Hepatocellular carcinoma beyond Barcelona clinic liver cancer resection criteria: resecting the aggressive tumor
Abstract
According to the Barcelona Clinic Liver Cancer (BCLC) staging system, surgical resection is recommended only for BCLC-0 and BCLC-A hepatocellular carcinoma (HCC). Nevertheless, several investigators have recently advocated for widening the resection criteria for HCC to select patients with BCLC-B and less frequently BCLC-C tumors. The available studies have reported a 5-year survival rate ranging from 25% to 63% following resection of select patients with multinodular HCC. The role of liver resection for macrovascular invasive HCC still remains unclear. The present review aimed to summarize the available evidence regarding the outcomes of patients who underwent resection for BCLC-B/C HCC as well as highlight the proposed criteria for resection beyond the current BCLC guidelines.
Keywords
INTRODUCTION
Hepatocellular carcinoma (HCC) accounts for 80%-90% of primary liver malignancies and represents the fifth most common cancer worldwide[1]. In the United States, the incidence of HCC has been gradually increasing with model-based projections estimating that HCC will be the third most common cause of cancer-related deaths by 2040[2]. A number of staging systems have been proposed for HCC - including the American Joint Committee on Cancer (AJCC), French classification, Cancer of the Liver Italian Program (CLIP), Barcelona Clinic Liver Cancer (BCLC) and Hong-Kong Liver Cancer staging systems - aimed at defining prognosis and informing stage-appropriate treatments[3,4]. Although the standard classification of HCC has been based on the AJCC TNM staging, this system has its own limitations including the need for pathologic information to define stage (e.g., microvascular invasion only available after resection), as well as the lack of incorporating information about liver function and patient performance status to estimate prognosis.
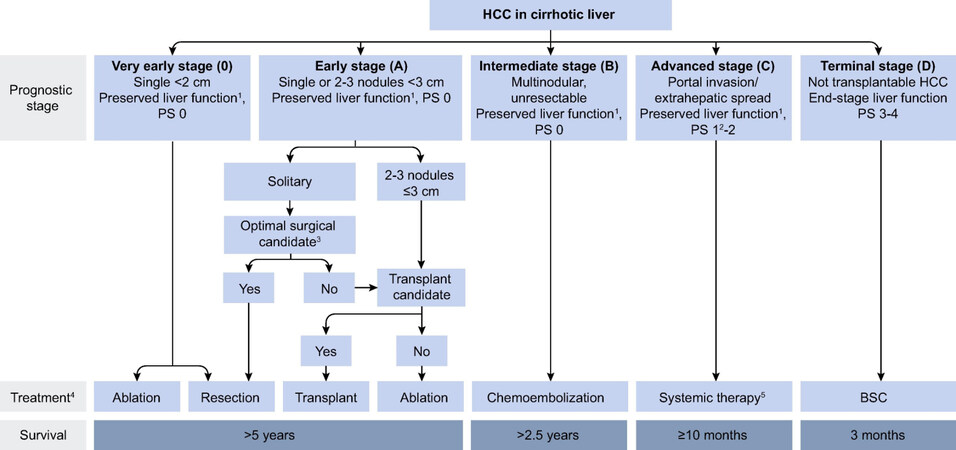
The BCLC staging schema has been widely used in the West and has been endorsed both by the American Association for the Study of Liver Diseases and the European Association for the Study of Liver guidelines[5-7]. The BCLC classification is more complicated than the AJCC staging system in that it includes information related to the extent of disease/tumor burden (i.e., size and number of tumors, extrahepatic spread), as well as information on liver function (i.e., Child Pugh class) and patient performance status (i.e., ECOG class) to define disease stage [Figure 1][6]. Apart from being a staging classification, the BCLC system is also used to guide stage-appropriate treatment recommendations[6]. In particular, according to the BCLC system, surgical resection is recommended for BCLC-0 and BCLC-A HCC, whereas patients with BCLC-B and BCLC-C HCC are recommended to undergo transarterial chemoembolization (TACE) and sorafenib, respectively[6,7].
