Expression and function of collagens in intrahepatic cholangiocarcinoma
Abstract
Intrahepatic cholangiocarcinoma (iCCA) is an aggressive malignancy that originates from the biliary tract located within the liver parenchyma. While the incidence rate of iCCA is increasing worldwide, the existing therapeutic options still remain limited. Thus, the spread of iCCA is the foremost cause of treatment failure representing a major clinical challenge. The aggressive nature and refractoriness of the iCCA are strictly related to the desmoplastic tumor microenvironment, in which cancer cells are surrounded by inflammatory cells, cancer-associated fibroblasts, and the aberrant deposition of members of the collagen family. In recent years, accumulating evidence revealed that remodeling of collagens is pivotal in driving the dissemination of desmoplastic cancers. In this review, we describe the expression profile of collagens and their unique architecture in iCCA and how this can dictate neoplastic cell behavior. The emerging view argues for specific strategies aimed at targeting the collagen architecture that may be useful to hamper iCCA metastasis.
Keywords
INTRODUCTION
Intrahepatic cholangiocarcinoma (iCCA) is a subtype of liver cancer that originates from the epithelial cells lining the biliary tree of the liver[1]. iCCA has the highest lethality among all human primary liver cancer with a dismal prognosis. The 5-year survival rate for iCCA is about 10%, which is further reduced following metastatic spread to the regional lymph nodes[2-4]. Despite the increasing incidence rate of iCCA, surgical resection remains the main therapeutic option to date. However, emerging data indicate that liver transplantation may be an option for iCCA patients with early-stage disease[1,5]. The evidence further supports that radioembolization by injecting Yttrium-90-loaded microspheres through the hepatic arteries may be an effective strategy linked to liver transplantation[6].
One of the unique hallmarks of iCCA is the desmoplastic reaction, which is key in promoting the malignant behavior of this cancer type[7,8]. Desmoplasia in iCCA causes the accumulation of an atypical remodeled extracellular matrix (ECM) along with scarce blood vascularization and abundant lymphatic bed[9-11]. This distinct tumor microenvironment (TME) makes the iCCA refractory to chemotherapy and promotes an early intrahepatic or lymph node metastatization, limiting the surgical resection suitable to less than one-third of patients[4,12]. Desmoplastic TME arises from the accumulation of α-smooth muscle actin positive (α-SMA+) cancer-associated fibroblasts (CAFs) in surrounding ducts and glandular structures of malignant cholangiocytes[13]. CAFs in iCCA are a heterogeneous population of stromal mesenchymal cells derived from various cellular lineages, including activated hepatic stellate cells, liver portal fibroblasts and bone-marrow-derived precursor cells that are attracted from iCCA and induced to adopt a CAF phenotype in the tumor area[14-16]. CAF activation is mediated by soluble factors frequently secreted by both cancer and inflammatory cells, such as platelet-derived growth factor-D (PDGF-D). Contrary to normal cholangiocytes, malignant cells secrete high levels of PDGF-D upon hypoxic stimulation. This stimulates chemotaxis in fibroblasts through the binding to its cognate receptor PDGFRβ , which in turn leads to Rho GTPase and JNK activation[17]. Once this tumor environment is formed, the reciprocal communication between CAFs and iCCA cells supports the tumor progression through the production of pro-invasive growth factors, chemokines, cytokines, and antiangiogenic and lymphangiogenic factors[10,11,18-20]. Most importantly, CAFs release high amounts of ECM proteins and ECM-modifying enzymes, causing a fibrogenic response, which is fundamental for desmoplasia[9,21,22]. Thus, the core of the desmoplastic stroma is represented by a prominent accumulation of collagen molecules[23]. However, while collagens are the most abundant components of the tumor reactive stroma, the relationship between their structure and function in tumor progression has received little attention until recently. In fact, in tumor types, such as breast, pancreatic, and prostate cancer, collagens are no longer considered as a dense and rigid scaffolding that surrounds the tumor mass. Instead, they constitute an active and dynamic network that supports and drives tumor cells for autonomous proliferation, invasion, and migration[24]. However, despite a collagen-enriched TME, this topic is not sufficiently highlighted in iCCA, and the impact of collagen on disease progression remains to be elucidated. In this review, we shed light on the structural and functional role of collagens and their importance in iCCA growth and dissemination.
