Biomarkers for living donor liver transplants in hepatocellular carcinoma
Abstract
Liver transplantation is one of the more definitive treatments for hepatocellular carcinoma (HCC). In the United States, liver transplantation has historically been focused on deceased donor organs, and tumor burden is used for risk-stratifying patients on the transplant waitlist. Living donor liver transplantation (LDLT) is gaining popularity in the United States and has long been practiced in Asian countries. To improve outcomes of overall survival and disease-free survival post-living donor liver transplantation, surrogates of tumor biology are now being regarded to be as important as tumor burden. This article reviews the different surrogates of tumor biology and discusses their role in the application of LDLT for advanced HCC.
Keywords
INTRODUCTION
Liver transplantation is a potential treatment for those with hepatocellular carcinoma (HCC). The Milan Criteria and University of California San Francisco (UCSF) criteria allow patients to be appropriately selected for liver transplantation and remain the benchmark in many institutions. These criteria used solely tumor burden (size and number) and were developed specifically in the deceased donor liver transplantation (DDLT) setting to help prioritize patients on the transplant waiting list and allow for fair allocation of the limited organs in the organ pool[1,2]. As many cases which exceed the Milan criteria have good prognoses, multiple attempts have been made to expand criteria, initially through the expansion of tumor burden. These criteria for living donor liver transplantation (LDLT) were not reported until 2007 and 2008, as the Tokyo and Asan criteria, respectively[3,4]. In recent years, partly due to very long waitlist times, LDLT has become a popular option to bypass the long waiting process in anticipation of liver transplantation.
Living donor liver transplantation
In LDLT, a specific donor liver is dedicated to one specific recipient. This arrangement is made through a more personal process than DDLT. In LDLT, as the donor is still living, there are additional risks of donor-related complications, even mortality. LDLT donor death is approximated to be around 0.20%[5], while donor complication rates can be as high as 80%[6-10]. Keeping in mind the living donor’s risks, a recent International Liver Transplantation Society (ILTS) statement declared that the accepted ethical justification for LDLT is a 5-year survival probability of at least 60%[11].
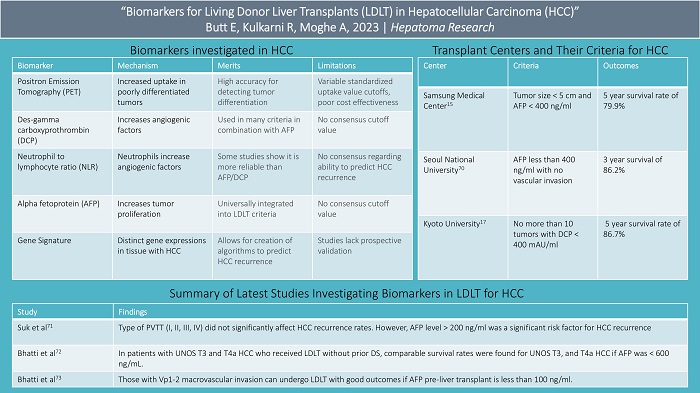
Further results comparing LDLT to DDLT in terms of overall survival (OS) and disease-free survival (DFS) are not in agreement, as some studies show worse outcomes with LDLT while others show no difference[12,13]. Regardless, many large LDLT institutions have attempted to expand their LDLT criteria to increase the number of eligible recipients with the aid of tumor biology[3,4,14-16]. For example, the initial Kyoto criteria included recipients with fewer than 10 tumors, with the largest tumor being smaller than 5 cm and a protein induced by vitamin K absence-II (PIVKA-II) or des-gamma carboxyprothrombin (DCP) level less than 400 mAU/ml[17]. These criteria provided an 82% 5-year post-LDLT survival rate. The National Cancer Center - Korea criteria included negative positron emission tomography (PET) scan and tumor diameter smaller than 10 cm for LDLT recipients, which allows for an 84% 5-year post LDLT survival rate compared to 60% for those outside these criteria[18].