Despite the wide acceptance of the BCLC system in clinical practice, several investigators have questioned whether certain patients with BCLC-B HCC may benefit more from surgical resection vs. other locoregional therapies (i.e., TACE)[8-10]. To date, however, there are no established criteria regarding which patients will benefit the most from resection beyond the current BCLC criteria. We sought to characterize the available evidence regarding outcomes of patients who underwent resection beyond the current BCLC criteria. In addition, we sought to summarize the proposed criteria for resection beyond the current BCLC guidelines.
RESECTION BEYOND BCLC CRITERIA: IS IT JUSTIFIED IN SELECT PATIENTS?
Over the past decade, significant advances in diagnostic methods, surgical techniques and perioperative care have been made in the field of hepatopancreatobiliary (HPB) surgery. In turn, HPB surgeons have attempted to push the limits of resectability of liver tumors and, in particular, HCC over time[10,11]. In fact, major hepatectomies have been increasingly performed for large, multinodular tumors, as well as tumors invading the major vasculature[12]. Recently, there has also been a growing interest in strategies that could facilitate resection of lesions previously considered unresectable[13]. In turn, treatment recommendations have been updated over the years to align with the available evidence and clinical practice worldwide. In particular, the BCLC system was updated in 2011 to designate single large HCC (≥ 5 cm) as resectable disease (i.e., BCLC-A rather than BCLC-B stage), acknowledging that resection is safe, feasible and should be considered the treatment of choice for single large tumors[6].
More recently, several investigators have suggested that resection of select BCLC-B/C tumors (i.e., beyond the BCLC guidelines) may be both safe and technically feasible in select patients[8-10,14]. In fact, previous institutional series have reported acceptable long-term outcomes following resection of HCC beyond the current guidelines[8-10,14-16]. In a large observational study of the East-West HCC study group, Torzilli et al.[15] analyzed data from patients undergoing resection of HCC at 10 tertiary referral centers worldwide. Of note, the authors demonstrated a 5-year survival of 57% following resection of BCLC-B HCC, which was similar to the survival of patients with BCLC-0/A tumors (5-year survival: 61%)[15]. In addition, the authors noted that almost one-half of patients had multinodular, large or macrovascular invasive HCC (BCLC-0/A: n = 1012; BCLC-B: n = 737; BCLC-C: n = 297); the data highlighted how - in real life clinical practice - surgery is not infrequently performed for patients with HCC beyond the BCLC resection criteria[15]. In a separate a propensity score matching analysis, Hsu et al.[16] reported a 5-year survival of 43% following resection vs. 15% following TACE for patients with BCLC-B HCC (n = 146 each group). Similarly, a multicenter study from Japan demonstrated that hepatic resection for BCLC-B HCC was independently associated with improved outcomes (compared with TACE) after adjusting for all other patient- and disease-related characteristics (HR = 0.56, 95%CI: 0.35-0.91)[17]. Of note, the benefit of resection was more pronounced among patients with a Child-Pugh score ≤ 5 who had less than 3 tumors (HR = 0.38, 95%CI: 0.23-0.64)[17]. Another large multi-institutional analysis of 1259 patients with BCLC B/C HCC confirmed the superiority of resection over TACE for BCLC B/C HCC (5-year survival: 39% vs. 16%, P < 0.001)[18].
Importantly, a recent meta-analysis of 18 studies [1 randomized controlled trial (RCT), 5 propensity score matching non-randomized comparative trials (NRCTs) and 12 NRCTs] demonstrated a survival benefit associated with hepatic resection vs. TACE for patients with BCLC-B/C HCC (HR = 0.59, 95%CI: 0.51-0.67)[10]. The benefit of surgical resection was noted in all subgroup analyses, including analyses stratified by BCLC stage (BCLC-B, HR = 0.53, 95%CI: 0.43-0.65; BCLC-C, HR = 0.67, 95%CI: 0.59-0.77), as well as study type (RCT + PSM NRCT, HR = 0.65, 95%CI: 0.53-0.78; all studies, HR = 0.59, 95%CI: 0.51-0.67)[10]. Although this meta-analysis further called into question the recommended treatment algorithm proposed by the current BCLC classification schema[10], it was later criticized for inconsistencies in inclusion criteria/definition of BCLC stages [i.e., 39%-86% of patients had single large tumors (> 5 cm) in the BCLC-B group], overlapping populations among individual studies, as well as sequential treatments offered to patients (i.e., not only surgery or TACE) that prevented a “true” comparison of surgery vs. TACE for intermediate or advanced stage HCC[19,20].