COLLAGEN TYPES AND THEIR ABERRANT EXPRESSION IN iCCA
Collagen proteins are the most abundant ECM components, representing up to 30% of the total protein in humans[25]. They are grouped in a family composed of twenty-eight different types, each comprising a triple helix selected among the forty-six distinct polypeptide alpha chains[26].
The classification of collagens is based on common domain homology and their function, represented in the following seven categories [Table 1]: fibrillar collagens, fibril-associated collagens with interrupted triple helices (FACITs), network-forming collagens, membrane-associated collagens with interrupted triple helices (MACITs), anchoring fibrils, beaded-filament-forming collagens, and multiple triple-helix domains and interruptions/endostatin-producing collagens (MULTIPLEXIN)[27].
Collagen types and their expression in iCCA
| Category | Type | Type expressed in healthy liver | Type expressed in HCC | Type expressed in iCCA* | Chain detected in iCCA* |
| Fibrillar collagens | I II III V XI XXIV XXVII | I III V XXIV | I II III V XXVII | I III V | COL1A1; COL1A2 COL3A1 COL5A1; COL5A2 |
| FACITs | IX XII XIV XVI XIX XX XXI XXII | IX XII XIV XVI XIX XXI XXII | XIV XVI XXII | XII XIV | COL12A1 COL14A1 |
| Network-forming collagens | IV VIII X | IV VIII X | IV X | IV | COL4A1; COL4A2 |
| Beaded-filament-forming collagens | VI XVI XXVIII | VI XVI XXVIII | VI XVI | VI | COL6A1; COL6A2; COL6A3 |
| MULTIPLEXIN | XV XVIII | XV XVIII | XV XVIII | XVIII | N/A |
| Anchoring fibrils | VII | VII | VII | ||
| MACITs | XIII XVII XXIII XXV | XIII XXV |
The biosynthesis, secretion and degradation of collagen types are finely tuned in a dynamic equilibrium, which dictates the composition and function of tissue-specific ECM scaffold[26]. However, in the tumor desmoplastic reaction, this equilibrium is disturbed by an aberrant collagen deposition due to cross-linking[28]. Consequently, the ECM remodeling results in a pathological condition with a stiff scaffold, in which tissue morphogenesis is lost and malignant progression is favored[29,30]. Extensive collagen deposition in iCCA has been in fact reported since 1987[31]. Hence, the iCCA TME constitutes a distinct collagen pattern that differs profoundly from those expressed in healthy liver and hepatocellular carcinoma (HCC)[32,33].
The collagen categories are discussed below, except the anchoring fibrils and MACITs, which are not yet detected in the iCCA stroma [Table 1].
Fibrillar collagens
Fibrillar collagens are the main structural elements of the interstitial ECM surrounding tissues, including dermis, bone, and tendon[34]. It forms heterotrimeric (type I and type V collagens) or homotrimeric triple helices (type III collagen), which are self-assembled into fibrils to either shape strong and thick fibers (mainly type I) or reticular fibers (type III)[34,35]. In contrast to the well-differentiated HCC, representing the most common type of primary liver cancer, the iCCA ECM displays fibrillar collagen expression 70-fold more than in the tumor stroma[36]. In particular, type I and type III collagen are the predominant components, with fiber scores of 1.62 and 0.85, respectively. By contrast, in the HCC, these collagens are poorly represented (0.002 and 0.02)[36]. Notably, while type I collagen is detected both in the tumoral and non-tumoral tissues of iCCA patients, type III collagen is only found in the iCCA stroma[9]. Moreover, iCCA stroma shows a slight decrease in the type V collagen, known to regulate fibril diameters that prevent the further addition of collagen molecules[9,37]. Both in normal and tumoral tissues, fibrillar collagens are not only involved in tensile strength, but they also provide chemical and mechanical signals to promote cell migration, adhesion, tissue growth and repair. In line with this, by investigating the role of fibrillar collagen expression in the iCCA tumor stroma, we have recently demonstrated how iCCA cell behavior can be modified in terms of tumor cell migration through type III collagen[9].