ROLE OF TUMOR BIOLOGY AND THE USE OF BIOMARKERS
Frequently, small HCC tumors may have more aggressive features leading to worse outcomes after liver transplantation, while larger tumors may have less aggressive features resulting in better post-transplant outcomes. Hence, tumor biology rather than tumor number or size is thought to better risk-stratify those undergoing liver transplantation. One method to examine the tumor biology of HCC in a patient is obtaining tissue via a liver biopsy. Although liver biopsy is the gold standard used to investigate the biological behavior of HCC, there are downsides to biopsy, and hence, HCCs are rarely biopsied. Besides the obvious risk of tumor seeding/spread, a pre-transplant tumor biopsy may underestimate tumor differentiation as HCC is highly heterogeneous[19]. In addition, it is difficult to assess microvascular invasion in a single biopsy due to sampling bias[20]. Therefore, surrogate markers of tumor biology such as PET, PIVKA-II, neutrophil to lymphocyte ratio (NLR), and alpha-fetoprotein (AFP) are very useful. We will review these in more detail in this manuscript.
Positron-emission tomography
Positron-emission tomography (PET) avidity is one of the methods used to assess tumor biology. The more poorly differentiated HCC cells are, the higher their uptake of 18F-fluorodeoxyglucose [(18F)FDG][21,22]. Therefore, PET avidity is typically associated with high levels of AFP and microvascular invasion. Because normal liver physiology is associated with some uptake of 18F-FDG, the definition of PET positivity is not universally established. Objective markers to attempt to define positivity have been used, which include the tumor-to-normal liver ratio maximum standardized uptake value (SUV), the maximum SUV of the tumor, and the ratio of the tumor SUV max to the normal liver mean SUV max. Optimal cut-offs for these values are still being debated[23,24].
Despite this lack of uniformity, PET avidity is used by many institutions as a surrogate of tumor biology as it has a high accuracy for detecting tumor differentiation (54 to 71%) and microvascular invasion (68 to 88%)[25-30]. In addition, PET avidity has allowed providers to predict HCC recurrence in advanced HCC. HCC with tumor burden beyond Milan/UCFS criteria but those which display non-avid [18F]FDG PET activity are shown to have a lower recurrence of HCC compared to patients with high avidity but within the conventional criteria[31,32]. Specifically, for those with high avidity, the recurrence-free survival rate 3 years after undergoing liver transplantation has been shown to be 35 to 57% versus 84 to 94% in those with non-avid PET scans[26-29,33]. The use of PET in selection criteria for LDLT was strongly recommended per the ILTS consensus in 2020[11]. However, cost-effectiveness, low sensitivity, and variable SUV cut-offs are barriers to incorporating the widespread use of PET avidity in transplant criteria[34].
Protein induced by vitamin K absence-II / Des-gamma carboxyprothrombin
PIVKA-II or DCP is a form of prothrombin created in the absence of vitamin K. It is produced in large quantities in the setting of malignant hepatocytes. The abnormal prothrombin upregulates angiogenic factors which in turn increase the risk of metastasis and microvascular invasion[35-38]. Consequently, it has been shown to correlate with the degree of HCC malignancy, like PET avidity.
DCP is useful for predicting the risk of HCC recurrence after liver transplantation. Although there is no consensus cut-off value, it has been successfully incorporated into many criteria in combination with AFP. For example, the Kyushu and Kyoto criteria combine different levels of DCP and AFP to provide patients beyond the Milan criteria with survival rates over 80% who otherwise would not be able to undergo liver transplantation[37,39,40]. DCP’s integration with AFP has been shown to be more effective than using one or the other alone. For instance, the Mayo Clinic reported a hazard ratio of HCC recurrence after liver transplantation of 2.8 and 3.5 when using AFP and DCP alone, respectively. With the combination of the two values, the hazard ratio increased to 5.2[41]. The integration of PIVKA-II into LDLT selection criteria was strongly recommended by the ILTS in 2020[11].
Neutrophil-lymphocyte ratio
Neutrophil-lymphocyte ratio (NLR) is the ratio of neutrophils to lymphocytes in the blood. The rationale behind using NLR to measure tumor aggressiveness is thought to be explained by neutrophils upregulating angiogenic factors[42]. NLR is a widely used marker of tumor biology and is accepted by many as an independent risk factor for the recurrence of HCC post-transplant[43]. As a surrogate of tumor biology, it has shown its greatest efficacy in combination with other tumor markers[43-48].
The association between NLR and HCC recurrence was first shown in 2009 by Halazun et al.[49]. A meta-analysis by Xu et al. 9 years later showed that an elevated NLR correlated with higher odds of vascular invasion (Odds ratio 2.39) and lower recurrence-free survival after liver transplantation with a hazard ratio of 3.77[50]. This analysis also found that those with HCC outside the Milan criteria more frequently had an elevated NLR compared to those within the Milan criteria. The study suggested a cut-off value of 4 when incorporating NLR into liver transplantation criteria. While some have shown that NLR is a more reliable predictor of HCC recurrence than AFP or DCP[51], others have shown that DCP and AFP have larger predictive power than NLR[43]. As a result, there is not a universal consensus in terms of its consistency in predicting HCC recurrence or how it compares in relation to AFP or DCP[43,51-53].