Despite data favoring resection over TACE for select patients with BCLC-B tumors, the majority of available data derive from retrospective analyses that are subject to selection bias. Thus, definitive conclusions relative to superiority of resection over TACE cannot be made with certainty. Of note, for non-surgical candidates, combination multimodality therapy (i.e., TACE + RFA) may be associated with acceptable outcomes. A recent systematic review and meta-analysis of 8 retrospective studies and one randomized controlled trial compared oncologic outcomes of combination therapy (i.e., TACE + RFA) vs. surgical resection of HCC[21]. Following propensity score matching, there were no differences in 1-, 3- and 5-year OS and DFS among patients receiving combination therapy vs. surgical resection; TACE + RFA was, however, associated with lower morbidity vs. resection[21]. While BCLC criteria may be too restrictive, rigorous case selection to identify the best candidates for surgical resection is critical to achieve acceptable outcomes among patients with HCC beyond the BCLC guidelines. Of note, the majority of studies have analyzed single large HCC (currently considered BCLC-A) and multinodular HCC together - further confusing interpretation of the results and limiting the ability to know the “true” benefit of resection for multinodular HCC (i.e., true BCLC-B tumors)[10,15,18].
LIVER RESECTION FOR BCLC-B TUMORS (PURELY MULTINODULAR HCC)
According to the BCLC staging schema, patients with multiple HCC should be treated with TACE when transplantation is contraindicated (i.e., HCC exceeding Milan criteria)[6]. Although a number of studies have reported on outcomes following resection of BCLC-B tumors[8-10,14-16,22], only a handful of these studies have consistently used the latest BCLC classification, referring to BCLC-B HCC as purely multinodular tumors[8,23-26]. Among the few available studies, 5-year survival following resection of only patients with multinodular HCC have ranged from 25% to 63%[8,23-26]. However, both cirrhotic and non-cirrhotic populations have been included in these respective analyses[8,23-26]. While resection for multifocal HCC in cirrhotic patients is generally not feasible or recommended, at least one study did demonstrate acceptable outcomes in well selected cirrhotic patients[27]. In particular, a multi-institutional study of 1066 cirrhotic patients noted that that liver resection for multinodular HCC could be safely performed among well-selected patients (30-day mortality: 1.9%) at experienced centers with a 5-year OS of 34.6%[27].
In a study of only patients with multiple tumors, Ho et al.[23] demonstrated that patients who underwent surgical resection (n = 294) had a better 5-year survival (36.6%) vs. those treated with TACE (11.0%) (n = 367) or chemotherapy/supportive care (0.7%) (n = 404). In another study, Wada et al.[8] examined 85 patients with multifocal BCLC-B HCC and reported a 5-year OS of 63.4% following curative-intent resection. On PSM analysis, patients with BCLC-B HCC (n = 80) had a 5-year survival of 63% after resection vs. only 22% among patients who received non-surgical treatment (n = 80)[24]. A separate multi-institutional analysis analyzed 814 patients who underwent curative-intent resection of HCC at major HPB centers[25]. In this study, 157 patients underwent resection for multinodular BCLC-B HCC and had similar outcomes as those who underwent resection for a single large tumor (BCLC-A HCC) (5-year survival: 49.9% vs. 56.9%, P = 0.259)[25]. Of note, the lack of survival difference among patients with multinodular BCLC-B HCC (i.e., theoretically unresectable HCC) vs. a single large HCC (i.e., resectable HCC) persisted even after adjusting for competing factors (HR = 0.83, 95%CI: 0.54-1.28, P = 0.40)[25]; these data suggested that select patients with multinodular HCC may indeed benefit from resection when treated at major HPB centers. In a different study, up to 37.6% of patients with multinodular BCLC-B HCC achieved “statistical cure” (i.e., mortality risk reached a level expected in the general population) following curative-intent resection[28], highlighting that surgery may indeed provides a chance of “cure” for select BCLC-B patients.