FACITs
FACITs are short and flexible collagens that contain subunits of the IX, XII, XIV, XVI, XIX, and XXI types[27]. They share the features of proteoglycans and function as single molecules with fibrillar collagens, linking together fibers with the other components of the ECM[34]. In the iCCA stroma, the main represented FACITs are the type XII collagens. Compared to the non-cancerous tissue, which expresses type XIV and XXI alpha-1 chains, the tumor stroma from the iCCA patients was shown to exclusively express collagen type XII alpha-1 chains[9]. Type XII collagens are known to directly link to type I collagen fibers through a bridge of decorins, which are proteoglycans with anti-tumoral activities. Given that the expression of these proteoglycans is significantly reduced in many human cancers, including iCCA[38,39], the switch from type XIV to type XII collagen may be required for the collagen fiber remodeling in the iCCA ECM.
Network-forming collagens
The basement membrane is a pericellular matrix. As an anchorage for epithelial cells, it holds them together and separates them from the surrounding stroma[26]. In normal liver, this sheet-like structure is mainly composed of type IV collagen, which shapes a network together with glycoproteins (i.e., laminins and nidogens) and proteoglycans (perlecan)[40]. Compared to the adjacent non-tumor fibrous tissue, an increased amount of type IV collagens has been observed in iCCA[20,41]. By contrast, other components of the basement membrane (i.e., the large basement membrane heparan sulfate proteoglycan perlecan and laminins) were found to be downregulated[9]. This encapsulates the tumor mass in a physical barrier that initially hinders cancer cells from disseminating. However, during iCCA progression, cancer cells proteolytically perforate this dense type IV collagen network resulting in its progressive dismantle, thus leading to invasiveness[42].
Beaded-filament-forming collagens
Type VI collagen is the most representative member of the beaded-filament-forming collagens[43], and it displays a widespread expression in various tissues. Beyond contributing to the formation of a network of beaded microfilaments with other ECM components, they exert a bridging and anchoring role for cells both in the pericellular and interstitial matrices[44]. Thus, although being a minor component of the ECM, this collagen type is essential for tissue integrity. Accordingly, its accumulation contributes to the distorted architecture of the liver, particularly in hepatic fibrosis[45,46]. Like in the hepatic parenchyma, the type VI collagens composed of alpha-1, alpha-2 and alpha-3 chains have been observed in the iCCA tissue. However, unexpectedly, their expression showed similar levels to the adjacent non-cancerous tissues[9]. However, the expression of type VI collagen in tumors shows a contradictory pattern. While it has been found to be overexpressed in many solid tumors, where it plays a role in tumor growth and metastasis, in other cancer types, such as fibrosarcoma, its expression was not observed in the tumor stroma[47,48]. Considering that type VI collagen is mostly located near or within the blood vessels of the tumor stroma[47], it is likely that the angioinhibitory microenvironment in the iCCA may hamper its overexpression.
MULTIPLEXIN
The type XV and type XVIII collagens are the only two members of the MULTIPLEXIN/endostatin-producing collagens[49]. They are expressed in all vascular and epithelial basement membranes and share a C-terminal non-collagenous domain, the endostatin module. Therefore, beyond their structural role, MULTIPLEXINs are precursors of endostatin. Once released, they act as endogenous inhibitors of angiogenesis by downregulating components of the vascular endothelial growth factor (VEGF) signaling. The latter promotes the synthesis of thrombospondin 1 (THBS1), which is responsible for angiogenesis inhibition in iCCA[10,50]. Expectedly, both the tumor and stromal cells in cholangiocarcinoma express a high level of collagen XVIII[51]. Of note, in primary liver cancer, two variants of the type XVIII collagen, namely the SHORT and LONG forms, are differentially expressed: tumor hepatocytes express the LONG form, whereas cholangiocarcinoma cells express the SHORT form[52].
