complication rate is reported to be 1-5%, and these include radiation-induced pneumonitis, cholecystitis, and other gastrointestinal complications. Another complication is radioembolization-induced liver disease, a form of veno-occlusive disease characterised by jaundice and ascites up to eight weeks following treatment with an incidence as high as 15% in cirrhotic patients after TARE[95].
Indications and efficacy
Table 2 provides a summary of selected randomised controlled trials of transcatheter therapy in the treatment of HCC.
Randomised controlled trials of transcatheter therapies in HCC
| Author | Year | Patients | HCC stage/size | Experimental Arms | Endpoints | Outcomes |
| TACE | ||||||
| Llovet et al.[96] | 2002 | 112 | CP A/B Okuda I/II | TAE (n = 37) vs. TACE (n = 40) vs. BSC (n = 35) | OS (2 years) | 50% vs. 63% vs. 27% (TACE vs. BSC, P = 0.009) |
| Lo et al.[97] | 2002 | 80 | Okuda I/II | TACE (n = 40) vs. BSC (n = 40) | OS (3 years) | 26% vs. 3%; P = 0.002 |
| Kudo et al.[100] (BRISK-TA trial) | 2014 | 502 | CP A/B 5 cm | TACE or DEB-TACE plus brivanib (n = 249) vs. TACE plus placebo (n = 253) | OS | 26.4 months vs. 26.1 months HR: 0.90; 95%CI: 0.66-1.23; P = 0.53 |
| Lencioni et al.[143] (SPACE trial) | 2016 | 307 | BCLC B | DEB-TACE plus sorafenib (n = 154) vs. DEB-TACE plus placebo (n = 153) | TTP | 5.6 months vs. 5.5 months HR: 0.797; 95%CI: 0.59-1.08; P = 0.072 |
| Meyer et al.[99] (TACE-2 trial) | 2017 | 313 | BCLC B | DEB-TACE plus sorafenib (n = 157) vs. DEB-TACE plus placebo (n = 156) | PFS | 7.8 months vs. 7.7 months HR: 1.03; 95%CI: 0.75-1.42; P = 0.85 |
| Kudo et al.[104] (ORIENTAL trial) | 2018 | 889 | BCLC B | TACE plus orantinib (n = 445) vs. TACE plus placebo (n = 444) | OS | 31.1 months vs. 32.3 months HR: 1.09; 95%CI: 0.88-1.35; P = 0.435 |
| Ikeda et al.[105] | 2018 | 257 | LCSGJ TNM II/III | TACE with miriplatin (n = 129) vs. TACE with epirubicin (n = 128) | OS | 36.5 months vs. 37.1 months HR: 1.01; 95%CI: 0.73-1.40; P = 0.946 |
| Park et al.[159] (STAH trial) | 2019 | 339 | mUICC III/Iva/IVb | TACE plus sorafenib (n = 170) vs. sorafenib (n = 169) | OS | 12.8 months vs. 10.8 months HR: 0.91; 95%CI: 0.69-1.21; P = 0.29 |
| Kudo et al.[144] (TACTICS trial) | 2020 | 156 | BCLC B/C < 10 cm | TACE plus sorafenib (n = 80) vs. TACE (n = 76) | mPFS | 25.2 months vs. 13.5 months HR: 0.59; 95%CI: 0.41-0.87; P = 0.006 |
| TARE | ||||||
| Salem et al.[108] | 2016 | 45 | BCLC A/B | TARE (n = 24) vs. TACE (n = 21) | TTP | > 26 months vs. 6.8 months HR: 0.12; 95%CI: 0.027-0.55; P = 0.001 |
| Vilgrain et al.[114] (SARAH trial) | 2017 | 459 | CP A/B BCLC C | TARE (n = 237) vs. sorafenib (n = 222) | OS | 8 months vs. 9.9 months HR: 1.15; 95%CI: 0.94-1.41; P = 0.18 |
| Chow et al.[115] (SIRveNIB trial) | 2018 | 360 | BCLC B/C | TARE (n = 182) vs. sorafenib (n = 178) | OS | 8.8 months vs. 10.0 months HR: 1.12; 95%CI: 0.9-1.4; P = 0.36 |
| Ricke et al.[116] (SORAMIC trial) | 2019 | 424 | BCLC A/B/C | TARE plus sorafenib (n = 216) vs. sorafenib (n = 208) | OS | 12.1 months vs. 11.4 months HR: 1.01; 95%CI: 0.81-1.25; P = 0.95 |
TAE, TACE and DEB-TACE
The AASLD, EASL and APASL guidelines are consistent in recommending TACE for patients with intermediate stage HCC (BCLC stage B), defined as patients with multinodular disease, performance status 0, Child-Pugh class A or B cirrhosis and without portal vein invasion or extrahepatic disease[8,11,16]. These recommendations stem from the results of two landmark trials demonstrating significant benefit in overall survival for patients treated with TACE compared with best supportive care alone. The European trial, a multicentre trial of 112 patient in Barcelona (median tumour size 5 cm), demonstrated a significantly improved two-year survival of 63% in the TACE group compared to 25% in the best supportive care group (P = 0.009)[96]. The Asian trial, a single-centre trial of 80 patients in Hong Kong (median tumour size 7 cm), showed that TACE was associated with a marked tumour response, significantly improved survival (57% at one year, 31% at two years and 26% at three years in TACE group versus 32% at one year, 11% at two years and 3% at three years; P = 0.002) and a significant survival benefit (relative risk of death 0.49; 95%CI: 0.29-0.81; P = 0.006)[97]. A meta-analysis of seven RCTs including 545 patients showed that the two-year survival was significantly better with transarterial embolization compared to control (odds ratio (OR) 0.53; 95%CI: 0.32-0.89; P = 0.017). Further, sensitivity analysis showed a significant benefit of TACE with chemotherapeutic agents (OR 0.42; 95%CI: 0.20-0.88) over bland embolization alone (OR 0.59; 95%CI: 0.29-1.20)[98].
While the outcomes of TACE and DEB-TACE are more favourable than best supportive care in selected patients, the superiority of DEB-TACE over TACE is yet to be proven. Several trials assessing TACE and DEB-TACE reported median survival of 19-38 months with no significant difference in overall survival between the two techniques[96,97,99-105]. The PRECISION V study, however, demonstrated significant improvement in tumour response, reduction in serious liver toxicity and reduced doxorubicin-related adverse events in the DEB-TACE group compared to conventional TACE for selected patients (Child-Pugh class B, performance status 1, bilobar disease, recurrent disease)[106]. Despite these promising results, further research is required to assess the efficacy and cost-effectiveness of DEB-TACE[16].
Apart from the treatment of patients with intermediate stage HCC, TACE is the most common locoregional therapy used for downstaging or bridging treatment in order to meet or stay within the accepted transplant criteria[79]. There is currently no consensus on either the role or the optimal choice of locoregional therapy for downstaging, as non-comparative studies have not yet demonstrated superiority of one form of locoregional therapy over the other[79]. TACE is preferred over ablative therapies in some centres due to the small but real risk of tumour seeding associated with ablative therapies. In the United States, the United Network for Organ Sharing (UNOS) has set out its own HCC criteria for organ allocation and has stratified patients into UNOS stage 1 (one nodule, 1-2 cm), UNOS stage 2 (Milan criteria) and UNOS stage 3 (beyond Milan criteria). A recent review examined the role of locoregional therapy in HCC patients awaiting liver transplantation[79]. The authors concluded that, in UNOS stage 1 HCCs, locoregional therapy is only recommended for patients with high levels of AFP or rapidly enlarging lesions. For UNOS stage 2 HCCs, locoregional therapy is recommended if the waiting time is > 6 months, there is rapid tumour growth or when AFP > 500 ng/ml. Finally, for UNOS stage 3 HCCs, only well-selected patients would benefit from liver transplantation after successful downstaging, with acceptable survival and tumour recurrence rates.
