Distinctive magnetic resonance imaging findings of hepatocellular carcinoma after hepatitis C virus eradication with direct-acting antivirals
Abstract
Aim: The aim of the present study was to evaluate the characteristics of the magnetic resonance imaging features of hepatocellular carcinoma (HCC) that developed early after the eradication of hepatitis C virus (HCV) by direct-acting antiviral (DAA) treatment.
Methods: This study included 26 patients who achieved sustained viral response with DAA and developed HCC thereafter within one year (DAA-SVR HCC). The radiologic characteristics of these patients were evaluated by contrast-enhanced magnetic resonance imaging, including diffusion-weighted imaging (DWI) and T2-weighted imaging (T2WI). For comparison, 80 HCC patients with positive HCV RNA (HCV-positive HCC) were included. Among 42 patients where tumor biopsy was available, histological grade and radiologic findings were compared.
Results: The rates of high intensity on DWI and T2WI were significantly higher in DAA-SVR HCC compared to HCV-positive HCC (DWI: 100% vs. 67.5%, P < 0.001; T2WI: 92.6% vs. 67.5%, P = 0.01). HCC with high intensity on DWI or T2WI was more likely to have moderately or poorly differentiated HCC compared to well-differentiated HCC (DWI: 69.7% vs. 30.3%, P = 0.02; T2WI: 66.7% vs. 27.3%, P = 0.03).
Conclusion: High intensity on DWI and hyperintensity on T2WI were distinctive features of HCC that developed within one year after the end of DAA treatment.
Keywords
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancer types[1] and the major cause of cancer-related deaths[2]. Chronic liver disease and cirrhosis are closely associated with the development of HCC[3]. In patients with chronic hepatitis C virus (HCV) infection, sustained viral response (SVR) can be observed in more than 90% of the cases by antiviral therapy using direct-acting antivirals (DAAs)[4]. However, it remains controversial whether HCC that developed after the eradication of HCV with DAA regimens differs from those that developed during active infection with HCV. Although the incidence of HCC development may not be suppressed or enhanced by the eradication of HCV with DAA[5], the question remains whether there are differences in the biological characteristics, such as histology or radiologic findings, or the clinical course after curative treatment. Imaging features of HCC after SVR with DAA have not been studied in detail.
For the diagnosis of HCC, contrast-enhanced magnetic resonance imaging (MRI) using gadolinium ethoxybenzyl diethylenetriamine penta-acetic acid (Gd-EOB-DTPA) is often used[6-9]. MRI has an advantage over computer tomography (CT) in that various images, including diffusion-weighted images (DWI) or T2-weighted images (T2WI), can be obtained in addition to the vascularity information of HCC nodules. DWI is imaging associated with the restriction of water molecule movement and reflects the histopathologic features of organs and tissues by virtue of high scan speed. It is widely known that DWI is valuable for the diagnosis and differential diagnosis of neoplasm in the liver, including small (< 2 cm) HCC[10]. In this study, we evaluated the MRI features of HCC that developed within one year after the end of DAA treatment.
Methods
Patients
In our hospital cohort, 695 patients with chronic hepatitis C were treated with DAA regimens and achieved SVR between October 2014 and September 2016. At baseline and after SVR, the surveillance for HCC was performed using ultrasonography, contrast-enhanced CT, or EOB-MRI. Among these 695 patients, 26 patients developed HCC within one year after the end of DAA therapy (DAA-SVR HCC). All of these patients had no previous history of HCC treatment. For comparison, 80 HCC patients with positive HCV RNA (HCV-positive HCC) were selected from 208 consecutive HCC patients with HCV infection who were treated for the first time from December 2011 and February 2018 according to the following inclusion criteria: (1) HCV RNA positive at the time of HCC diagnosis; (2) Child-Pugh A; (3) tumor diameter ≤ 3 cm and number of nodules ≤ 3; (4) no vascular invasion and no extrahepatic metastasis; (5) EOB-MRI available; and (6) radiofrequency ablation (RFA) was performed. This study was conducted according to the ethical principles of the Declaration of Helsinki and STROBE guidelines. This study was approved by the Ethics Committees of Musashino Red Cross Hospital (approval number 28077).