COLLAGEN MODIFYING PROTEINS IN iCCA
Collagen biosynthesis, modification and degradation are pivotal processes for ECM remodeling in fibrogenic disorders, including desmoplastic cancers[26]. Moreover, increased collagen deposition and cross-linking lead to ECM stiffening, which in turn disrupts tissue morphogenesis and promotes tumor progression and metastasis. The lysyl oxidase (LOX) family members are secreted copper-dependent amine oxidases known to play a key role in the post-translational modification of collagens. Further, elastin catalyzes the covalent cross-linking of these fibers[53]. Collagen cross-linking is essential for the stability and tensile strength of fibrils and fibers, thus lending a structural platform to sustain tumor proliferation, epithelial-mesenchymal transition (EMT), and migration and invasion of liver cancers[54]. Recently, the LOX homolog 2 (LOXL2) has been characterized as a member of the LOX-like (LOXL) family involved in iCCA progression[54]. In a cohort of 107 surgical resections of iCCA patients, Bergeat et al. showed that the level of both mRNA and protein of LOXL2 was upregulated in the tumor stroma[55]. Their findings further suggest that a high expression of LOXL2 is related to the dismal prognosis of iCCA.
In addition to cross-linking, collagen degradation by matrix metalloproteinases (MMPs) is an important process for ECM remodeling. Through the mobilization from ECM-associated reservoirs, the MMPs are further crucial for increasing the level of growth factors and cytokines, primarily the VEGFs[26]. In tumorigenesis, MMPs are released by a variety of cells, such as CAFs, endothelial, inflammatory, and cancer cells. They promote the invasiveness of tumors because of basement membrane degradation[56]. In iCCA, the MMP1, 2, 3 and 9 are known to be overexpressed, while the tissue inhibitor of metalloproteinases 3 (TIMP3) is downregulated[9,57]. This further highlights the role and impact of these ECM regulators in promoting iCCA cell invasion and dissemination.
Among the collagen-modifying proteins, matricellular proteins are pivotal in mediating collagen deposition, maturation and organization, beyond facilitating cell-ECM interaction to sustain cell migration[26]. An aberrant expression of these proteins, such as periostin, THBS1 and 2, secreted protein acidic and rich in cysteine (SPARC), has long been detected in the iCCA stroma. However, their precise role is recently being unveiled[22]. For instance, SPARC, which behaves as a collagen chaperone in the regulation of collagen fiber formation and organization[58], has been observed to regulate the malignant cell behavior of the iCCA through the activation of the PI3K-AKT signaling[59]. Periostin, a matricellular protein that strongly correlates with reduced survival of iCCA patients, has been shown to induce EMT in iCCA cells via the integrin α5β1/TWIST‐2 axis[60]. Further, THBS1 and 2, two matricellular proteins with antiangiogenic properties known to be expressed in the iCCA stroma, have been shown to contribute to the lymphatic vessel formation through vascular cell trans-differentiation and to iCCA cell proliferation, as well as lymph node dissemination in a xenograft mouse model[11].
ARCHITECTURE OF COLLAGEN FIBERS IN iCCA
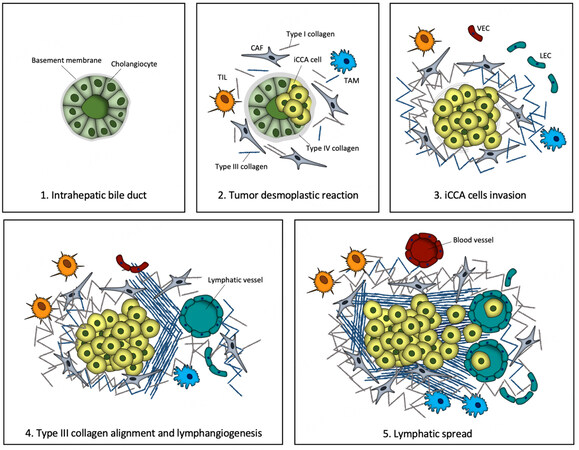
The iCCA progression and metastasization are gradual and progressive events in which malignant cholangiocytes acquire a mesenchymal-like phenotype with increased mobility and ability to cross the basement membrane. Consequently, this leads to cancer cell dissemination via the lymphatic system exploiting tumor-induced lymphangiogenesis [Figure 1].