TARE
The indications and contraindications of TARE are similar to those of TACE[85]. TARE has gained wide acceptance in many centres, particularly in the United States where there is institutional expertise and availability. A meta-analysis of five studies including 553 patients with unresectable HCC showed that TARE is a safe alternative to TACE with comparable complication profile, partial or complete response rates and survival rates[107]. In a randomised study of 45 HCC patients of BCLC stages A or B, TARE was found to provide significantly longer time to progression than conventional TACE (> 26 months vs. 6.8 months; P = 0.0012) with no difference in survival (18.6 months vs. 17.7 months; P = 0.99)[108]. In a prospective study of 1000 patients treated with TARE over 15 years, the overall survival for Child-Pugh class A and B patients was 47.3 and 27 months, respectively[109]. For patients with advanced HCC with portal vein thrombosis, cohort studies have shown that TARE is a safe approach as it does not have the macro-embolic effect that is seen in TACE[110-113]. For HCC patients with BCLC stage C, three RCTs designed for superiority testing have compared TARE with sorafenib with a primary endpoint of overall survival. The French SARAH trial[114] and the Asia-Pacific SIRveNIB trial[115] evaluated TARE versus sorafenib, whereas the European SORAMIC trial[116] compared TARE plus sorafenib versus sorafenib alone. All three studies failed to show survival benefit with TARE, either in isolation or in combination, over sorafenib alone, and hence TARE is not recommended in BCLC stage C by society guidelines[8,11,16]. However, emerging evidence on dosimetry in radioembolization has challenged these negative trials, in which no personalised dosimetry was used. The recently published DOSISPHERE-01 trial was a randomised, multicentre, open-label phase 2 trial, in which 56 patients with unresectable locally advanced HCC within strict selection criteria were randomised and treated with either standard dosimetry (120 20 Gy) targeted to the perfused lobe or personalised dosimetry ( 205 Gy) targeted to the index lesion[117]. Compared with standard dosimetry, personalised dosimetry had significantly improved objective response rate (71% vs. 36%, P = 0.0074) and comparable serious adverse events (Grade 3, 20% vs. 33%), suggesting personalised dosimetry is likely to improve outcomes in patients with unresectable locally advanced HCC[117].
Regarding the role of TARE in downstaging to meet transplant criteria, a retrospective single-centre study of 86 patients showed that it was significantly more likely to achieve downstaging with TARE compared with TACE (58% vs. 31%). In this study, both TARE and TACE showed a comparable time to progression (33.3 months vs. 18.2 months; P = 0.098), but TARE outperformed TACE in terms of partial response rates (61% vs. 37%), event-free survival (17.7 months vs. 7.1 months; P = 0.0017) and overall survival (41.6 months vs. 19.2 months; P = 0.008)[118]. Another study comparing post-transplant outcomes in 103 HCC patients within Milan criteria undergoing TARE or TACE as a bridge to transplantation demonstrated a trend towards improved three-year survival in the TARE group (92.9% vs. 75.7%; P = 0.052)[119]. Microvascular invasion, however, was statistically different between the two groups and was observed in 3.6% of explants in the TARE group as opposed to 27% in the TACE group (P = 0.013). Further, the number of treatment sessions is significantly lower in the TARE group (1.46% vs. 2.43%; P = 0.001) despite no difference in time on the waitlist.
The recently published LEGACY study substantiated the role of TARE in the management of HCC across different stages, from downstaging for the resection patient to bridging for the transplant patient and standalone treatment for the patient with BCLC stage C disease[120]. This multicentre, single-arm, retrospective study demonstrated the clinical efficacy of TARE in patients with solitary unresectable HCC < 8 cm and preserved performance status. In this study, 162 patients underwent TARE, 21% of whom as bridging therapy to transplantation, 6.8% as downstaging therapy to resection and the rest as standalone primary treatment. The objective response rate (best response) was 88% (CI: 82.4-92.4), with 62.2% (CI: 54.1-69.8) exhibiting a duration of response of 6 months[120]. The three-year overall survival was also promising, reported at 86.6% for all patients and 92.8% for those patients who had undergone neoadjuvant radioembolization with subsequent resection or transplantation[120].
RADIATION THERAPY
Background
Stereotactic body radiotherapy (SBRT) is a form of external beam radiotherapy that accurately delivers high-dose radiation in one or few treatment fractions to the target tumour while limiting the radiation dose to the adjacent non-target liver[17]. Despite the highly conformal treatment, some irradiation to normal liver parenchyma is inevitable. This results in radiation-induced liver disease, a veno-occlusive disease which has been observed in up to 44% of patients within three months with a mortality rate as high as 13%[121-124]. Other complications relate to irradiation of non-target tissue including ulceration, perforation, stenosis to adjacent hollow viscera, biliary injury and, rarely, injury to the spinal cord[17].
Indications and efficacy
For patients with early tumours, the AASLD guidelines accept SBRT as a treatment option with level 2 evidence[8], although neither EASL nor APASL recommends this therapy[11,16]. A recent meta-analysis comparing RFA and SBRT in 1755 patients with early HCC revealed that the two techniques achieved similar local disease control, but overall survival was significantly better in patients treated with RFA (OR:1.43; 95%CI:1.05-1.95; P = 0.023)[125]. The two-year local disease control with SBRT as primary treatment has been reported to be up to 81% by several non-comparative studies and small RCTs[126]. For patients with intermediate HCC, the evidence for SBRT is limited and conflicting. A cohort study comparing TACE with SBRT in 188 patients with medium-sized HCC (3-8 cm) demonstrated comparable local control and survival[127]. Another propensity score-matched study of 209 patients favoured SBRT over TACE in terms of two-year local control (91% vs. 23%; HR:66.5; P = 0.008) and toxicity (8% vs.13%; P = 0.05), but they had similar overall survival[128]. For patients with advanced HCC, an RCT comparing TACE plus SBRT versus sorafenib alone in 90 patients with macrovascular invasion showed that the combination group had improved progression-free survival, objective response rate, time to progression and overall survival[129]. Further, SBRT has been used in place of TACE as a bridging therapy to transplantation. A retrospective study of 379 HCC patients (44% within Milan criteria) who had undergone SBRT, RFA or TACE showed that the overall five-year survival (61% for SBRT and RFA and 56% for TACE) and dropout rates from the transplant waiting list (SBRT 16.7%, RFA 16.8% and TACE 20.2%) were similar for all three treatment modalities[130]. This study suggests that SBRT is a safe and feasible alternative for selected patients with HCC as a bridge to liver transplantation.
COMBINATION THERAPIES
As RFA is the only locoregional therapy that can achieve complete response and comparable survival rates as surgical resection in selected patients, much research has been dedicated to the utility of combination therapies with the goal to improve observed response rate and survival rates in patients with intermediate or advanced HCC. Ongoing trials investigating the combination of locoregional therapies plus immune therapies and systemic therapies are summarised in Table 3.