Alpha-fetoprotein and downstaging
AFP is thought to upregulate tumor proliferation by activating cell adhesion molecules and signaling tyrosine protein kinases which accelerate cellular growth[54,55]. AFP has been almost universally integrated into LDLT criteria and is commonly used to assess tumor biology. It can help predict post-transplant HCC recurrence and survival times in patients with advanced HCC, specifically in patients who undergo downstaging, which involves locoregional therapy (LRT) such as radiofrequency ablation or trans-arterial chemoembolization, to shrink the tumor dimensions to meet acceptable transplantation criteria[56,57]. For example, in those with AFP larger than 1,000 ng/ml, studies have shown that reducing this level to less than 100 ng/ml with DS allows a 5-year survival rate of 88% versus 67% in those whose AFP level only fell to 101-499 ng/ml with downstaging[58]. Halazun et al provided similar results showing that those with initial AFP 200-1000 which dropped to below 200 with LRT had better post-liver transplantation results[59]. Other studies have shown that AFP greater than 1,000 ng/ml prior to liver transplantation showed higher rates of HCC recurrence after liver transplantation even when HCC met UCSF criteria[56,60,61].
The roles of downstaging (DS) in LDLT are controversial. Historically, macrovascular invasion was an absolute contraindication to LDLT. However, the use of tumor biology and its surrogates have allowed those with macrovascular invasion to undergo LDLT if they have good tumor biology. For example, even in patients with advanced HCC and a tumor thrombus in segmental branches, an AFP 10-100 ng/ml and a great response to DS have been shown to be effective predictors of longer survival times for recipients of LDLT compared to those without sufficient DS response and AFP levels[56,62-64].
Although some pre-liver transplantation criteria have been proposed across the world for LDLT, the absence of widely accepted criteria for DS in LDLT in those with advanced HCC remains an issue. Many patients with advanced HCC are reasonable candidates for LDLT, while up to 1/5 of those within traditional Milan criteria are poor candidates for LDLT[65]. AFP is a useful tool that can be used to better identify those in either group. Although there is no consensus on the AFP cut-off value, the integration of AFP into LDLT and DDLT selection criteria has been strongly recommended by ILTS[11].
Gene signature
Gene signature is another surrogate of tumor biology. Using silico gene expression analysis, scientists are able to observe which genes occur more frequently in HCC[66]. Genes that occur most frequently in HCC are grouped together to allow for the creation of multigene signature models to better inform clinicians of HCC management[67]. Patients with certain gene signatures are then stratified into different tiers based on their outcomes regarding HCC.
A study by Wang et al. investigated candidate genes selected from Gene Expression Profiling Interactive Analysis (GEPIA)[68]. These genes and their association with survivability and HCC were examined through reverse transcription PCR with cDNA microarrays. Seven genes of interest were compared with two reference genes to create a low, intermediate, and high-risk group of patients with HCC. The 3-year overall survival rate was 20.6% in the high risk, 74.5% in the intermediate risk, and 88.9% in the low-risk group. Another study by Son et al. identified the expression of five different genes with the use of quantitative reverse transcription PCR and found that certain combinations of genes (HMGA1 and MPZL1) better predicted HCC recurrence versus other combinations with an AUC (0.807, 95% confidence interval = 0.681-0.899)[66]. They also found certain combinations of genes to be upregulated in patients with microvascular invasion, while other genes, such as MPZL1 and SNRPB, were correlated to the degree of tumor differentiation. Overexpression of genes such as RAGCAP1 significantly affected overall survivability, while HMGA1 significantly affected DFS. Lastly, a review by Pinto-Marques et al. investigated a 4-gene signature in combination with different clinical variables to create an algorithm to identify which patients had over 99% DFR at 5 years, with 16-24% of these patients being outside clinical criteria[69]. Although gene signature is promising, many of the prior studies lack prospective validation, thus limiting its widespread use in clinical practice and LDLT.