HCC diagnosis
The radiologic diagnosis for HCC was based on the Japan Society of Hepatology[11], the American Association for the Study of Liver Disease[12], and the European Association for the Study of the Liver[13] guidelines. Typical radiographic findings on dynamic study (CT or MRI) or needle biopsy were used for diagnosis. Magnetic resonance examinations were performed in all patients using a 1.5 T scanner (Signa Excite HD; GE Healthcare, USA). Gd-EOB-DTPA was used as a contrast agent. The contrast material was administered as a bolus at a dose of 0.1 mL/kg body weight at a rate of 2 mL/s followed by flushing with 20 mL saline solution using a power injector. Images of the arterial phase, portal venous phase, late phase, and hepatobiliary phase (HPB) were obtained 18 s, 60 s, 150 s, and 20 min after the peak time, respectively, after contrast injection. DWI was acquired before HPB with b values of 0 and 1000 s/mm2. Apparent diffusion coefficient (ADC) maps were obtained automatically by two images of b values of 0 and 1000 s/mm2. Dynamic CT scan with a section thickness of 2 mm was performed. For triple-phase dynamic CT scans, arterial, portal, and equivalent phases were set at 35, 70, and 150 s, respectively, after injection of the contrast agent. Board-certified radiologists diagnosed HCC based on typical patterns, such as an early-phase hyperattenuation area and a late-phase hypoattenuation area on dynamic study. Pathologic diagnosis was confirmed by a certified pathologist who was unaware of the patient’s clinical data.
RFA procedure
RFA was percutaneously performed by using local anesthesia. We used an internally water-cooled 17 G cooled-tip electrode with an impedance-controlled generator (Cosman generator, Cool-Tip System, Radionics, Burlington, MA, USA). When the target nodule was more than 20 mm in diameter, multiple needle insertions and multiple ablations of one nodule were performed.
Statistical analysis
All statistical analyses were performed using Easy R (EZR) version 3.4.1 (Saitama Medical Center, Jichi Medical University, Saitama, Japan)[14], a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). P < 0.005 was considered statistically significant. Fisher’s exact test, paired t test, and Mann-Whitney U test were adopted to determine the differences between the two indexes. Fisher’s exact test was used to evaluate the intensity of DWI and T2WI. Survival curves were estimated using the Kaplan-Meier method. Overall survival (OS) was defined as the period between the treatment date of HCC and the patient’s death or last visit, and progression-free survival (PFS) was defined as the period between the treatment date of HCC and the recurrence confirmation date of dynamic CT or EOB-MRI.
Results
Comparison of demographics and laboratory data
The baseline clinical characteristics of all patients are shown in Table 1. Among them, 26 patients achieved SVR with DAAs before HCC diagnosis (DAA-SVR HCC) and HCV RNA was detected in the remaining 80 patients (HCV-positive HCC). The median age of all patients was 75 years old and the median tumor size was 18 (8-29) mm. Serum alanine aminotransferase (ALT; 18.5 vs. 53.0 IU/mL, P = 0.001) and α-fetoprotein (AFP; 3.42 vs. 25.3, P < 0.001) were significantly lower in DAA-SVR HCC patients than in HCV-positive HCC patients and the mean platelet count was higher in DAA-SVR HCC patients than in HCV-positive HCC patients (149 × 103/μL vs. 102 × 103/μL, P = 0.009). There was no significant difference in age, tumor size, tumor number, albumin, total bilirubin, and prothrombin time international normalized ratio between DAA-SVR and HCV-positive HCC patients. The median value of Wisteria floribunda agglutinin-positive Mac-2 binding protein (WFA ± M2BP) in 25 patients with DAA-SVR HCC was 1.48.