Current trials for combination therapy in HCC
| Trial | NCT Identifier | Phase | BCLC stage | Patients | Progress | Primary outcome | LR therapy | Immune/ Systemic therapy |
| TACE + immune/systemic | ||||||||
| EMERALD-1 | NCT03778957 | 3 | B/C | 710 | Recruiting | PFS | TACE | Durvalumab Bevacizumab |
| LEAP-012 | NCT04246177 | 3 | B/C | 950 | Recruiting | OS/PFS | TACE | Pembrolizumab + Lenvatinib |
| TACE-3 | NCT04268888 | 2/3 | B | 522 | Recruiting | OS/TTP | TACE/TAE | Nivolumab |
| CheckMate 74W | NCT04340193 | 3 | B | 26 | Active, not recruiting | OS/TTP | TACE | Nivolumab Ipilimumab |
| PETAL | NCT03397654 | 1/2 | B | 26 | Recruiting | AE | TACE | Pembrolizumab |
| IMMUTACE | NCT03572582 | 2 | B | 49 | Active, not recruiting | ORR | TACE | Nivolumab |
| TACE + IO | NCT03638141 | 2 | B | 30 | Recruiting | ORR | DEB-TACE | Durvalumab + Tremelimumab |
| DEB-TACE + IO | NCT03143270 | 1 | B | 14 | Recruiting | AE | DEB-TACE | Nivolumab |
| TARE + immune/systemic | ||||||||
| NASIR-HCC | NCT03380130 | 2 | B/C | 41 | Completed | AE | TARE | Nivolumab |
| HCRN: GI15-225 | NCT03099564 | 1 | B/C | 30 | Completed | PFS | TARE | Pembrolizumab |
| Y90 TARE + IO | NCT04541173 | 2 | B | 128 | Recruiting | PFS | TARE | Bevacizumab + Atezolizumab |
| SBRT + immune/systemic | ||||||||
| RTOG-1112 | NCT01730937 | 3 | B/C | 193 | Active, not recruiting | OS | SBRT | Sorafenib |
| ISBRT01 | NCT04167293 | 2/3 | C | 116 | Recruiting | PFS | SBRT+ TACE/HAI | Sintilimab |
Combination of different locoregional therapies
Several studies have demonstrated that combination therapy of RFA and TACE is superior to monotherapy of either technique alone. The hypothesis for improved efficacy in cases where RFA is performed before TACE is that the increased blood flow and vascular permeability observed in areas of sublethal heating at the peripheries of the ablation zone would facilitate delivery of the chemotherapeutic agent at relatively higher concentrations[131]. In cases where TACE is performed before RFA, the heat-sink effect is decreased by limiting hepatic arterial flow induced by TACE, thereby yielding a more complete central necrosis by RFA, especially for tumours > 3 cm[132]. A retrospective study of 83 patients with solitary tumours measured 2-3 cm showed that the one-, three and five-year local tumour progression-free survival rates were significantly higher in the combination group (95%, 86% and 83%, respectively) compared to the RFA-alone group (78%, 61% and 53%, respectively; P < 0.001), although the overall survival and major complication rates were similar[133]. A single-centre RCT comparing RFA plus TACE versus RFA alone in 189 patients with HCC < 7 cm showed a significantly better overall survival (P = 0.002) and recurrence-free survival (P = 0.009) in the combination group[134]. Similarly, another single-centre RCT comparing RFA plus intra-tumoral iodine-125 versus RFA alone in 136 patients with HCC 3 cm showed significant differences in overall survival (P = 0.003) and cumulative recurrence (P = 0.004), again favouring combination therapy[135]. Despite such encouraging findings, the lack of high-level evidence meant that adoption of combination locoregional therapies as standard clinical practice is yet to be realised.
Combination of locoregional therapy with immune therapies and systemic therapies
For years, sorafenib was the only systemic agent available for the treatment of unresectable HCC. The emergence of new immune therapy and systemic therapy agents has changed the landscape of HCC management. Currently, immune checkpoint inhibitors such as anti-PD1 (nivolumab, pembrolizumab and nivolumab plus ipilimumab) and anti-PDL1 (atezolizumab), as well as anti-VEGF (bevacizumab), antibodies have been approved only for the treatment of advanced HCC[19]. The rationale for combining locoregional therapy with immunotherapy is that locoregional therapies have an immune modulation effect on the tumour microenvironment, transforming the inherent immunosuppressive nature of the liver microenvironment into an immuno-supportive niche, in which checkpoint inhibitor therapy might be more effective[18]. There are multiple pre-clinical and clinical studies testing the feasibility and efficacy of combining immune checkpoint blockade with locoregional therapies. Several preclinical studies involved loading tyrosine kinase inhibitors (e.g., sunitinib and vandetanib) and anti-VEGF antibodies onto drug-eluting beads and have shown promising results in halting tumour growth[136-139]. In vitro and animal studies of HCC are also underway to investigate the combination of innate and adaptive immune therapies with RFA, MWA and cryoablation[140].
Several clinical trials investigating the efficacy of combining locoregional therapy and systemic therapies have so far been disappointing. The HEAT study, a global randomised, double-blind, dummy-controlled trial, compared RFA plus intravenous lyso-thermosensitive liposomal doxorubicin (LTLD) versus RFA alone[141]. This study recruited 701 patients with early HCC and showed no statistical difference in either progression-free survival or overall survival. Another phase III trial, the OPTIMA study, explored whether the addition of LTLD to RFA lasting over 45 min in solitary tumours would increase overall survival compared to RFA alone, was terminated early due to futility[18]. TACE in combination with systemic therapy for the treatment of intermediate HCC has also been subject of several clinical trials. Two European trials, the TACE2 and SPACE trials, tested the combination of DEB-TACE plus sorafenib against DEB-TACE alone and placebo, respectively. The TACE2 trial (294 patients) showed that combination therapy did not significantly improve progression-free survival or overall survival and interim futility analysis led to trial termination[142]. The SPACE trial (307 patients) showed that, although combination therapy was technically feasible, it did not improve time to tumour progression in a meaningful manner compared to DEB-TACE alone[143]. Another phase II RCT, the Japanese TACTICS trial, included 156 patients with unresectable HCC and compared TACE plus sorafenib versus TACE alone[144]. The combination group was shown to have significantly better progression-free survival compared to the TACE alone group (25.2 months vs. 13.5 months; P = 0.006). However, in this study, the definition of progression-free survival was unconventional and was defined as time to untreatable (unTACEable) progression. Further, overall survival was not analysed as only 73.6% of overall survival events were reached. There is therefore insufficient evidence to recommend the use of sorafenib in combination with TACE in the treatment of advanced HCC at present and alternative strategies, namely combining immune therapy with TACE, are being explored. Ongoing trials examining TACE plus immune therapy include the EMERALD-1, LEAP-012, CheckMate 74W and TACE-3 trials[18,53].
While the goal of combining locoregional therapy with other immune/systemic therapies is to improve the observed response rate locally to the target lesion, the rationale for neoadjuvant and adjuvant therapy is to reduce the risk of recurrence including intrahepatic recurrence, de novo HCC and appearance of extrahepatic metastatic lesions. The role of immune therapies and systemic therapies in addition to resection or locoregional therapy in both neoadjuvant and adjuvant settings is an area of great research interest and has been comprehensively reviewed in two recent reviews[145,146] and therefore is not repeated here.
ASSESSMENT OF TREATMENT RESPONSE
Assessing treatment response after locoregional therapy is critical for determining prognosis and informing future management. The goal of post-treatment imaging is to recognise residual or recurrent tumour and to identify complications of therapy[17]. Computed tomography (CT) and magnetic resonance imaging (MRI) are the mainstay of HCC diagnosis and in assessing treatment response. It is recognised that interpretation of post-treatment imaging findings can be challenging as it depends on the type(s) of treatment, the number of rounds of treatment, the magnitude of treatment response, the time interval after treatment and the cumulative effect of therapy on underlying liver function[17]. The World Health Organisation (WHO) was the first to develop treatment response criteria by assessing tumour burden based on size[147]. This was then improved by the Response Evaluation Criteria in Solid Tumours (RECIST) which clarified size measurement by using the sum of the longest diameters of target measurable lesions based on a one-dimensional measurement only[148]. Meanwhile, as locoregional therapies were developed to target arterialisation of HCC, it was recognised that such locoregional therapies result in significant changes, i.e., necrosis, within the tumour but have little effect on tumour size. New criteria, namely modified RECIST (mRECIST)[149], EASL criteria [142] and the Liver Imaging Reporting and Data System (LI-RADS) Treatment Response (LR-TR) algorithm[150], emerged to focus on the viable parts of tumours hence providing more appropriate feedback to guide patient management. Table 4 provides a summary of the treatment response based on the abovementioned criteria. The following discussion focuses on mRECIST and LR-TR as most clinicians and researchers use mRECIST rather than EASL criteria for the assessment of HCC after locoregional therapy as it is reproducible and simpler[147,151], while LR-TR is consistent with and fully integrated into the AASLD clinical practice guidelines[8].