Figure 1. Tumor progression and collagen patterns in iCCA. (1) Intrahepatic bile duct is composed of polarized cholangiocytes residing on a basement membrane; (2) In the desmoplastic reaction, cancer-associated fibroblasts (CAFs), tumor-infiltrating lymphocytes (TILs) and tumor-associated macrophages (TAMs) are co-opted with iCCA cells by secreting a huge variety of soluble factors. Subsequently, the activity of CAFs leads to type I and type III collagen deposition in the interstitial matrix and to the thickening of the basement membrane by type IV collagen; (3) Neoplastic cell invasion is accomplished by basement membrane dismantling; fibrillar collagens acquire a TACS-1-like structure surrounding the tumor boundary; lymphatic endothelial cells (LECs) and vascular endothelial cells (VECs) populate the tumor reactive stroma; (4) Gradually, type III collagen fibers become aligned and organized tangentially to the tumor mass. Paracrine signals released by TAMs, CAFs and iCCA cells lead to predominant sprouting of lymphatic vessels over the blood vasculature; (5) To complete the invasion-metastasis cascade, the aligned type III collagen organizes perpendicularly to the tumor boundary and acts as a binary track for neoplastic cells to escape toward the neo-formed lymph vessels.
These events are orchestrated by the desmoplastic reaction, by which the increased fibrillar collagen deposition, beyond contributing to elevated stiffness, also creates discrete structural patterns, so-called tumor-associated collagen signatures (TACS)[61]. TACS were first described in 2006 as a three type of peculiar and dynamic collagen fiber reorganization around the tumor mass in breast cancer, which direct cell migration into and through the stroma[61]. Initially, a dense collagen fiber concentration surrounds the tumor mass with a wavy and curly architecture (TACS-1). Subsequently, by exerting tension on the matrix, the CAFs organize collagen fibrils into aligned sheets and arrange cables in parallel to the tumor boundary (TACS-2). Finally, collagen fibers are vertically oriented with respect to the tumor mass, thus providing a conduit for carcinoma cell invasion (TACS-3)[61,62]. In recent years, the existence of these structural collagen patterns has been verified in other desmoplastic solid tumors (i.e., prostate and pancreatic cancers), in which elevated collagen deposition is a typical hallmark[63,64]. More recently, dense and aligned collagen fibers in desmoplastic stroma have also been detected in iCCA by Carpino et al.[9]. Here, type III collagen was identified as the main component of the aligned fibers, suggesting a specific involvement of the reticular fibers in the iCCA-associated ECM remodeling [Figure 1]. Moreover, the same study discovered that iCCA cell motility on type III collagen was significantly increased with respect to type I collagen, indicating that COL3A1 chains act as binary tracks providing contact guidance cues for iCCA cell migration and invasion. These findings suggest that the increased stiffness of ECM, resulting from dense, cross-linked and aligned fibrillar collagen deposition, may promote focal adhesion assembly and enhance cytoskeletal function in cancer cells, which favors proliferation, migration, and invasion[65]. However, as these observations are only a starting point, many questions remain to be addressed, including what is the extent of colocalization between TACS and the lymphatic bed in the iCCA TME? Are the aligned reticular fibers a peculiar feature of iCCA? What is the relationship between aligned reticular fibers and the early metastatic spread? Are TACS present in the extrahepatic subtypes of cholangiocarcinoma? Thus, future studies are needed to clarify this important but unresolved topic in the frame of cholangiocarcinoma.
Even if collagen organization is considered as a promising biomarker for evaluating desmoplastic cancers progression, it is poorly employed in clinical diagnosis. However, advanced microscopy technologies, such as polychromatic polarization microscopy (PPM), are now available to examine the collagen arrangements in diagnosis and prognosis of many diseases[66]. In fact, PPM allows visualizing collagen in unstained tissue specimens, which could complement other imaging techniques due to its highly sensitive detection of cellular and nuclear structures[66]. Accordingly, this system could potentially provide an overview of TACS and may be part of a regular pathology analysis of tissue slides in monitoring cancer progression in patients.