CRITERIA INCORPORATING TUMOR BIOLOGY
Different criteria incorporating various markers of tumor biology to predict HCC survival and recurrence after liver transplantation have been created. Specifically, for LDLT, many Asian institutions have created criteria incorporating tumor biology to predict outcomes and recurrence in those beyond the Milan criteria. Samsung Medical Center uses criteria incorporating tumor size less than 5 centimeters and an AFP less than 400 ng/ml with no limitation on the tumor number[15]. One- and five-year survival rates were 92.2% and 79.9%, respectively[15]. Seoul National University uses criteria incorporating a preoperative AFP of less than 400 ng/ml with no vascular invasion with a 3-year survival rate of 86.2%[70]. Asan Medical Center’s LDLT criteria incorporate no more than 6 tumors, each no larger than 5 centimeters, with no gross vascular invasion, producing a five-year survival rate of 81.6%[3]. The University of Tokyo uses criteria that allow the number of tumors to be no more than 5, no larger than 5 cm, with a 94% recurrence-free survival rate after LDLT[4]. Kyoto University uses similar criteria, except they allow the number of tumors to reach 10 if PIVKA-II levels are less than 400 mAU/ml[17]. This criterion results in a 5-year survival rate of 86.7%. Lastly, a study in Japan regarding 653 patients found that patients with HCC extending past the Milan criteria had an 84.3% 5-year disease-free survival as long as AFP levels were no larger than 200 ng/ml and PIVKA-II levels were no larger than 100 mAU/ml[40].
RECENT STUDIES INVESTIGATING THE IMPACT OF TUMOR BIOLOGY IN LDLT FOR ADVANCED HCC
A study by Suh et al. investigated the post-transplant recurrence rates for HCC in patients undergoing LDLT for HCC with portal vein tumor thrombus (PVTT)[71]. The type of PVTT (I, II, III, IV) did not significantly affect HCC recurrence rates. However, AFP level > 200 ng/ml was a significant risk factor for HCC recurrence. All patients with AFP > 200 ng/ml and PVTT had recurrence in 2 years post-LDLT, while those with PVTT but AFP level less than or equal to 200 ng/ml had a 3-year overall survival rate of 87.5% and disease-free survival rate of 65.6% 3 years after LDLT.
Regarding DDLT, transplant eligibility for T3–T4 HCC requires successful downstaging (DS). LDLT can be considered selectively in these patients without DS, but its role is not clearly defined. A more recent by Bhatti et al. looked carefully at the role of tumor biology in patients with advanced HCC based on UNOS staging who received LDLT without prior DS[72]. 5-year recurrence-free survival was compared in patients with advanced HCC. The recurrence rate of HCC in T3, T4a, and T4b HCC was 16.1, 5.9, and 37.5%, respectively. It was shown that T4b HCC (macrovascular invasion) and AFP > 600 ng/mL were significant predictors of recurrence. When excluding patients with AFP > 600 ng/mL, the 5-year recurrence-free survival for T3, and T4a HCC was 86% and 92%, respectively. The study found that in patients with UNOS T3 and T4a HCC who received LDLT without prior DS, comparable survival rates were found for UNOS T3, and T4a HCC if AFP was < 600 ng/mL.
Lastly, Bhatti et al. investigated the role of tumor biology in LDLT patients with advanced HCC and macrovascular invasion[73]. Patients that met A-VENA criteria[74] for macrovascular invasion were included in the study. A-VENA criteria were proposed by Sherman et al., which accurately differentiates bland portal veisn thrombosis from tumor portal vein thrombosis in patients who meet at least three of the following: venous expansion, thrombus enhancement, an AFP > 1,000 ng/dl, adjacency to HCC, and neovascularity. Regarding PVTT, LDLT was considered in patients with tumor thrombosis in segmental branches (Vp1-2) or lobar branches (Vp3)[74]. The role of AFP was studied in patients who met UCSF criteria, their center-specific USCF+ criteria (largest tumor diameter ≤ 10 cm, any tumor number, AFP ≤ 1,000 ng/ml), and those with macrovascular invasion. The recurrence rate of HCC was 13% for those within UCSF criteria versus 36% for those within UCSF+ criteria. In the UCSF+ group, the recurrence rate decreased from 36% to 27% when patients with AFP greater than 600 ng/ml were excluded.