To date, only one RCT has been published on surgery vs. TACE for multifocal HCC beyond Milan criteria[26]. This RCT analyzed 173 patients with multiple HCC beyond Milan criteria who were treated at the Eastern Hepatobiliary Surgery Hospital in China between 2008-2010[26]. In the intention-to-treat analysis, liver resection (n = 88) outperformed TACE (n = 85); specifically, 3-year survival was 51.5% after resection vs. 18.1% following TACE[26], even though the two groups were similar in terms of age, AFP levels, proportion of patients with cirrhosis, Child Pugh class, number and size of tumors[26]. Data from this RCT corroborated findings from previous retrospective analyses and suggested that hepatic resection may indeed be better than TACE for select patients with multinodular HCC beyond the Milan criteria.
LIVER RESECTION FOR BCLC-C TUMORS (HCC WITH MACROVASCULAR INVASION)
Resection of HCC with macrovascular invasion is technically challenging and the long-term survival benefit is still unclear. Macrovascular invasion is strongly related to an increased risk of intra- and extra-hepatic metastases and, in turn, inferior outcomes among patients with HCC[29]. Although previous studies have suggested a steady increase in the number of major hepatectomies performed at major HPB centers for tumors invading the major vasculature[12], in most surgical series, only approximately 5%-15% and 3%-4% of patients appear to have portal vein tumor thrombosis or hepatic vein invasion, respectively[30]. The postoperative morbidity and mortality following resection of HCC associated with macrovascular invasive can be significant, ranging from 30%-37% and 3%-8%, respectively[30,31].
In a multicenter analysis, Pawlik et al.[30] reported a median survival of 11 months (5-year survival: 10%) among patients who underwent hepatectomy for HCC with major portal or hepatic vein invasion, which exceeded the survival of historical patient cohorts treated with non-surgical therapies (median survival with sorafenib ~6 months)[32]. In another series of 17 patients with HCC and macrovascular invasion,
PROPOSED CRITERIA TO IDENTIFY CANDIDATES FOR RESECTION BEYOND CURRENT BCLC CRITERIA
To date there are no established criteria to identify the best candidates for resection beyond the BCLC guidelines. In turn, it is mostly up to the individual surgeon to recommend a more invasive approach to patients who would otherwise be served with non-surgical treatments. While the benefit from resection of tumors with major vascular invasion (i.e., BCLC-C) is still unclear, there is more evidence to suggest a potential benefit for select patients with multinodular BCLC-B HCC [Table 1].
Criteria for selecting patients for resection beyond current BCLC criteria
| Ref. (Year) | Criteria | Survival |
| Tsilimigras et al.[39] (2020) | BCLC-B and medium TBS* | 5-year OS: 58.9% |
| Wada et al.[8] (2016) | 2-3 lesions, < 5 cm | 5-year OS: 75.2% |
| Kudo et al.[40] (2015) | “Kinki criteria”: BCLC-B1: Child Pugh 5-6, within up-to-7 criteria | - |
| Tsilimigras et al.[42] (2020) | BCLC-B and pTBS ≤ 11 | 5-year OS: 60.1% |
| Tsilimigras et al.[43] (2020) | Low TBS-based score** | 5-year OS: 80.1% |
By analyzing a large multi-institutional database, our own group recently utilized tumor burden score (TBS) - a relatively novel tool that is based on the Pythagorean theorem and takes both tumor size and number into account (α2 + β2 = γ2, where α = maximum tumor diameter, β = number of tumors and γ = TBS) - to further subdivide BCLC stages[39]. Interestingly, patients with BCLC-B HCC who had a medium TBS had long-term survival that was comparable with those who had BCLC-A HCC and a medium TBS; in fact, patients with BCLC-B HCC who had a medium TBS proved to have an even better survival than patients with BCLC-A HCC and high TBS (i.e., theoretically earlier stage tumors)[39]. As such, TBS might be a valuable adjunct to further sub-classify the current BCLC stages and help identify which patients may likely benefit the most from resection of HCC that is beyond the BCLC guidelines[39]. To this point, in another study, Wada et al.[8] proposed 3 types of multiple HCC based on the number and size of tumors: type I (up to 3 lesions < 5 cm); type II (up to 3 lesions > 5 cm or 4 lesions of any size); type III (≥ 5 lesions of any size)[8]. Although all patients had BCLC-B tumors, patients with type I disease had the best long-term outcomes (5-year survival; type I: 75.2%, type II: 63.0%, type III: 37.1%, P < 0.001)[8].