Patients’ characteristics
| DAA-SVR HCC (n = 26) | HCV-positive HCC (n = 80) | P value | |
|---|---|---|---|
| Age (years), median (range) | 75 (63-83) | 75 (44-93) | 0.27 |
| Sex (male/female) | 10/16 | 43/37 | 0.26 |
| Number of tumors (single/multiple) | 21/5 | 60/20 | 0.61 |
| Tumor diameter (cm), median (range) | 18.0 (10-29) | 17.6 (8.0-29) | 0.7 |
| BCLC stage A/B/C | 26/0/0 | 80/0/0 | 1.0 |
| AFP (ng/mL) | 3.4 (2.0-6116.5) | 25.3 (2.0-1660) | < 0.001 |
| PIVKA-II (mAU/mL) | 19.0 (12-94) | 53.0 (13-245) | 0.2 |
| Albumin (g/dL) | 4.0 (2.9-4.7) | 3.7 (2.8-273) | 0.6 |
| ALT (IU/mL) | 18.0 (12-94) | 53 (13-245) | < 0.001 |
| Platelet (103/μL) | 149 (45-189) | 102 (33-344) | 0.009 |
| Total bilirubin (mg/dL) | 0.7 (0.3-2.0) | 0.8 (0.3-2.6) | 0.71 |
| PT% | 91 (57-113) | 89 (31-120) | 0.24 |
| Child-Pugh score 5/6 | 25/1 | 68/12 | 0.18 |
| Fib 4 index | 3.22(0.55-11.5) | 6.01(1.66-25.2) | < 0.001 |
Imaging features
The imaging features are shown in Table 2. There was no difference between DAA-SVR and HCV-positive HCC in terms of features of arterial phase, late phase, or HPB. In contrast, all DAA-SVR HCC was depicted as high intensity on DWI, whereas only 67.5% HCV-positive HCC was found (P < 0.001). Moreover, high intensity on T2WI was significantly higher in DAA-SVR HCC than in HCV-positive HCC (92.6% vs. 67.5%, P = 0.01). The typical imaging features of DAA-SVR HCC are shown in Figure 1. We also measured the ADC values of HCC lesions on DWI; however, there was no significant difference between the two groups.
Image features of EOB-MRI at HCC diagnosis
| Early high | Late low | HBP low | T2WI high | DWI high | |
|---|---|---|---|---|---|
| DAA-SVR HCC (n = 26) | 19/26 (73.0%) | 24/26 (92.3%) | 24/26 (92.3%) | 24/26 (92.3%) | 26/26 (100%) |
| HCV-positive HCC (n = 80) | 63/74 (85.1%) | 74/74 (100%) | 70/74 (94.6%) | 54/80 (67.5%) | 54/80 (67.5%) |
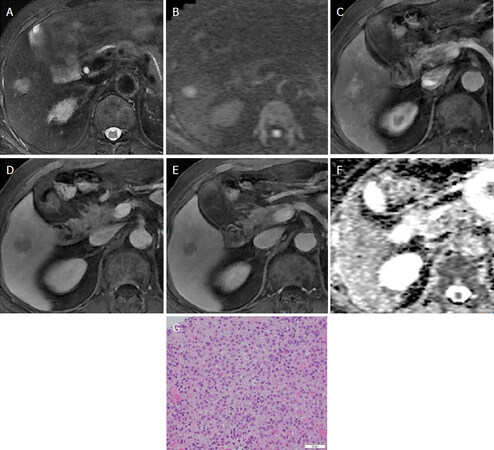
Figure 1. Hepatocellular carcinoma in an 82-year-old male. In hepatic segment VI, a 2-cm nodule shows: A: hyperintensity on axial T2-weighted imaging; B: hyperintensity on diffusion-weighted imaging (b value = 800 s/mm2); C: hyperintensity on arterial phase; D: hypointensity on late phase; E: hypointensity on hepatobiliary phase; F: hypointensity on apparent diffusion coefficient map; G: pathology was moderate
Tumor biopsy was performed in 9 patients with DAA-SVR HCC and 33 patients with HCV-positive HCC. In nine patients with DAA-SVR HCC, four patients showed well-differentiated HCC and five patients were diagnosed with moderately or poorly differentiated HCC. In 33 patients with HCV-positive HCC, 13 patients showed well-differentiated HCC and 20 patients were diagnosed with moderately or poorly differentiated HCC. There were significant associations with MRI and pathologic findings. HCC with high intensity on DWI or T2WI was more likely to have moderately or poorly differentiated HCC compared to well-differentiated HCC (DWI: 69.7% vs. 30.3%, P = 0.02; T2WI: 66.7% vs. 27.3%, P = 0.03).