Patients who underwent DS were characterized as being low risk (AFP less than or equal to 100 ng/ml and good response to DS) or high risk (AFP greater than 100 ng/ml or poor response to DS). In LDLT patients with macrovascular invasion who underwent DS, 80% of those in the low-risk group and 20% in the high-risk group remained alive at the end of a 5-year period after transplant. Patients who were not eligible to undergo DS were also divided into high-risk (AFP > 100 or Vp3 macrovascular invasion) or low-risk (AFP less than equal to 100 ng/ml and Vp1-2 macrovascular invasion) groups. All patients in the low-risk group were alive at the end of the 5-year period after transplant, while only 1/9 of patients in the high-risk group were alive at the time.
The study supported the idea that incorporating AFP into criteria can allow for acceptable survival in patients with advanced HCC who undergo LDLT. Specifically, those with Vp1-2 macrovascular invasion can undergo LDLT with good outcomes if AFP pre-liver transplantation is less than 100 ng/ml.
These results have changed Shifa International Hospital’s protocol to incorporate the following: 1. All patients without evidence of extrahepatic disease and main portal vein tumor thrombosis but with AFP > 1000 ng/ml undergo DS; 2. All patients with macrovascular invasion have a staging PET scan, and in those who undergo DS, only those whose AFP drops below 100 ng/ml after DS undergo LDLT; 3. After PET, in those not eligible for DS, those with a low AFP and Vp1-2 macrovascular invasion can still be considered for LDLT.
CONCLUSION
In patients with advanced HCC, LDLT can be a potential treatment option. The use of biomarkers for tumor biology is helping to expand the patient selection in those undergoing LDLT. As major advancements continue to be made in the detection of effective biomarkers for tumor biology, progress towards expanded criteria for LDLT in patients with advanced HCC will continue to be made. This progress will hopefully propel the Western world towards increasing the number of living donor liver transplants for HCC, following suit with their counterparts in the East[75].
DECLARATIONS
Authors’ ContributionsMade substantial contributions to the gathering of information, analyzing of information, authorship, and editing of this article: Butt E, Kulkarni R, Akshata M
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9.
2. Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394-403.
3. Lee SG, Hwang S, Moon DB, et al. Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center. Liver Transpl 2008;14:935-45.
4. Sugawara Y, Tamura S, Makuuchi M. Living donor liver transplantation for hepatocellular carcinoma: Tokyo University series. Dig Dis 2007;25:310-2.
5. Trotter JF, Adam R, Lo CM, Kenison J. Documented deaths of hepatic lobe donors for living donor liver transplantation. Liver Transpl 2006;12:1485-8.
6. Cho JY, Suh KS, Kwon CH, et al. Outcome of donors with a remnant liver volume of less than 35% after right hepatectomy. Liver Transpl 2006;12:201-6.
7. Hwang S, Lee SG, Lee YJ, et al. Lessons learned from 1,000 living donor liver transplantations in a single center: how to make living donations safe. Liver Transpl 2006;12:920-7.
8. Lo CM, Fan ST, Liu CL, et al. Lessons learned from one hundred right lobe living donor liver transplants. Ann Surg 2004;240:151-8.
9. Marsh JW, Gray E, Ness R, Starzl TE. Complications of right lobe living donor liver transplantation. J Hepatol 2009;51:715-24.
10. Yi NJ, Suh KS, Cho JY, et al. Three-quarters of right liver donors experienced postoperative complications. Liver Transpl 2007;13:797-806.
11. Mehta N, Bhangui P, Yao FY, et al. Liver Transplantation for hepatocellular carcinoma. working group report from the ILTS transplant oncology consensus conference. Transplantation 2020;104:1136-42.
12. Grant RC, Sandhu L, Dixon PR, Greig PD, Grant DR, McGilvray ID. Living
13. Liang W, Wu L, Ling X, et al. Living donor liver transplantation versus deceased donor liver transplantation for hepatocellular carcinoma: a meta-analysis. Liver Transpl 2012;18:1226-36.
14. Ito T, Takada Y, Ueda M, et al. Expansion of selection criteria for patients with hepatocellular carcinoma in living donor liver transplantation. Liver Transpl 2007;13:1637-44.
15. Kwon CH, Kim DJ, Han YS, et al. HCC in living donor liver transplantation: can we expand the Milan criteria? Dig Dis 2007;25:313-9.
16. Soejima Y, Taketomi A, Yoshizumi T, et al. Extended indication for living donor liver transplantation in patients with hepatocellular carcinoma. Transplantation 2007;83:893-9.