Recently, Kudo et al.[40] proposed the “Kinki criteria” to further subclassify BCLC-B tumors. According to these criteria, patients with Child-Pugh score 5-6 who have tumors within the up-to-7 criteria were classified as BCLC-B1, patients with Child-Pugh score 5-6 beyond the up-to-7 criteria as BCLC-B2 and patients with Child-Pugh score 8-9 within or beyond up-to-7 criteria were classified as BCLC-B3[40]. According to this proposed subclassification, patients with BCLC-B1 HCC should be recommended resection, while B2 and B3 HCC should be treated with ablation, TACE, or sorafenib[40]. The proposed Kinki system has been subsequently validated in an external cohort[41].
Recently, machine learning methods have been utilized to identify subgroups of patients with BCLC-B HCC who may benefit the most from resection[42]. Among all patient- and tumor-related factors, the classification and regression tree (CART) model demonstrated that radiologic and pathologic TBS were the most important predictors of outcomes among BCLC-B patients in the pre- and post-operative setting, respectively[42]. Of note, patients with BCLC-B HCC and pathologic TBS ≤ 11 (n = 111) had a 5-year survival of 60.1%, whereas patients with BCLC-B HCC and TBS > 11 (n = 39) had a 5-year survival of 13.9%, further validating the utility of TBS in identifying the best candidates for resection beyond the BCLC criteria[42]. In turn, TBS-based risk scores have been proposed to enhance prognostication among patients undergoing resection for multinodular HCC beyond Milan criteria[43]. Specifically, combining TBS, ASA class, presence of cirrhosis, AFP levels, tumor grade and presence of lymphovascular invasion into a single formula, the prognosis of patients with multinodular HCC beyond Milan criteria can be accurately predicted[43]. In particular, patients with a low TBS-based risk score had the best 5-year survival (80.1%) followed by those with medium- (37.2%) and high-risk scores (not reached) (P < 0.001)[43]. The TBS-based risk score has been validated externally with excellent accuracy to predict long-term outcomes (5-year survival; low risk score: 66.3% vs. medium risk score: 25.2% vs. high risk score: not reached, P < 0.001)[43]. Collectively, the data suggest that patients with low or medium TBS-based risk score may benefit the most relative to long-term outcomes after curative-intent resection for multinodular HCC beyond the Milan criteria.
CONCLUSION
Although the BCLC guidelines recommend resection for only BCLC-0/A tumors, accumulating evidence has suggested that surgery should not be a priori denied to patients with multinodular BCLC-B HCC. The role of surgical resection for patients with macrovascular invasive HCC remains controversial. The current data emphasize the need for further refinement of the current BCLC classification and proposed treatment algorithms.
DECLARATIONS
Authors’ contributionsPawlik both made substantial contributions to the concept, design, and production of the manuscript: Tsilimigras DI, Pawlik TM
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestBoth authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2021.
REFERENCES
1. Lafaro KJ, Demirjian AN, Pawlik TM. Epidemiology of hepatocellular carcinoma. Surg Oncol Clin N Am 2015;24:1-17.
2. Rahib L, Wehner MR, Matrisian LM, Nead KT. Estimated projection of US cancer incidence and death to 2040. JAMA Netw Open 2021;4:e214708.
3. Marrero JA, Fontana RJ, Barrat A, et al. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology 2005;41:707-16.
4. O'Neil BH, Venook AP. Hepatocellular carcinoma: the role of the North American GI Steering Committee Hepatobiliary Task Force and the advent of effective drug therapy. Oncologist 2007;12:1425-32.
5. Cillo U, Vitale A, Grigoletto F, et al. Prospective validation of the Barcelona Clinic Liver Cancer staging system. J Hepatol 2006;44:723-31.
6. Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182-236.
7. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723-50.
8. Wada H, Eguchi H, Noda T, et al. Selection criteria for hepatic resection in intermediate-stage (BCLC stage B) multiple hepatocellular carcinoma. Surgery 2016;160:1227-35.