Assessment of HCC response to therapy according to image-based response criteria
| Whole tumour size-based criteria | Viable tumour size-based criteria | Viable tumour appearance-based criteria | |||
| WHO | RECIST 1.0 & 1.1 | mRECIST | EASL | LR-TR* | |
| Complete response | Disappearance of all lesions | Disappearance of all target lesions RECIST 1.1 added: disappearance of pathological lymph nodes | Disappearance of intratumoral arterial enhancement and pathological lymph nodes | Disappearance of any intratumoral enhancement (arterial and portal) in all target lesion(s) | LR-TR Nonviable: No lesional enhancement, or treatment-specific expected enhancement pattern |
| Partial response | 50% decrease in sum of cross-product of target lesion(s) | 30% decrease in sum of maximum diameter of target lesion(s) | 30% decrease in sum of diameters of viable (arterial enhancing) target lesion(s) | 50% decrease in sum of diameters of viable target lesion(s) | LR-TR Equivocal: Enhancement atypical for treatment-specific expected enhancement pattern and not meeting criteria for probably viable or definitely viable LR-TR Viable: Nodular, mass-like, or thick irregular tissue in or along the treated lesion with any of the following: APHE, or washout, or enhancement similar to pre-treatment |
| Stable disease | Neither PR nor PD | Neither PR nor PD | Neither PR nor PD | Neither PR nor PD | Not stated |
| Progressive disease | > 25% increase in sum of cross-product of target lesion(s) | > 20% increase in sum of diameters RECIST 1.1 added: must have at least 5mm absolute increase in sum | > 20% increase in sum of diameters of viable (arterial enhancing) target lesion(s) | 25% increase in sum of enhancing area or appearance of new lesion | Not stated |
The LR-TR algorithm categorises response of HCC to locoregional therapy, which are non-evaluable, non-viable, equivocal and viable[150]. Unlike RECIST criteria that focus on disease progression on a systemic level, the focus of the LR-TR algorithm is on a lesion level, whereby the enhancement pattern of the tumour is assessed for viability[152]. Although mRECIST has historically been used for the assessment of HCC after locoregional therapy, it differs from the LR-TR algorithm in that it lacks the “equivocal” category and the additional features for diagnosing tumour viability[149]. mRECIST uses solely the presence of arterial phase hyperenhancement (APHE) to diagnose viability, while the LR-TR algorithm incorporates additional features namely washout and enhancement similar to pre-treatment [150]. A recent meta-analysis of 10 studies comprising of 971 patients assessing the accuracy of each imaging feature of the LR-TR viable category showed that the pooled sensitivity and diagnostic odds ratio were the highest for APHE, followed by washout appearance and then enhancement similar to pre-treatment[153]. It also showed that the diagnostic performance of APHE was significantly different depending on the type of reference standard and MRI contrast agent[153]. Consensus is yet to be reached on whether the LR-TR algorithm is at least equivalent, if not superior, in predicting HCC viability after locoregional therapy compared to mRECIST. A recent retrospective study of 52 cirrhotic patients with 71 lesions showed that the LR-TR algorithm demonstrated high specificity and low to moderate sensitivity for the detection of viable HCC after TACE, without significant difference in diagnostic performance between the LR-TR algorithm and mRECIST[154]. Another retrospective study included 114 patients with 206 lesions who underwent liver transplantation after locoregional therapy and liver explants were used as reference. This study found that the LR-TR algorithm showed better diagnostic performance than mRECIST on CT, whereas the LR-TR algorithm and mRECIST showed comparable performance on MRI[155].
It is beyond the scope of this review to discuss in detail the technical requirements for post-treatment CT and MRI and the diagnostic performance of mRECIST and the LR-TR algorithm, but a few salient points of response assessment are discussed here. First, for patients treated with TACE, lipiodol retention may mask enhancement at surveillance CT and MRI should be considered in challenging cases[17]. Second, subtraction images in MRI may be helpful in differentiating true enhancement of viable tumour from pseudo-enhancement caused by locoregional therapy induced coagulative necrosis[17]. Third, complete radiological response after locoregional therapy is not equivalent to complete pathological response, due to the presence of tumour microsatellites within the cancer niche that are undetectable by imaging[18]. Fourth, perilesional APHE in the early post-treatment period after radiation therapy (SBRT and TARE) could be due to pseudoprogression, a phenomenon when tumour size and enhancement are transiently increased, which can be challenging to distinguish between true residual tumour viability[17,156]. Finally, assessment of treatment response should be delayed for > 3 months if immunotherapy is given in combination, again due to the phenomenon of pseudoprogression[157,158]
CONCLUSIONS
HCC is the sixth most common cancer and the third cause of cancer-related deaths worldwide. Although overall survival has improved in recent years, prognosis is still poor for patients with advanced disease. Surgical resection and liver transplantation remain the cornerstone for cure in early-stage disease, but there is an expanding role of locoregional therapies in the management of HCC. Ablative therapies have become the first-line treatment, as recommended by society guidelines for non-surgical patients with very early HCC achieving curative outcomes, while transarterial embolization techniques offer major benefit for patients with intermediate HCC. Although patients with advanced HCC are currently limited to systemic therapy, the combination of locoregional and immune therapies shows promise in HCC management.
DECLARATIONS
Authors’ contributionsThe main text of the manuscript was written by Janet WC Kung. Kelvin KC Ng helped edit the manuscript. Both authors contributed to the final version of the manuscript.
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49.
2. Ng KK, Lam CM, Poon RT, Ai V, Tso WK, Fan ST. Thermal ablative therapy for malignant liver tumors: a critical appraisal. J Gastroenterol Hepatol 2003;18:616-29.
3. Roayaie S, Jibara G, Tabrizian P, et al. The role of hepatic resection in the treatment of hepatocellular cancer. Hepatology 2015;62:440-51.
4. Tsilimigras DI, Mehta R, Guglielmi A, et al. Recurrence beyond the Milan criteria after curative-intent resection of hepatocellular carcinoma: a novel tumor-burden based prediction model. J Surg Oncol 2020;122:955-63.
5. Wei T, Zhang XF, Xue F, et al. Multi-institutional development and external validation of a nomogram for prediction of extrahepatic recurrence after curative-intent resection for hepatocellular carcinoma. Ann Surg Oncol 2021;28:7624-33.
6. Portolani N, Coniglio A, Ghidoni S, et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg 2006;243:229-35.
7. Zhou Y, Sui C, Li B, et al. Repeat hepatectomy for recurrent hepatocellular carcinoma: a local experience and a systematic review. World J Surg Oncol 2010;8:55.
8. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358-80.
9. Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2016;2:16018.
10. Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology 2019;156:477-91.e1.
11. Association for the Study of the Liver, Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182-236.
12. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the american association for the study of liver diseases. Hepatology 2018;68:723-50.
13. Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE study. Liver Int 2015;35:2155-66.
14. Grieco A, Pompili M, Caminiti G, et al. Prognostic factors for survival in patients with early-intermediate hepatocellular carcinoma undergoing non-surgical therapy: comparison of Okuda, CLIP, and BCLC staging systems in a single Italian centre. Gut 2005;54:411-8.
15. Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong liver cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology 2014;146:1691-700.e3.
16. Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 2017;11:317-70.
17. Voizard N, Cerny M, Assad A, et al. Assessment of hepatocellular carcinoma treatment response with LI-RADS: a pictorial review. Insights Imaging 2019;10:121.
18. Llovet JM, De Baere T, Kulik L, et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2021;18:293-313.
19. Llovet JM, Villanueva A, Marrero JA, et al. AASLD Panel of Experts on Trial Design in HCC. Trial design and endpoints in hepatocellular carcinoma: AASLD consensus conference. Hepatology 2021;73 Suppl 1:158-91.
20. Sasaki A, Kai S, Iwashita Y, Hirano S, Ohta M, Kitano S. Microsatellite distribution and indication for locoregional therapy in small hepatocellular carcinoma. Cancer 2005;103:299-306.
21. Lee MJ, Mueller PR, Dawson SL, et al. Percutaneous ethanol injection for the treatment of hepatic tumors: indications, mechanism of action, technique, and efficacy. AJR Am J Roentgenol 1995;164:215-20.
22. Joseph FB, Baumgarten DA, Bernardino ME. Hepatocellular carcinoma: CT appearance after percutaneous ethanol ablation therapy. Work in progress. Radiology 1993;186:553-6.
23. Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut 2005;54:1151-6.
24. Shiina S, Teratani T, Obi S, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology 2005;129:122-30.
25. Minami Y, Kudo M. Radiofrequency ablation of hepatocellular carcinoma: a literature review. Int J Hepatol 2011;2011:104685.
26. Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol 2000;174:323-31.
27. Lin ZY, Li GL, Chen J, Chen ZW, Chen YP, Lin SZ. Effect of heat sink on the recurrence of small malignant hepatic tumors after radiofrequency ablation. J Cancer Res Ther 2016;12:C153-8.
28. Poulou LS, Botsa E, Thanou I, Ziakas PD, Thanos L. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Hepatol 2015;7:1054-63.
29. Poggi G, Tosoratti N, Montagna B, Picchi C. Microwave ablation of hepatocellular carcinoma. World J Hepatol 2015;7:2578-89.
30. Bertot LC, Sato M, Tateishi R, Yoshida H, Koike K. Mortality and complication rates of percutaneous ablative techniques for the treatment of liver tumors: a systematic review. Eur Radiol 2011;21:2584-96.
31. Nakagomi R, Tateishi R, Shiina S, et al. Drastically reduced neoplastic seeding related to radiofrequency ablation for hepatocellular carcinoma. Am J Gastroenterol 2014;109:774-6.
32. Yu J, Liang P, Yu XL, Cheng ZG, Han ZY, Dong BW. Needle track seeding after percutaneous microwave ablation of malignant liver tumors under ultrasound guidance: analysis of 14-year experience with 1462 patients at a single center. Eur J Radiol 2012;81:2495-9.
33. Wang CC, Kao JH. Artificial ascites is feasible and effective for difficult-to-ablate hepatocellular carcinoma. Hepatol Int 2015;9:514-9.
34. Bischof JC, Rubinsky B. Large ice crystals in the nucleus of rapidly frozen liver cells. Cryobiology 1993;30:597-603.
35. Whittaker DK. Mechanisms of tissue destruction following cryosurgery. Ann R Coll Surg Engl 1984;66:313-8.
36. Brown NJ, Bayjoo P, Reed MW. Effect of cryosurgery on liver blood flow. Br J Cancer 1993;68:10-2.
37. Rubinsky B, Lee C, Bastacky J, Onik G. The process of freezing and the mechanism of damage during hepatic cryosurgery. Cryobiology 1990;27:85-97.
38. Lencioni R, Crocetti L, Narayanan G. Irreversible electroporation in the treatment of hepatocellular carcinoma. Tech Vasc Interv Radiol 2015;18:135-9.
39. O'Neill CH, Martin RCG 2nd. Cardiac synchronization and arrhythmia during irreversible electroporation. J Surg Oncol 2020;122:407-11.
40. Nikfarjam M, Christophi C. Interstitial laser thermotherapy for liver tumours. Br J Surg 2003;90:1033-47.
41. Muralidharan V, Malcontenti-Wilson C, Christophi C. Effect of blood flow occlusion on laser hyperthermia for liver metastases. J Surg Res 2002;103:165-74.
42. Liu DL, Svanberg K, Wang I, Andersson-engels S, Svanberg S. Laser doppler perfusion imaging: New technique for determination of perfusion and reperfusion of splanchnic organs and tumor tissue. Lasers Surg Med 1997;20:473-9.
43. Diana M, Schiraldi L, Liu YY, et al. High intensity focused ultrasound (HIFU) applied to hepato-bilio-pancreatic and the digestive system-current state of the art and future perspectives. Hepatobiliary Surg Nutr 2016;5:329-44.
44. Zhou Y, Gao XW. Variations of bubble cavitation and temperature elevation during lesion formation by high-intensity focused ultrasound. J Acoust Soc Am 2013;134:1683-94.
45. Lam VW, Ng KK, Chok KS, et al. Risk factors and prognostic factors of local recurrence after radiofrequency ablation of hepatocellular carcinoma. J Am Coll Surg 2008;207:20-9.
46. Lee DH, Lee JM, Lee JY, et al. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology 2014;270:900-9.
47. Lencioni R, Cioni D, Crocetti L, et al. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology 2005;234:961-7.
48. N'Kontchou G, Mahamoudi A, Aout M, et al. Radiofrequency ablation of hepatocellular carcinoma: long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology 2009;50:1475-83.
49. Sala M, Llovet JM, Vilana R, et al. Barcelona Clínic Liver Cancer Group. Initial response to percutaneous ablation predicts survival in patients with hepatocellular carcinoma. Hepatology 2004;40:1352-60.
50. Rossi S, Ravetta V, Rosa L, et al. Repeated radiofrequency ablation for management of patients with cirrhosis with small hepatocellular carcinomas: a long-term cohort study. Hepatology 2011;53:136-47.
51. Brunello F, Veltri A, Carucci P, et al. Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: a randomized controlled trial. Scand J Gastroenterol 2008;43:727-35.
52. Shiina S, Tateishi R, Arano T, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol 2012;107:569-77; quiz 578.
53. Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021;7:6.
54. Livraghi T, Meloni F, Di Stasi M, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology 2008;47:82-9.
55. Lencioni RA, Allgaier HP, Cioni D, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology 2003;228:235-40.
56. Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or = 4 cm. Gastroenterology 2004;127:1714-23.
57. Giorgio A, Di Sarno A, De Stefano G, Scognamiglio U, Farella N, Mariniello A, Esposito V, Coppola C, Giorgio V. Percutaneous radiofrequency ablation of hepatocellular carcinoma compared to percutaneous ethanol injection in treatment of cirrhotic patients: an Italian randomized controlled trial. Anticancer Res 2011;31:2291-5.
58. Ng KKC, Chok KSH, Chan ACY, et al. Randomized clinical trial of hepatic resection versus radiofrequency ablation for early-stage hepatocellular carcinoma. Br J Surg 2017;104:1775-84.
59. Xu XL, Liu XD, Liang M, Luo BM. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma: systematic review of randomized controlled trials with meta-analysis and trial sequential analysis. Radiology 2018;287:461-72.
60. Izumi N, Hasegawa K, Nishioka Y, et al. A multicenter randomized controlled trial to evaluate the efficacy of surgery vs. radiofrequency ablation for small hepatocellular carcinoma (SURF trial). Journal of Clinical Oncology 2019:37.