TARGETING COLLAGENS AND COLLAGEN-MODIFYING PROTEINS FOR CANCER TREATMENT
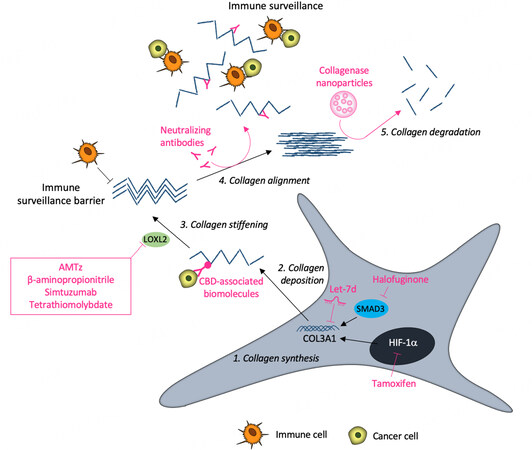
Given that the desmoplastic stroma of the iCCA is pivotal in tumor progression, targeting its aberrant expression of collagen might be considered a potential therapeutic strategy in iCCA, as in other types of solid desmoplastic tumors. To this end, collagen-based targeting approaches can either be directly exerted on collagen(s) or through the modulation of collagen-interacting molecules[67]. Many proteins interact with collagens by means of specific collagen-binding domains (CBD). Thus, antibodies, drugs, or cytokines engineered with a CBD can target and release the CBD-conjugated biomolecules into the collagen scaffold of the TME[68]. This strategy allows circumventing many downsides, such as the off-target effects and the toxicity related to systemic administration, thereby improving the therapeutic effectiveness of the biomolecules[68]. In particular, in the frame of squamous carcinoma, the conjugation of the EGFR binding fragment of cetuximab to a CBD allowed for specific targeting and improved penetration in the tumor area[69]. A similar approach has been applied in order to conjugate a CBD to immune checkpoint inhibitor antibodies and fusion to interleukin (IL)-2 and IL-12[70].
Other strategies aim at promoting tumor collagen degradation, either by collagenase treatment (also encapsulated in nanoparticles and hydrogels) or by modulating collagen structure and biosynthesis[68]. For instance, in order to reduce tumor-associated ECM stiffness, the components of the LOXL family may be considered as attractive drug targets. However, while several strategies have been developed to lower LOXL activity, most of them are in the preclinical phase and require further studies. Currently, the in vivo models offer promising therapeutic tools aimed at improving or analyzing compounds with known targets or the development of novel molecules. In this perspective, the tools developed for lowering LOXL activity mainly include the use of inhibitors. Among them, a series of 2-aminomethylene-5-sulfonylthiazole (AMTz, dual inhibitors of LOX and LOXL2), β-aminopropionitrile and simtuzumab (an anti-LOXL2 antibody) show an ability to delay tumor growth[68,71]. Further, as copper is crucial for LOX activity, copper chelators are currently assessed as therapeutic tools to inhibit LOX activities. For instance, in a recent phase II clinical trial, the copper chelation ability of the tetrathiomolybdate allowed for reducing collagen cross-linking in breast cancer patients[72]. In the frame of the iCCA, the knockdown of LOXL2 reduced both tumor growth and angiogenesis in a xenograft mouse model[73].