9. Bhandare MS, Patkar S, Shetty N, et al. Liver resection for HCC outside the BCLC criteria. Langenbecks Arch Surg 2018;403:37-44.
10. Hyun MH, Lee YS, Kim JH, et al. Hepatic resection compared to chemoembolization in intermediate- to advanced-stage hepatocellular carcinoma: a meta-analysis of high-quality studies. Hepatology 2018;68:977-93.
11. Lim C, Salloum C, Osseis M, et al. Short-term outcomes following hepatectomy for hepatocellular carcinoma within and beyond the BCLC guidelines: a prospective study. HPB (Oxford) 2018;20:222-30.
12. Tsilimigras DI, Sahara K, Moris D, et al. Assessing textbook outcomes following liver surgery for primary liver cancer over a 12-year time period at major hepatobiliary centers. Ann Surg Oncol 2020;27:3318-27.
13. She WH, Chok K. Strategies to increase the resectability of hepatocellular carcinoma. World J Hepatol 2015;7:2147-54.
14. Guo H, Wu T, Lu Q, et al. Surgical resection improves long-term survival of patients with hepatocellular carcinoma across different Barcelona Clinic Liver Cancer stages. Cancer Manag Res 2018;10:361-9.
15. Torzilli G, Belghiti J, Kokudo N, et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations? Ann Surg 2013;257:929-37.
16. Hsu CY, Hsia CY, Huang YH, et al. Comparison of surgical resection and transarterial chemoembolization for hepatocellular carcinoma beyond the Milan criteria: a propensity score analysis. Ann Surg Oncol 2012;19:842-9.
17. Tada T, Kumada T, Toyoda H, et al. Role of hepatic resection in patients with intermediate-stage hepatocellular carcinoma: a multicenter study from Japan. Cancer Sci 2017;108:1414-20.
18. Zhong JH, Ke Y, Gong WF, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg 2014;260:329-40.
19. Labgaa I, Demartines N, Melloul E. Surgical resection versus transarterial chemoembolization for intermediate stage hepatocellular carcinoma (BCLC-B): an unsolved question. Hepatology 2019;69:923.
20. Mo DC, Jia RR, Zhong JH. Letter to the Editor: hepatic resection compared to chemoembolization in intermediate- to advanced-stage hepatocellular carcinoma: a comment for moving forward. Hepatology 2019;70:446-7.
21. Gui CH, Baey S, D'Cruz RT, Shelat VG. Trans-arterial chemoembolization + radiofrequency ablation versus surgical resection in hepatocellular carcinoma - A meta-analysis. Eur J Surg Oncol ;46(5):763-771.
22. Moris D, Felekouras E. Ignore reality but not the consequences of its ignorance: Broaden guidelines in surgery of hepatocellular carcinoma. Hepatology 2017;65:1772-3.
23. Ho MC, Huang GT, Tsang YM, et al. Liver resection improves the survival of patients with multiple hepatocellular carcinomas. Ann Surg Oncol 2009;16:848-55.
24. Kim H, Ahn SW, Hong SK, et al. Survival benefit of liver resection for Barcelona Clinic Liver Cancer stage B hepatocellular carcinoma. Br J Surg 2017;104:1045-52.
25. Tsilimigras DI, Bagante F, Sahara K, et al. Prognosis after resection of barcelona clinic liver cancer (BCLC) Stage 0, A, and B hepatocellular carcinoma: a comprehensive assessment of the current BCLC classification. Ann Surg Oncol 2019;26:3693-700.
26. Yin L, Li H, Li AJ, et al. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan Criteria: a RCT. J Hepatol 2014;61:82-8.
27. Li ZL, Yu JJ, Guo JW, et al. Liver resection is justified for multinodular hepatocellular carcinoma in selected patients with cirrhosis: a multicenter analysis of 1,066 patients. Eur J Surg Oncol 2019;45:800-7.
28. Tsilimigras DI, Bagante F, Moris D, et al. Defining the chance of cure after resection for hepatocellular carcinoma within and beyond the Barcelona Clinic Liver Cancer guidelines: a multi-institutional analysis of 1,010 patients. Surgery 2019;166:967-74.
29. Kanda M, Tateishi R, Yoshida H, et al. Extrahepatic metastasis of hepatocellular carcinoma: incidence and risk factors. Liver Int 2008;28:1256-63.