61. Xia Y, Li J, Liu G, et al. Long-term effects of repeat hepatectomy vs percutaneous radiofrequency ablation among patients with recurrent hepatocellular carcinoma: a randomized clinical trial. JAMA Oncol 2020;6:255-63.
62. Majumdar A, Roccarina D, Thorburn D, Davidson BR, Tsochatzis E, Gurusamy KS. Management of people with early- or very early-stage hepatocellular carcinoma: an attempted network meta-analysis. Cochrane Database Syst Rev 2017;3:CD011650.
63. Yu J, Yu XL, Han ZY, et al. Percutaneous cooled-probe microwave versus radiofrequency ablation in early-stage hepatocellular carcinoma: a phase III randomised controlled trial. Gut 2017;66:1172-3.
64. Vietti Violi N, Duran R, Guiu B, et al. Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: a randomised controlled phase 2 trial. The Lancet Gastroenterology & Hepatology 2018;3:317-25.
65. Chong CCN, Lee KF, Cheung SYS, et al. Prospective double-blinded randomized controlled trial of Microwave versus RadioFrequency Ablation for hepatocellular carcinoma (McRFA trial). HPB (Oxford) 2020;22:1121-7.
66. Tan W, Deng Q, Lin S, Wang Y, Xu G. Comparison of microwave ablation and radiofrequency ablation for hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia 2019;36:264-72.
67. Glassberg MB, Ghosh S, Clymer JW, et al. Microwave ablation compared with radiofrequency ablation for treatment of hepatocellular carcinoma and liver metastases: a systematic review and meta-analysis. Onco Targets Ther 2019;12:6407-38.
68. Facciorusso A, Di Maso M, Muscatiello N. Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia 2016;32:339-44.
69. Wang C, Wang H, Yang W, et al. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology 2015;61:1579-90.
70. Ooka Y, Kanai F, Okabe S, et al. Gadoxetic acid-enhanced MRI compared with CT during angiography in the diagnosis of hepatocellular carcinoma. Magn Reson Imaging 2013;31:748-54.
71. Brown KT, Nevins AB, Getrajdman GI, et al. Particle embolization for hepatocellular carcinoma. J Vasc Interv Radiol 1998;9:822-8.
72. Yasui D, Murata S, Ueda T, et al. Novel treatment strategy for advanced hepatocellular carcinoma: combination of conventional transcatheter arterial chemoembolization and modified method with portal vein occlusion for cases with arterioportal shunt: a preliminary study. Acta Radiol 2018;59:266-74.
73. Roth GS, Teyssier Y, Abousalihac M, et al. Idarubicin vs doxorubicin in transarterial chemoembolization of intermediate stage hepatocellular carcinoma. World J Gastroenterol 2020;26:324-34.
74. Guan YS, He Q, Wang MQ. Transcatheter arterial chemoembolization: history for more than 30 years. ISRN Gastroenterol 2012;2012:480650.
75. Marelli L, Stigliano R, Triantos C, et al. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? Cardiovasc Intervent Radiol 2007;30:6-25.
76. Pleguezuelo M, Marelli L, Misseri M, et al. TACE versus TAE as therapy for hepatocellular carcinoma. Expert Rev Anticancer Ther 2008;8:1623-41.
77. Miraglia R, Pietrosi G, Maruzzelli L, et al. Efficacy of transcatheter embolization/chemoembolization (TAE/TACE) for the treatment of single hepatocellular carcinoma. World J Gastroenterol 2007;13:2952-5.
78. Bruix J, Llovet JM, Castells A, et al. Transarterial embolization versus symptomatic treatment in patients with advanced hepatocellular carcinoma: results of a randomized, controlled trial in a single institution. Hepatology 1998;27:1578-83.
79. Thuluvath PJ, To C, Amjad W. Role of locoregional therapies in patients with hepatocellular cancer awaiting liver transplantation. Am J Gastroenterol 2021;116:57-67.
80. Varela M, Real MI, Burrel M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol 2007;46:474-81.
81. Brown KT, Do RK, Gonen M, et al. Randomized trial of hepatic artery embolization for hepatocellular carcinoma using doxorubicin-eluting microspheres compared with embolization with microspheres alone. J Clin Oncol 2016;34:2046-53.
82. Sangro B. Chemoembolization and radioembolization. Best Pract Res Clin Gastroenterol 2014;28:909-19.
83. Wigmore SJ, Redhead DN, Thomson BN, et al. Postchemoembolisation syndrome--tumour necrosis or hepatocyte injury? Br J Cancer 2003;89:1423-7.
84. Paye F, Farges O, Dahmane M, Vilgrain V, Flejou JF, Belghiti J. Cytolysis following chemoembolization for hepatocellular carcinoma. Br J Surg 1999;86:176-80.
85. Makary MS, Khandpur U, Cloyd JM, Mumtaz K, Dowell JD. Locoregional therapy approaches for hepatocellular carcinoma: recent advances and management strategies. Cancers (Basel) 2020;12:1914.
86. Chan AO, Yuen MF, Hui CK, Tso WK, Lai CL. A prospective study regarding the complications of transcatheter intraarterial lipiodol chemoembolization in patients with hepatocellular carcinoma. Cancer 2002;94:1747-52.
87. Garwood ER, Fidelman N, Hoch SE, Kerlan RK Jr, Yao FY. Morbidity and mortality following transarterial liver chemoembolization in patients with hepatocellular carcinoma and synthetic hepatic dysfunction. Liver Transpl 2013;19:164-73.
88. Wu GC, Perng WC, Chen CW, Chian CF, Peng CK, Su WL. Acute respiratory distress syndrome after transcatheter arterial chemoembolization of hepatocellular carcinomas. Am J Med Sci 2009;338:357-60.
89. Zivin SP, Elias Y, Ray CE Jr. Tumor lysis syndrome and primary hepatic malignancy: case presentation and review of the literature. Semin Intervent Radiol 2015;32:3-9.
90. Brown DB, Geschwind JF, Soulen MC, Millward SF, Sacks D. Society of Interventional Radiology position statement on chemoembolization of hepatic malignancies. J Vasc Interv Radiol 2009;20:S317-23.
91. Bhangoo MS, Karnani DR, Hein PN, et al. Radioembolization with Yttrium-90 microspheres for patients with unresectable hepatocellular carcinoma. J Gastrointest Oncol 2015;6:469-78.
92. Kim YC, Kim YH, Uhm SH, et al. Radiation safety issues in y-90 microsphere selective hepatic radioembolization therapy: possible radiation exposure from the patients. Nucl Med Mol Imaging 2010;44:252-60.
93. Kennedy AS, Nutting C, Coldwell D, Gaiser J, Drachenberg C. Pathologic response and microdosimetry of (90)Y microspheres in man: review of four explanted whole livers. Int J Radiat Oncol Biol Phys 2004;60:1552-63.
94. Sangro B, Iñarrairaegui M, Bilbao JI. Radioembolization for hepatocellular carcinoma. J Hepatol 2012;56:464-73.
95. Gil-Alzugaray B, Chopitea A, Iñarrairaegui M, et al. Prognostic factors and prevention of radioembolization-induced liver disease. Hepatology 2013;57:1078-87.
96. Llovet JM, Real MI, Montaña X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002;359:1734-9.
97. Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002;35:1164-71.
98. Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 2003;37:429-42.
99. Meyer T, Fox R, Ma YT, et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. The Lancet Gastroenterology & Hepatology 2017;2:565-75.
100. Kudo M, Han G, Finn RS, et al. Brivanib as adjuvant therapy to transarterial chemoembolization in patients with hepatocellular carcinoma: a randomized phase III trial. Hepatology 2014;60:1697-707.