17. Kaido T, Ogawa K, Mori A, et al. Usefulness of the Kyoto criteria as expanded selection criteria for liver transplantation for hepatocellular carcinoma. Surgery 2013;154:1053-60.
18. Lee SD, Lee B, Kim SH, et al. Proposal of new expanded selection criteria using total tumor size and (18)F-fluorodeoxyglucose - positron emission tomography/computed tomography for living donor liver transplantation in patients with hepatocellular carcinoma: The national cancer center Korea criteria. World J Transplant 2016;6:411-22.
19. Pawlik TM, Gleisner AL, Anders RA, Assumpcao L, Maley W, Choti MA. Preoperative assessment of hepatocellular carcinoma tumor grade using needle biopsy: implications for transplant eligibility. Ann Surg 2007;245:435-42.
20. Rodríguez-Pérez C, Molina-Montes E, Verardo V, et al. Changes in dietary behaviours during the COVID-19 outbreak confinement in the Spanish COVIDiet study. Nutrients 2020;12:1730.
21. Lin CC, Chen CL. Living donor liver transplantation for hepatocellular carcinoma achieves better outcomes. Hepatobiliary Surg Nutr 2016;5:415-21.
22. Torizuka T, Tamaki N, Inokuma T, et al.
23. Ahn SY, Lee JM, Joo I, et al. Prediction of microvascular invasion of hepatocellular carcinoma using gadoxetic acid-enhanced MR and (18)F-FDG PET/CT. Abdominal imaging 2014;40:843-51.
24. Lin CY, Liao CW, Chu LY, et al. Predictive value of 18F-FDG PET/CT for vascular invasion in patients with hepatocellular carcinoma before liver transplantation. Clin Nucl Med 2017;42:e183-7.
25. Bailly M, Venel Y, Orain I, Salamé E, Ribeiro MJ. 18F-FDG PET in liver transplantation setting of hepatocellular carcinoma: predicting histology? Clin Nucl Med 2016;41:e126-9.
26. Kornberg A, Freesmeyer M, Bärthel E, et al. 18F-FDG-uptake of hepatocellular carcinoma on PET predicts microvascular tumor invasion in liver transplant patients. Am J Transplant 2009;9:592-600.
27. Kornberg A, Küpper B, Tannapfel A, et al. Patients with non-[18 F]fludeoxyglucose-avid advanced hepatocellular carcinoma on clinical staging may achieve long-term recurrence-free survival after liver transplantation. Liver Transpl 2012;18:53-61.
28. Lee SD, Kim SH, Kim SK, Kim YK, Park SJ. Clinical impact of 18F-fluorodeoxyglucose positron emission tomography/computed tomography in living donor liver transplantation for advanced hepatocellular carcinoma. Transplantation 2015;99:2142-9.
29. Lee SD, Kim SH, Kim YK, et al. (18)F-FDG-PET/CT predicts early tumor recurrence in living donor liver transplantation for hepatocellular carcinoma. Transpl Int 2013;26:50-60.
30. Yang SH, Suh KS, Lee HW, et al. The role of (18)F-FDG-PET imaging for the selection of liver transplantation candidates among hepatocellular carcinoma patients. Liver Transpl 2006;12:1655-60.
31. Bhangui P, Saigal S, Gautam D, et al. Incorporating tumor biology to predict hepatocellular carcinoma recurrence in patients undergoing living donor liver transplantation using expanded selection criteria. Liver Transpl 2021;27:209-21.
32. Hsu CC, Chen CL, Wang CC, et al. Combination of FDG-PET and UCSF criteria for predicting HCC recurrence after living donor liver transplantation. Transplantation 2016;100:1925-32.
33. Lee JW, Paeng JC, Kang KW, et al. Prediction of tumor recurrence by 18F-FDG PET in liver transplantation for hepatocellular carcinoma. J Nucl Med 2009;50:682-7.
34. Lu RC, She B, Gao WT, et al. Positron-emission tomography for hepatocellular carcinoma: Current status and future prospects. World J Gastroenterol 2019;25:4682-95.
35. Gao FJ, Cui SX, Chen MH, et al. Des-gamma-carboxy prothrombin increases the expression of angiogenic factors in human hepatocellular carcinoma cells. Life Sci 2008;83:815-20.
36. Poté N, Cauchy F, Albuquerque M, et al. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol 2015;62:848-54.
37. Shirabe K, Itoh S, Yoshizumi T, et al. The predictors of microvascular invasion in candidates for liver transplantation with hepatocellular carcinoma-with special reference to the serum levels of des-gamma-carboxy prothrombin. J Surg Oncol 2007;95:235-40.