Halofuginone is a collagen biosynthesis inhibitor that blocks fibroblast Smad3 activation under the transforming growth factor beta 1 (TGF-β1) signaling. In a pancreatic cancer model, its use improved drug delivery and immune infiltration[74]. Among the other anti-fibrotic drugs, tamoxifen, a selective estrogen receptor modulator, was found to reduce the levels of hypoxia-inducible factor-1α (HIF-1α) in pancreatic cancer, thus decreasing the collagen deposition, fiber alignment, and the tumor tissue stiffness[75]. According to some investigations, collagen and immune cell infiltration can represent a link to increase the efficiency of the immunotherapy approaches, since the non-responders to conventional therapies urgently need new therapeutic treatment strategies. Many studies highlighted the relationship between the dense, aligned collagen and the inhibition of T cell migration into the TME. Thus, the treatment with neutralizing antibodies that interfere with collagen fiber alignment can encourage immune cell infiltration and, consequently, inhibit cancer development. Recently, it has been revealed how the inhibition of LOX enzymatic activity, in combination with anti-programmed cell death protein 1 (anti-PD-1) administration, is able to improve CD8+ T cell infiltration in tumors[76].
Until now, little is known about direct collagen-targeted therapy to specifically counteract iCCA progression. The investigation of the role and function of biomolecules in disease conditions may be essential for several cellular processes, contributing to the maintenance of cell homeostasis[77]. However, in renal cell carcinoma, COL3A1 has been shown to be a target of let-7 miRNA, suppressing cancer cell proliferation, dissemination, and tumor macrophage infiltration[78]. Accordingly, Yu et al. have investigated the expression of long non-coding (lnc) RNAs and mRNAs in several iCCA tissue samples in comparison to adjacent normal tissues and discovered an overexpression of lncRNA LIM and cysteine-rich domains 1 antisense RNA1 (LMCD-AS1) in cancerous tissues[79]. Most importantly, a strong positive correlation of LMCD1-AS1 and type VI collagen α-3 chain (COL6A3) in iCCA tissues was observed, suggesting that the oncogenic role of LMCD1-AS1 may be partly dependent on COL6A3 expression. This study describes the LMCD1-AS1/miR-345–5p/COL6A3 axis as a potential new scenario in specific stroma-targeting therapies to counteract iCCA progression. Figure 2 illustrates a schematic representation of the described therapeutic strategies.
CONCLUSIONS
Collagens are among the most ubiquitous and abundant proteins in human tissues. However, until recent years, evidence has highlighted that collagens play crucial roles in iCCA progression. First, specific collagens and collagen-associated proteins represent a reservoir of cellular mediators that, once released, enhance the iCCA cell malignant behavior and promote tumor-associated lymphangiogenesis. Second, collagen density provides a stiff scaffold to support iCCA cell growth and, as a physical barrier, to escape from the immune T-cell surveillance. Finally, the supramolecular reorganization of the aligned type III collagen during iCCA progression acts as a binary track for cancer cell escape toward neo-formed lymph vessels. Taken together, we believe that deeper investigations on the structural and functional properties of the iCCA-associated collagens will lay the ground for the development of novel targeted therapeutic strategies as well as new tools of pharmacological intervention for this malignancy.
DECLARATIONS
Authors’ contributionsMade substantial contributions to the conception and writing of the manuscript: Colasanti T, Vakifahmetoglu-Norberg H, Mancone C
Availability of data and materialsNot applicable.
Financial support and sponsorshipThis work was supported by AMMF - The Cholangiocarcinoma Charity and by Sapienza University of Rome-Fondi di Ateneo.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. Banales JM, Marin JJG, Lamarca A, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol 2020;17:557-88.
2. Bertuccio P, Malvezzi M, Carioli G, et al. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol 2019;71:104-14.
3. Jutric Z, Johnston WC, Hoen HM, et al. Impact of lymph node status in patients with intrahepatic cholangiocarcinoma treated by major hepatectomy: a review of the National Cancer Database. HPB 2016;18:79-87.
4. Cadamuro M, Romanzi A, Guido M, et al. Translational value of tumor-associated lymphangiogenesis in cholangiocarcinoma. J Pers Med 2022;12:1086.
5. Kodali S, Saharia A, Ghobrial RM. Liver transplantation and intrahepatic cholangiocarcinoma: time to go forward again? Curr Opin Organ Transplant 2022;27:320-8.
6. Aliseda D, Martí-Cruchaga P, Zozaya G, et al. Liver resection and transplantation following yttrium-90 radioembolization for primary malignant liver tumors: a 15-year single-center experience. Cancers 2023;15:733.