Prognosis after curative HCC treatment
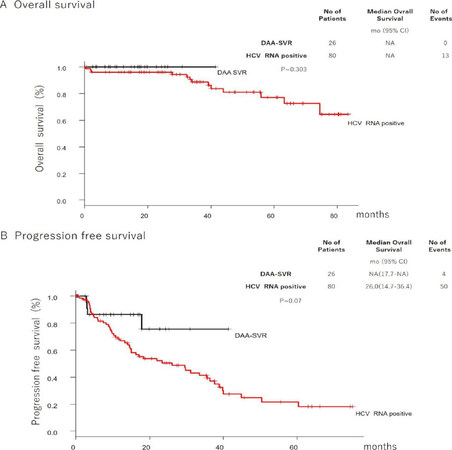
Among patients with DAA-SVR HCC, 1 patient received resection, 2 patients were treated by transcatheter arterial chemoembolization, 1 patient was not treated, and the remaining 22 patients were treated with RFA. All patients with HCV-positive HCC were treated with RFA. OS and PFS were not different between DAA-SVR and HCV-positive HCC [Figure 2].
Discussion
The present study evaluated the imaging features of HCC that developed early after the eradication of HCV by DAA therapy. All of these HCC showed high intensity on MRI DWI and more than 90% showed high intensity on T2WI, which was significantly different from HCC with positive HCV RNA. The stage of HCC, such as the number of HCC nodules and the maximum diameter, was not different between these two groups, indicating that high intensity on DWI or T2WI on MRI is the characteristic imaging feature of HCC after DAA treatment.
The high signal intensity on DWI or T2WI is reported to be linked to the pathology of HCC. Among small hepatic nodules with hypovascularity on arterial phase, high intensity on DWI or T2WI is the characteristic feature of HCC compared to dysplastic nodules[15,16]. High signal intensity on T2WI correlates with increased intratumoral arterial supply and decreased intratumoral portal blood supply. Among HCC with hypovascularity on arterial phase, high signal intensity on T2WI was associated with histologic grade[17].
In a report that classified the signal intensity of DWI into three stages (iso/slight, moderate, and obvious) in 254 resected HCC, the prevalence of well-differentiated HCC was high and that of moderately or poorly differentiated HCC was low in the iso/slight intensity imaging group compared to the obvious intensity imaging group: the pathology of well/moderate/poor HCC was 38.9%/57.4%/3.7% for the iso/slight intensity group and 5.8%/77.4%/16.8% for the obvious intensity group[18]. Other reports showed that DWI features were linked to poor differentiation on pathology, vascular invasion, and recurrence risk factor after resection of small HCC[19,20]. Although the number of patients with the information of pathology was small in our study, HCC with high intensity on DWI or T2WI was more likely to have moderately or poorly differentiated HCC compared to well-differentiated HCC. Taken together, these results suggest that high intensity on DWI or T2WI, the characteristic feature of HCC that developed early after the eradication of HCV by DAA therapy, may be the hallmark of the possible malignant phenotype of HCC in general.
Despite having these imaging features of malignant potential, the prognosis of HCC after DAA treatment was comparable to HCV-positive HCC that received curative RFA therapy in terms of OS or PFS. The possible reason for this fair prognosis is that all DAA-SVR HCC was found in the early stage (mean diameter of 18 mm and 81% as single nodule), and actually 25 of 26 patients received curative therapy (resection or RFA in 23 patients and transcatheter arterial chemoembolization with curative consent in 2 patients). However, if these patients were found in a more advanced stage, the prognosis may have been worse due to rapid progression or by the inability to receive curative therapy. Mariño et al.[21] recently reported that HCC incidence was 3.73 HCC/100 person-years in 1123 cirrhotic patients treated with interferon (IFN)-free regimens and 72 patients developed HCC within a median of 10.3 months after starting antiviral treatment. Although some large-cohort studies and systemic reviews revealed that there were no significant differences in hepatocarcinogenesis after achieving SVR between patients treated with IFN and those treated with DAAs, there are still unknown mechanisms involved in the increased risk of HCC emergence in IFN-free regimens. Yoshimasu et al.[22] reported that the HCC occurrence rate after DAA treatment was very low and the recurrence rate was lower than that in previous IFN reports.