38. Wang SB, Cheng YN, Cui SX, et al. Des-gamma-carboxy prothrombin stimulates human vascular endothelial cell growth and migration. Clin Exp Metastasis 2009;26:469-77.
39. Takada Y, Ito T, Ueda M, et al. Living donor liver transplantation for patients with HCC exceeding the Milan criteria: a proposal of expanded criteria. Dig Dis 2007;25:299-302.
40. Todo S, Furukawa H, Tada M. Japanese Liver Transplantation Study Group. Extending indication: role of living donor liver transplantation for hepatocellular carcinoma. Liver Transpl 2007;13:S48-54.
41. Chaiteerakij R, Zhang X, Addissie BD, et al. Combinations of biomarkers and Milan criteria for predicting hepatocellular carcinoma recurrence after liver transplantation. Liver Transpl 2015;21:599-606.
42. Motomura T, Shirabe K, Mano Y, et al. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepatol 2013;58:58-64.
43. Shindoh J, Sugawara Y, Nagata R, et al. Evaluation methods for pretransplant oncologic markers and their prognostic impacts in patient undergoing living donor liver transplantation for hepatocellular carcinoma. Transpl Int 2014;27:391-8.
44. Harimoto N, Yoshizumi T, Shimagaki T, et al. Inflammation-based prognostic score in patients with living donor liver transplantation for hepatocellular carcinoma. Anticancer Res 2016;36:5537-42.
45. Lai Q, Castro Santa E, Rico Juri JM, Pinheiro RS, Lerut J. Neutrophil and platelet-to-lymphocyte ratio as new predictors of dropout and recurrence after liver transplantation for hepatocellular cancer. Transpl Int 2014;27:32-41.
46. Na GH, Kim DG, Han JH, et al. Inflammatory markers as selection criteria of hepatocellular carcinoma in living-donor liver transplantation. World J Gastroenterol 2014;20:6594-601.
47. Wang W, Ye Y, Wang T, et al. Prognostic prediction of male recipients selected for liver transplantation: with special attention to neutrophil to lymphocyte ratio. Hepatol Res 2016;46:899-907.
48. Yoshizumi T, Ikegami T, Yoshiya S, et al. Impact of tumor size, number of tumors and neutrophil-to-lymphocyte ratio in liver transplantation for recurrent hepatocellular carcinoma. Hepatol Res 2013;43:709-16.
49. Halazun KJ, Hardy MA, Rana AA, et al. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg 2009;250:141-51.
50. Xu ZG, Ye CJ, Liu LX, et al. The pretransplant neutrophil-lymphocyte ratio as a new prognostic predictor after liver transplantation for hepatocellular cancer: a systematic review and meta-analysis. Biomark Med 2018;12:189-99.
51. Halazun KJ, Najjar M, Abdelmessih RM, et al. Recurrence after liver transplantation for hepatocellular carcinoma: a new moral to the story. Ann Surg 2017;265:557-64.
52. Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost 2011;9:237-49.
53. Kusumanto YH, Dam WA, Hospers GA, Meijer C, Mulder NH. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis 2003;6:283-7.
54. Lu Y, Zhu M, Li W, et al. Alpha fetoprotein plays a critical role in promoting metastasis of hepatocellular carcinoma cells. J Cell Mol Med 2016;20:549-58.
55. Wang X, Wang Q. Alpha-fetoprotein and hepatocellular carcinoma immunity. Can J Gastroenterol Hepatol 2018;2018:9049252.
56. Bhatti ABH, Qureshi AI, Tahir R, et al. When to call it off: defining transplant candidacy limits in liver donor liver transplantation for hepatocellular carcinoma. BMC Cancer 2020;20:754.
57. Crocetti L, Bozzi E, Scalise P, et al. Locoregional treatments for bridging and downstaging hcc to liver transplantation. Cancers 2021;13:5558.
58. Mehta N, Dodge JL, Roberts JP, Hirose R, Yao FY. Alpha-fetoprotein decrease from > 1,000 to < 500 ng/ml in patients with hepatocellular carcinoma leads to improved posttransplant outcomes. Hepatology 2019;69:1193-205.