101. Okusaka T, Kasugai H, Shioyama Y, et al. Transarterial chemotherapy alone versus transarterial chemoembolization for hepatocellular carcinoma: a randomized phase III trial. J Hepatol 2009;51:1030-6.
102. Yu SC, Hui JW, Hui EP, et al. Unresectable hepatocellular carcinoma: randomized controlled trial of transarterial ethanol ablation versus transcatheter arterial chemoembolization. Radiology 2014;270:607-20.
103. Golfieri R, Giampalma E, Renzulli M, et al. PRECISION ITALIA STUDY GROUP. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer 2014;111:255-64.
104. Kudo M, Cheng A, Park J, et al. Orantinib versus placebo combined with transcatheter arterial chemoembolisation in patients with unresectable hepatocellular carcinoma (ORIENTAL): a randomised, double-blind, placebo-controlled, multicentre, phase 3 study. Lancet Gastroenterology & Hepatology 2018;3:37-46.
105. Ikeda M, Kudo M, Aikata H, et al. ; Miriplatin TACE Study Group. Transarterial chemoembolization with miriplatin vs. epirubicin for unresectable hepatocellular carcinoma: a phase III randomized trial. J Gastroenterol 2018;53:281-90.
106. Lammer J, Malagari K, Vogl T, et al. PRECISION V Investigators. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol 2010;33:41-52.
107. Lobo L, Yakoub D, Picado O, et al. Unresectable hepatocellular carcinoma: radioembolization versus chemoembolization: a systematic review and meta-analysis. Cardiovasc Intervent Radiol 2016;39:1580-8.
108. Salem R, Gordon AC, Mouli S, et al. Y90 Radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 2016;151:1155-63.e2.
109. Salem R, Gabr A, Riaz A, et al. Institutional decision to adopt Y90 as primary treatment for hepatocellular carcinoma informed by a 1,000-patient 15-year experience. Hepatology 2018;68:1429-40.
110. Hilgard P, Hamami M, Fouly AE, et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology 2010;52:1741-9.
111. Mazzaferro V, Sposito C, Bhoori S, et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology 2013;57:1826-37.
112. Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology 2010;138:52-64.
113. Sangro B, Carpanese L, Cianni R, et al. ; European Network on Radioembolization with Yttrium-90 Resin Microspheres (ENRY). Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology 2011;54:868-78.
114. Vilgrain V, Pereira H, Assenat E, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncology 2017;18:1624-36.
115. Chow PKH, Gandhi M, Tan SB, et al. Asia-Pacific Hepatocellular Carcinoma Trials Group. SIRveNIB: selective internal radiation therapy versus sorafenib in Asia-Pacific patients with hepatocellular carcinoma. J Clin Oncol 2018;36:1913-21.
116. Ricke J, Klümpen HJ, Amthauer H, et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J Hepatol 2019;71:1164-74.
117. Garin E, Tselikas L, Guiu B, et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. The Lancet Gastroenterology & Hepatology 2021;6:17-29.
118. Lewandowski RJ, Kulik LM, Riaz A, et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant 2009;9:1920-8.
119. Zori AG, Ismael MN, Limaye AR, et al. Locoregional therapy protocols with and without radioembolization for hepatocellular carcinoma as bridge to liver Transplantation. Am J Clin Oncol 2020;43:325-33.
120. Salem R, Johnson GE, Kim E, et al. Yttrium-90 radioembolization for the treatment of solitary, unresectable HCC: the LEGACY study. Hepatology 2021;74:2342-52.
121. Schaub SK, Hartvigson PE, Lock MI, et al. Stereotactic body radiation therapy for hepatocellular carcinoma: current trends and controversies. Technol Cancer Res Treat 2018;17:1533033818790217.
122. Liang SX, Zhu XD, Xu ZY, et al. Radiation-induced liver disease in three-dimensional conformal radiation therapy for primary liver carcinoma: the risk factors and hepatic radiation tolerance. Int J Radiat Oncol Biol Phys 2006;65:426-34.
123. Benson R, Madan R, Kilambi R, Chander S. Radiation induced liver disease: a clinical update. J Egypt Natl Canc Inst 2016;28:7-11.
124. Kimura T, Takahashi S, Takahashi I, et al. The time course of dynamic computed tomographic appearance of radiation injury to the cirrhotic liver following stereotactic body radiation therapy for hepatocellular carcinoma. PLoS One 2015;10:e0125231.
125. Lee J, Shin IS, Yoon WS, Koom WS, Rim CH. Comparisons between radiofrequency ablation and stereotactic body radiotherapy for liver malignancies: meta-analyses and a systematic review. Radiother Oncol 2020;145:63-70.
127. Shen PC, Chang WC, Lo CH, et al. Comparison of stereotactic body radiation therapy and transarterial chemoembolization for unresectable medium-sized hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2019;105:307-18.
128. Sapir E, Tao Y, Schipper MJ, et al. Stereotactic body radiation therapy as an alternative to transarterial chemoembolization for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2018;100:122-30.
129. Yoon SM, Ryoo BY, Lee SJ, et al. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: a randomized clinical trial. JAMA Oncol 2018;4:661-9.
130. Sapisochin G, Barry A, Doherty M, et al. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J Hepatol 2017;67:92-9.
131. Iezzi R, Pompili M, Posa A, Coppola G, Gasbarrini A, Bonomo L. Combined locoregional treatment of patients with hepatocellular carcinoma: state of the art. World J Gastroenterol 2016;22:1935-42.
132. Lu Z, Wen F, Guo Q, Liang H, Mao X, Sun H. Radiofrequency ablation plus chemoembolization versus radiofrequency ablation alone for hepatocellular carcinoma: a meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol 2013;25:187-94.
133. Kim JW, Kim JH, Won HJ, et al. Hepatocellular carcinomas 2-3 cm in diameter: transarterial chemoembolization plus radiofrequency ablation vs. radiofrequency ablation alone. Eur J Radiol 2012;81:e189-93.
134. Peng ZW, Zhang YJ, Chen MS, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol 2013;31:426-32.
135. Chen K, Chen G, Wang H, et al. Increased survival in hepatocellular carcinoma with iodine-125 implantation plus radiofrequency ablation: a prospective randomized controlled trial. J Hepatol 2014;61:1304-11.
136. Fuchs K, Bize PE, Dormond O, et al. Drug-eluting beads loaded with antiangiogenic agents for chemoembolization: in vitro sunitinib loading and release and in vivo pharmacokinetics in an animal model. J Vasc Interv Radiol 2014;25:379-87,387.e1.
137. Bize P, Duran R, Fuchs K, et al. Antitumoral effect of sunitinib-eluting beads in the rabbit VX2 tumor model. Radiology 2016;280:425-35.
138. Hagan A, Phillips GJ, Macfarlane WM, Lloyd AW, Czuczman P, Lewis AL. Preparation and characterisation of vandetanib-eluting radiopaque beads for locoregional treatment of hepatic malignancies. Eur J Pharm Sci 2017;101:22-30.
139. Sakr OS, Berndt S, Carpentier G, Cuendet M, Jordan O, Borchard G. Arming embolic beads with anti-VEGF antibodies and controlling their release using LbL technology. J Control Release 2016;224:199-207.
140. Slovak R, Ludwig JM, Gettinger SN, Herbst RS, Kim HS. Immuno-thermal ablations-boosting the anticancer immune response. J Immunother Cancer 2017;5:78.
141. Tak WY, Lin SM, Wang Y, et al. Phase III HEAT study adding lyso-thermosensitive liposomal doxorubicin to radiofrequency ablation in patients with unresectable hepatocellular carcinoma lesions. Clin Cancer Res 2018;24:73-83.
142. Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. Journal of Hepatology 2001;35:421-30.
143. Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol 2016;64:1090-8.