The AFP level and AFP-L3% were identified as important factors in predicting the occurrence/recurrence of HCC; thus, patients with such levels are still at risk of developing cancer after SVR. Villani et al.[23] reported that DAA administration induced an early increase in serum vascular endothelial growth factor (VEGF) and a change in the inflammatory pattern, coinciding with HCV clearance. Debes et al.[24] identified a set of 12 immune mediators whose levels were significantly higher in serum before DAA treatment of patients who eventually developed de novo HCC compared to controls. A panel of nine cytokines, measured in serum before treatment (MIG, IL22, TRAIL, APRIL, VEGF, IL3, TWEAK, SCF, and IL21), identified patients who developed de novo HCC with an area under the receiver operating characteristic curve value higher than 0.8. Further analyses of the mechanism also provide important information about HCV-induced carcinogenesis and the effects of DAAs. In this study, we focused on the HCV status; however, HCC recurrence and overall survival are associated with multiple factors including liver fibrosis, immune status, life style, and comorbidities.
Several studies revealed that the ADC value was a predictive factor of the histological grade of HCC[25-27]. However, in this study, we could not find a significant difference in ADC values between DAA-SVR and HCV-positive HCC. In contrast to these studies that included patients who received resection or transplantation, our patients had a smaller size of HCC, which led to inaccurate quantification values due to technical error.
There are some limitations in this study. The sample size was small and this study was conducted in a single center. DAA-SVR HCC patients received HCC screening within six months before administration of DAAs; however, the imaging modality was not uniform, including CT, MRI, or ultrasound. Therefore, we could not definitely exclude the possibility that small hepatic nodules that could be detected only by HPB of EOB-MRI were missed in some patients before DAA treatment. Tumor biopsy was performed only when the patients agreed to the procedure and the location of the tumor was not near large vessels, liver surface, and other organs. Therefore, the correlation between pathological and MRI features should be evaluated in a large cohort in which patients received liver resection.
Our study is the first to reveal the significant differences in MRI findings between DAA-SVR and HCV-positive HCC. According to our results, HCC that develop within one year after the end of DAA treatment would have unique imaging features that may be linked to malignant phenotype if not found and treated early. Today, most patients with HCV infection can achieve viral eradication with DAA therapy. However, in high-risk patients such as those with cirrhosis, the surveillance of HCC should be done at early time points after SVR to diagnose HCC at an early stage and curatively treat it with resection, RFA, or microwave ablation, which may lead to better OS in these patients.
Declarations
AcknowledgmentsWe really appreciate the meaningful suggestion from Dr. Utaroh Motosugi (Yamanashi university) and valuable pathological comments from Dr. Kotaro Matsunaga (St. Marianna University school of Medicine).
Authors’ contributionsMade substantial contributions to conception and design of the study and performed data analysis and interpretation: Shimizu T, Tsuchiya K, Kurosaki M
Conceptualization: Shimizu T, Tsuchiya K, Kurosaki M
Methodology: Yasui Y, Hisamatsu T
Data collection: Kirino S, Watakabe K, Osawa L, Okada M, Wang W, Higuchi M, Takaura K, Kaneko S, Tamaki N, Nakanishi H, Itakura J, Takahashi Y
Writing - original draft preparation: Shimizu T, Tsuchiya K,
Writing - review and editing: Kurosaki M
Supervision: Hisamatsu T, Izumi N
Availability of data and materialsThe original raw data used to support the findings of this study have not been made available because of the risk that will come into conflict with Personal Information Protection Law in Japan.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateThis study was approved by the ethical committee at Musashino Red Cross Hospital (approval number 28077). Written informed consent was obtained from each patient.