59. Halazun KJ, Tabrizian P, Najjar M, et al. Is it time to abandon the milan criteria?: results of a bicoastal US collaboration to redefine hepatocellular carcinoma liver transplantation selection policies. Ann Surg 2018;268:690-9.
60. DuBay D, Sandroussi C, Sandhu L, et al. Liver transplantation for advanced hepatocellular carcinoma using poor tumor differentiation on biopsy as an exclusion criterion. Ann Surg 2011;253:166-72.
61. Hameed B, Mehta N, Sapisochin G, Roberts JP, Yao FY. Alpha-fetoprotein level > 1,000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transpl 2014;20:945-51.
62. Assalino M, Terraz S, Grat M, et al. Liver transplantation for hepatocellular carcinoma after successful treatment of macrovascular invasion - a multi-center retrospective cohort study. Transpl Int 2020;33:567-75.
63. Lee KW, Suh SW, Choi Y, et al. Macrovascular invasion is not an absolute contraindication for living donor liver transplantation. Liver Transpl 2017;23:19-27.
64. Mehta N, Heimbach J, Harnois DM, et al. Validation of a risk estimation of tumor recurrence after transplant (RETREAT) score for hepatocellular carcinoma recurrence after liver transplant. JAMA Oncol 2017;3:493-500.
65. Prasad KR, Young RS, Burra P, et al. Summary of candidate selection and expanded criteria for liver transplantation for hepatocellular carcinoma: a review and consensus statement. Liver Transpl 2011;17 Suppl 2:S81-9.
66. Son JA, Ahn HR, You D, et al. Novel gene signatures as prognostic biomarkers for predicting the recurrence of hepatocellular carcinoma. Cancers 2022;14:865.
67. Zhou T, Cai Z, Ma N, et al. A novel ten-gene signature predicting prognosis in hepatocellular carcinoma. Front Cell Dev Biol 2020;8:629.
68. Wang J, Zhang Q, Shi F, et al. A seven-gene signature to predict prognosis of patients with hepatocellular carcinoma. Front Genet 2021;12:728476.
69. Pinto-Marques H, Cardoso J, Silva S, et al. A gene expression signature to select hepatocellular carcinoma patients for liver transplantation. Ann Surg 2022;276:868-74.
70. Suh KS, Cho EH, Lee HW, Shin WY, Yi NJ, Lee KU. Liver transplantation for hepatocellular carcinoma in patients who do not meet the Milan criteria. Dig Dis 2007;25:329-33.
71. Suh KS, Lee HW. Liver transplantation for advanced hepatocellular carcinoma: how far can we go? Hepat Oncol 2015;2:19-28.
72. Bhatti ABH, Waheed A, Khan NA. Living Donor Liver Transplantation for hepatocellular carcinoma: appraisal of the united network for organ sharing modified TNM staging. Front Surg 2020;7:622170.
73. Bhatti ABH, Naqvi W, Khan NY, et al. Living donor liver transplantation for advanced hepatocellular carcinoma including macrovascular invasion. J Cancer Res Clin Oncol 2022;148:245-53.
74. Sherman CB, Behr S, Dodge JL, Roberts JP, Yao FY, Mehta N. Distinguishing tumor from bland portal vein thrombus in liver transplant candidates with hepatocellular carcinoma: the A-VENA criteria. Liver Transpl 2019;25:207-16.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Butt E, Kulkarni R, Akshata M. Biomarkers for living donor liver transplants in hepatocellular carcinoma. Hepatoma Res 2023;9:20. http://dx.doi.org/10.20517/2394-5079.2023.25
AMA Style
Butt E, Kulkarni R, Akshata M. Biomarkers for living donor liver transplants in hepatocellular carcinoma. Hepatoma Research. 2023; 9: 20. http://dx.doi.org/10.20517/2394-5079.2023.25
Chicago/Turabian Style
Butt, Edward, Rupak Kulkarni, Moghe Akshata. 2023. "Biomarkers for living donor liver transplants in hepatocellular carcinoma" Hepatoma Research. 9: 20. http://dx.doi.org/10.20517/2394-5079.2023.25
ACS Style
Butt, E.; Kulkarni R.; Akshata M. Biomarkers for living donor liver transplants in hepatocellular carcinoma. Hepatoma. Res. 2023, 9, 20. http://dx.doi.org/10.20517/2394-5079.2023.25
About This Article
Copyright
Data & Comments
Data

 Cite This Article 7 clicks
Cite This Article 7 clicks










Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.