144. Kudo M, Ueshima K, Ikeda M, et al. TACTICS study group. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut 2020;69:1492-501.
145. Mathias-Machado M. C. dFLG. Neoadjuvant and adjuvant systemic treatment for hepatocellular carcinoma. Hepatoma Res 2021;7:67.
146. Brown ZJ, Tsung A. Adjuvant treatment of hepatocellular carcinoma after resection. Hepatoma Res 2021;7:68.
147. Gregory J, Dioguardi Burgio M, Corrias G, Vilgrain V, Ronot M. Evaluation of liver tumour response by imaging. JHEP Rep 2020;2:100100.
148. Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16.
149. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52-60.
150. Kielar A, Fowler KJ, Lewis S, et al. Locoregional therapies for hepatocellular carcinoma and the new LI-RADS treatment response algorithm. Abdom Radiol (NY) 2018;43:218-30.
151. Sato Y, Watanabe H, Sone M, et al. Japan Interventional Radiology in Oncology Study Group-JIVROSG. Tumor response evaluation criteria for HCC (hepatocellular carcinoma) treated using TACE (transcatheter arterial chemoembolization): RECIST (response evaluation criteria in solid tumors) version 1.1 and mRECIST (modified RECIST): JIVROSG-0602. Ups J Med Sci 2013;118:16-22.
152. Tovoli F, Renzulli M, Granito A, Golfieri R, Bolondi L. Radiologic criteria of response to systemic treatments for hepatocellular carcinoma. Hepat Oncol 2017;4:129-37.
153. Huh YJ, Kim DH, Kim B, Choi JI, Rha SE. Per-feature accuracy of liver imaging reporting and data system locoregional treatment response algorithm: a systematic review and meta-analysis. Cancers (Basel) 2021;13:4432.
154. Kierans AS, Najjar M, Dutruel SP, et al. Evaluation of the LI-RADS treatment response algorithm in hepatocellular carcinoma after trans-arterial chemoembolization. Clin Imaging 2021;80:117-22.
155. Seo N, Kim MS, Park MS, et al. Evaluation of treatment response in hepatocellular carcinoma in the explanted liver with liver imaging reporting and data system version 2017. Eur Radiol 2020;30:261-71.
156. Kellock T, Liang T, Harris A, et al. Stereotactic body radiation therapy (SBRT) for hepatocellular carcinoma: imaging evaluation post treatment. Br J Radiol 2018;91:20170118.
157. Ma Y, Wang Q, Dong Q, Zhan L, Zhang J. How to differentiate pseudoprogression from true progression in cancer patients treated with immunotherapy. Am J Cancer Res 2019;9:1546-53.
158. Llovet JM, Lencioni R. mRECIST for HCC: Performance and novel refinements. J Hepatol 2020;72:288-306.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Kung JWC, Ng KKC. Role of locoregional therapies in the management of patients with hepatocellular carcinoma. Hepatoma Res 2022;8:17. http://dx.doi.org/10.20517/2394-5079.2021.138
AMA Style
Kung JWC, Ng KKC. Role of locoregional therapies in the management of patients with hepatocellular carcinoma. Hepatoma Research. 2022; 8: 17. http://dx.doi.org/10.20517/2394-5079.2021.138
Chicago/Turabian Style
Kung, Janet Wui Cheung, Kelvin Kwok Chai Ng. 2022. "Role of locoregional therapies in the management of patients with hepatocellular carcinoma" Hepatoma Research. 8: 17. http://dx.doi.org/10.20517/2394-5079.2021.138
ACS Style
Kung, JWC.; Ng KKC. Role of locoregional therapies in the management of patients with hepatocellular carcinoma. Hepatoma. Res. 2022, 8, 17. http://dx.doi.org/10.20517/2394-5079.2021.138
About This Article
Copyright
Data & Comments
Data
 Cite This Article 66 clicks
Cite This Article 66 clicks



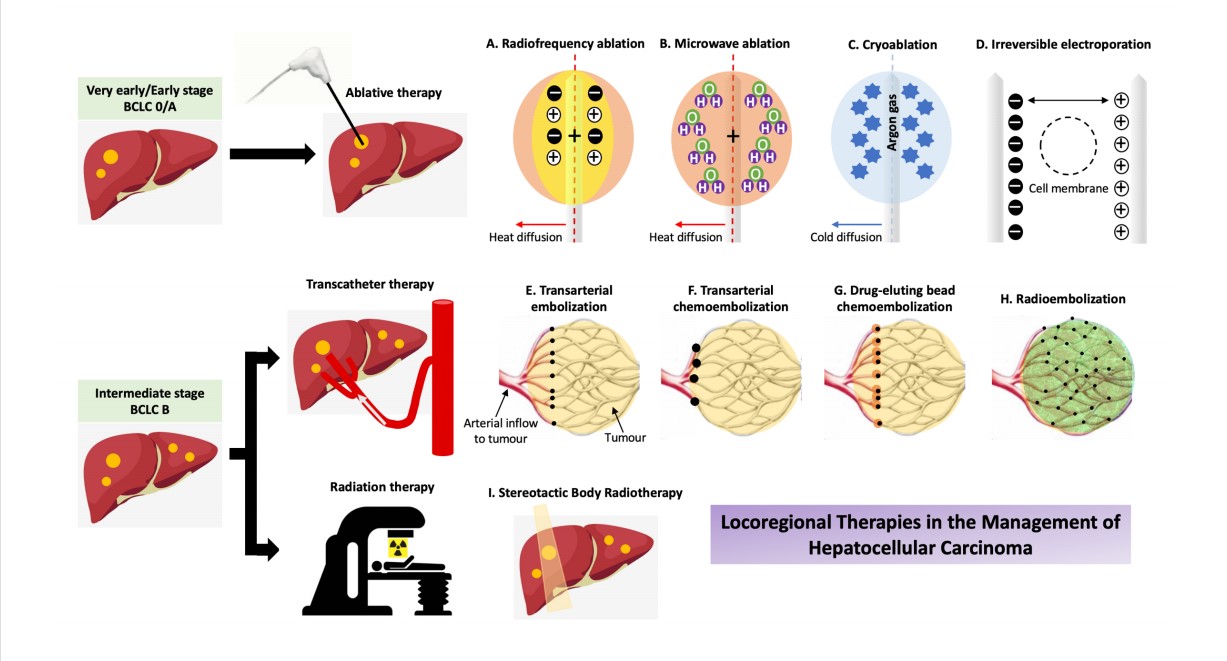

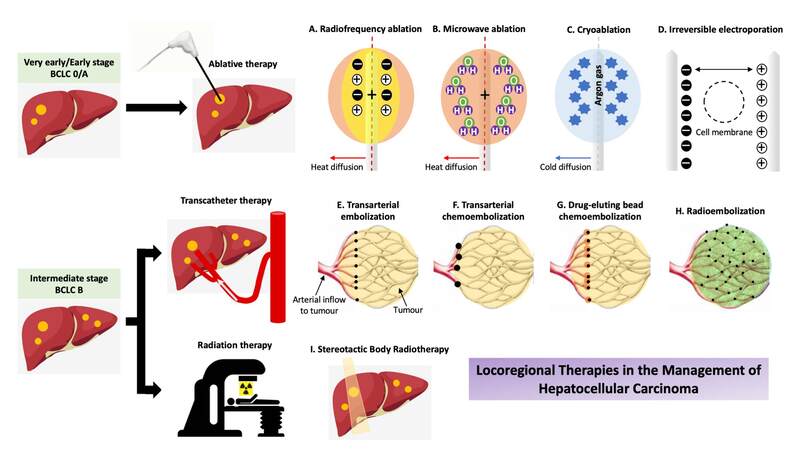










Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.