Consent for publicationNot applicable.
Copyright© The Author(s) 2020.
REFERENCES
2. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, et al. Estimates of worldwide burden of cancer in 2008: Globocan 2008. Int J Cancer 2010;127:2893-917.
3. Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut 2014;63:844-55.
4. Pawlotsky JM. New hepatitis C therapies: the toolbox, strategies, and challenges. Gastroenterology 2014;146:1176-92.
5. Toyoda H, Kumada T, Tada T, Mizuno K, Tanaka J, et al. The impact of HCV eradication by direct-acting antivirals on the transition of precancerous hepatic nodules to HCC: a prospective observational study. Liver Int 2019;39:448-54.
6. Huppertz A, Balzer T, Blakeborough A, Breuer J, Giovagnoni A, et al. Improved detection of focal liver lesions at MR imaging: multicenter comparison of gadoxetic acid enhanced MR images with intraoperative findings. Radiology 2004;230:266-75.
7. Kitao A, Matsui O, Yoneda N, Kozaka K, Shinmura R, et al. The uptake transporter OATP8 expression decreases during multistep hepatocarcinogenesis: correlation with gadoxetic acid enhanced MR imaging. Eur Radiol 2011;21:2056-66.
8. Kim HY, Choi JY, Kim CW, Bae SH, Yoon SK, et al. Gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging predicts the histological grade of hepatocellular carcinoma only in patients with Child-Pugh class A cirrhosis. Liver Transplant 2012;18:850-7.
9. Kogita S, Imai Y, Okada M, Kim T, Onishi H, et al. Gd-EOB-DTPA enhanced magnetic resonance images of hepatocellular carcinoma: correlation with histological grading and portal blood flow. Eur Radiol 2010;20:2405-13.
10. Lim KS. Diffusion-weighted MRI of hepatocellular carcinoma in cirrhosis. Clin Radiol 2014;69:1-10.
11. Kokudo N, Takemura N, Hasegawa K, Takayama T, Kubo S, et al. Clinical practice guidelines for hepatocellular carcinoma: the Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res 2019;49:1109-13.
12. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182-236.
13. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723-50.
14. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013;48:452-8.
15. Hwang J, Kim YK, Jeong WK, Choi D, Rhim H, et al. Nonhypervascular hypointense nodules at gadoxetic acid enhanced MR imaging in chronic liver disease. Radiology 2015;276:137-46.
16. Kwon HJ, Byun JH, Kim JY, Hong GS, Won HJ, et al. Differentiation of small (≤ 2 cm) hepatocellular carcinomas from small benign nodules in cirrhotic liver on gadoxetic acid-enhanced and diffusion-weighted magnetic resonance images. Abdom Imaging 2015;40:64-75.
17. Inoue T, Hyodo T, Murakami T, Takayama Y, Nishie A, et al. Hypovascular hepatic nodules showing hypointense on the hepatobiliary-phase image of Gd-EOB-DTPA-enhanced MRI to develop a hypervascular hepatocellular carcinoma: A nationwide retrospective study on their natural course and risk factors. Dig Dis 2013;31:472-9.
18. Chou CT, Chou JM, Chang TA, Huang SF, Chen CB, et al. Differentiation between dysplastic nodule and early-stage hepatocellular carcinoma: the utility of conventional MR imaging. World J Gastroenterol 2013;19:7433-9.
19. Jiang T, Xu JH, Zou Y, Chen R, Peng LR, et al. Diffusion-weighted imaging (DWI) of hepatocellular carcinomas: a retrospective analysis of the correlation between qualitative and quantitative DWI and tumor grade. Clin Radiol 2017;72:465-72.
20. Okamura S, Sumie S, Tonan T, Nakano M, Satani M, et al. Diffusion-weighted magnetic resonance imaging predicts malignant potential in small hepatocellular carcinoma. Dig Liver Dis 2016;48:945-52.