30. Pawlik TM, Poon RT, Abdalla EK, et al. Hepatectomy for hepatocellular carcinoma with major portal or hepatic vein invasion: results of a multicenter study. Surgery 2005;137:403-10.
31. Chok KS, Cheung TT, Chan SC, Poon RT, Fan ST, Lo CM. Surgical outcomes in hepatocellular carcinoma patients with portal vein tumor thrombosis. World J Surg 2014;38:490-6.
32. Worns MA, Koch S, Niederle IM, et al. The impact of patient and tumour baseline characteristics on the overall survival of patients with advanced hepatocellular carcinoma treated with sorafenib. Dig Liver Dis 2013;45:408-13.
33. Ruzzenente A, Capra F, Pachera S, et al. Is liver resection justified in advanced hepatocellular carcinoma? J Gastrointest Surg 2009;13:1313-20.
34. Costentin CE, Decaens T, Laurent A, et al. Sorafenib vs surgical resection for hepatocellular carcinoma with macrovascular invasion: a propensity score analysis. Liver Int 2017;37:1869-76.
35. Couri T, Pillai A. Goals and targets for personalized therapy for HCC. Hepatol Int 2019;13:125-37.
36. Inoue Y, Hasegawa K, Ishizawa T, et al. Is there any difference in survival according to the portal tumor thrombectomy method in patients with hepatocellular carcinoma? Surgery 2009;145:9-19.
37. Wang Y, Yuan L, Ge RL, Sun Y, Wei G. Survival benefit of surgical treatment for hepatocellular carcinoma with inferior vena cava/right atrium tumor thrombus: results of a retrospective cohort study. Ann Surg Oncol 2013;20:914-22.
38. Le Treut YP, Hardwigsen J, Ananian P, et al. Resection of hepatocellular carcinoma with tumor thrombus in the major vasculature. A European case-control series. J Gastrointest Surg 2006;10:855-62.
39. Tsilimigras DI, Moris D, Hyer JM, et al. Hepatocellular carcinoma tumour burden score to stratify prognosis after resection. Br J Surg 2020;107:854-64.
40. Kudo M, Arizumi T, Ueshima K, Sakurai T, Kitano M, Nishida N. Subclassification of BCLC B stage hepatocellular carcinoma and treatment strategies: proposal of Modified Bolondi's Subclassification (Kinki Criteria). Dig Dis 2015;33:751-8.
41. Arizumi T, Ueshima K, Iwanishi M, et al. Validation of Kinki Criteria, a modified substaging system, in patients with intermediate stage hepatocellular carcinoma. Dig Dis 2016;34:671-8.
42. Tsilimigras DI, Mehta R, Moris D, et al. Utilizing machine learning for pre- and postoperative assessment of patients undergoing resection for BCLC-0, A and B hepatocellular carcinoma: implications for resection beyond the BCLC guidelines. Ann Surg Oncol 2020;27:866-74.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Tsilimigras DI, Pawlik TM. Hepatocellular carcinoma beyond Barcelona clinic liver cancer resection criteria: resecting the aggressive tumor. Hepatoma Res 2021;7:63. http://dx.doi.org/10.20517/2394-5079.2021.51
AMA Style
Tsilimigras DI, Pawlik TM. Hepatocellular carcinoma beyond Barcelona clinic liver cancer resection criteria: resecting the aggressive tumor. Hepatoma Research. 2021; 7: 63. http://dx.doi.org/10.20517/2394-5079.2021.51
Chicago/Turabian Style
Tsilimigras, Diamantis I., Timothy M. Pawlik. 2021. "Hepatocellular carcinoma beyond Barcelona clinic liver cancer resection criteria: resecting the aggressive tumor" Hepatoma Research. 7: 63. http://dx.doi.org/10.20517/2394-5079.2021.51
ACS Style
Tsilimigras, DI.; Pawlik TM. Hepatocellular carcinoma beyond Barcelona clinic liver cancer resection criteria: resecting the aggressive tumor. Hepatoma. Res. 2021, 7, 63. http://dx.doi.org/10.20517/2394-5079.2021.51
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 39 clicks
Cite This Article 39 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.