21. Mariño Z, Darnell A, Lens S, Sapena V, Díaz A, et al. Time association between hepatitis C therapy and hepatocellular carcinoma emergence in cirrhosis: Relevance of non-characterized nodules. J Hepatol 2019;70:874-84.
22. Yoshimasu Y, Furuichi Y, Kasai Y, Takeuchi H, Sugimoto K, et al. Predictive factors for hepatocellular carcinoma occurrence or recurrence after direct-acting antiviral agents in patients with chronic hepatitis C. J Gastrointest Liver Dis 2019;28:63-71.
23. Villani R, Vendemiale G, Serviddio G. Molecular mechanisms involved in HCC recurrence after direct-acting antiviral therapy. Int J Mol Sci 2018;20:49.
24. Debes JD, van Tilborg M, Groothuismink ZMA, Hansen BE, Schulze Zur Wiesch J, et al. Levels of cytokines in serum associate with development of hepatocellular carcinoma in patients with HCV infection treated with direct-acting antivirals. Gastroenterology 2018;154:515-7.e3.
25. Nakanishi M, Chuma M, Hige S, Omatsu T, Yokoo H, et al. Relationship between diffusion-weighted magnetic resonance imaging and histological tumor grading of hepatocellular carcinoma. Ann Surg Oncol 2012;19:1302-9.
26. Shankar S, Kalra N, Bhatia A, Srinivasan R, Singh P, et al. Role of diffusion weighted imaging (DWI) for hepatocellular carcinoma (HCC) detection and its grading on 3T MRI: a prospective study. J Clin Exp Hepatol 2016;6:303-10.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Shimizu T, Tsuchiya K, Kurosaki M, Yasui Y, Kirino S, Watakabe K, Osawa L, Okada M, Wang W, Higuchi M, Takaura K, Kaneko S, Tamaki N, Nakanishi H, Itakura J, Takahashi Y, Hisamatsu T, Izumi N. Distinctive magnetic resonance imaging findings of hepatocellular carcinoma after hepatitis C virus eradication with direct-acting antivirals. Hepatoma Res 2020;6:12. http://dx.doi.org/10.20517/2394-5079.2019.48
AMA Style
Shimizu T, Tsuchiya K, Kurosaki M, Yasui Y, Kirino S, Watakabe K, Osawa L, Okada M, Wang W, Higuchi M, Takaura K, Kaneko S, Tamaki N, Nakanishi H, Itakura J, Takahashi Y, Hisamatsu T, Izumi N. Distinctive magnetic resonance imaging findings of hepatocellular carcinoma after hepatitis C virus eradication with direct-acting antivirals. Hepatoma Research. 2020; 6: 12. http://dx.doi.org/10.20517/2394-5079.2019.48
Chicago/Turabian Style
Shimizu, Takao, Kaoru Tsuchiya, Masayuki Kurosaki, Yutaka Yasui, Sakura Kirino, Keiya Watakabe, Leona Osawa, Mao Okada, Wan Wang, Mayu Higuchi, Kenta Takaura, Shun Kaneko, Nobuharu Tamaki, Hiroyuki Nakanishi, Jun Itakura, Yuka Takahashi, Tadakazu Hisamatsu, Namiki Izumi. 2020. "Distinctive magnetic resonance imaging findings of hepatocellular carcinoma after hepatitis C virus eradication with direct-acting antivirals" Hepatoma Research. 6: 12. http://dx.doi.org/10.20517/2394-5079.2019.48
ACS Style
Shimizu, T.; Tsuchiya K.; Kurosaki M.; Yasui Y.; Kirino S.; Watakabe K.; Osawa L.; Okada M.; Wang W.; Higuchi M.; Takaura K.; Kaneko S.; Tamaki N.; Nakanishi H.; Itakura J.; Takahashi Y.; Hisamatsu T.; Izumi N. Distinctive magnetic resonance imaging findings of hepatocellular carcinoma after hepatitis C virus eradication with direct-acting antivirals. Hepatoma. Res. 2020, 6, 12. http://dx.doi.org/10.20517/2394-5079.2019.48
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 0 clicks
Cite This Article 0 clicks










Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.