7. Brivio S, Cadamuro M, Strazzabosco M, Fabris L. Tumor reactive stroma in cholangiocarcinoma: the fuel behind cancer aggressiveness. World J Hepatol 2017;9:455-68.
8. Sirica AE, Gores GJ. Desmoplastic stroma and cholangiocarcinoma: clinical implications and therapeutic targeting. Hepatology 2014;59:2397-402.
9. Carpino G, Overi D, Melandro F, et al. Matrisome analysis of intrahepatic cholangiocarcinoma unveils a peculiar cancer-associated extracellular matrix structure. Clin Proteomics 2019;16:37.
10. Cadamuro M, Brivio S, Mertens J, et al. Platelet-derived growth factor-D enables liver myofibroblasts to promote tumor lymphangiogenesis in cholangiocarcinoma. J Hepatol 2019;70:700-9.
11. Carpino G, Cardinale V, Di Giamberardino A, et al. Thrombospondin 1 and 2 along with PEDF inhibit angiogenesis and promote lymphangiogenesis in intrahepatic cholangiocarcinoma. J Hepatol 2021;75:1377-86.
12. Cadamuro M, Fabris L, Zhang X, Strazzabosco M. Tumor microenvironment and immunology of cholangiocarcinoma. HR 2022;8:11.
13. Sirica AE. The role of cancer-associated myofibroblasts in intrahepatic cholangiocarcinoma. Nat Rev Gastroenterol Hepatol 2011;9:44-54.
14. Okabe H, Beppu T, Hayashi H, et al. Hepatic stellate cells may relate to progression of intrahepatic cholangiocarcinoma. Ann Surg Oncol 2009;16:2555-64.
15. Dranoff JA, Wells RG. Portal fibroblasts: underappreciated mediators of biliary fibrosis. Hepatology 2010;51:1438-44.
16. Quante M, Tu SP, Tomita H, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell 2011;19:257-72.
17. Cadamuro M, Nardo G, Indraccolo S, et al. Platelet-derived growth factor-D and Rho GTPases regulate recruitment of cancer-associated fibroblasts in cholangiocarcinoma. Hepatology 2013;58:1042-53.
18. da Cunha BR, Domingos C, Stefanini ACB, et al. Cellular interactions in the tumor microenvironment: the role of secretome. J Cancer 2019;10:4574-87.
19. Fabris L, Perugorria MJ, Mertens J, et al. The tumour microenvironment and immune milieu of cholangiocarcinoma. Liver Int 2019;39 Suppl 1:63-78.
20. Sulpice L, Rayar M, Desille M, et al. Molecular profiling of stroma identifies osteopontin as an independent predictor of poor prognosis in intrahepatic cholangiocarcinoma. Hepatology 2013;58:1992-2000.
21. Sirica AE, Almenara JA, Li C. Periostin in intrahepatic cholangiocarcinoma: pathobiological insights and clinical implications. Exp Mol Pathol 2014;97:515-24.
22. Sirica AE. Matricellular proteins in intrahepatic cholangiocarcinoma. hepatobiliary cancers: translational advances and molecular medicine. Elsevier; 2022. pp. 249-81.
23. Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol 2012;196:395-406.
24. Mohan V, Das A, Sagi I. Emerging roles of ECM remodeling processes in cancer. Semin Cancer Biol 2020;62:192-200.
25. Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci 2010;123:4195-200.
26. Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. Extracellular matrix structure. Adv Drug Deliv Rev 2016;97:4-27.
28. Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 2014;15:786-801.
29. Levental KR, Yu H, Kass L, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009;139:891-906.
30. Maller O, Drain AP, Barrett AS, et al. Tumour-associated macrophages drive stromal cell-dependent collagen crosslinking and stiffening to promote breast cancer aggression. Nat Mater 2021;20:548-59.
31. Al-Zuhair AG, Al-Adnani MS, Al-Bader AA, Francis IM. Expression of connective tissue stromal elements in human cholangiocarcinomas. an immunohistochemical and ultrastructural study. J Submicrosc Cytol 1987;19:321-7.
33. Lai KK, Shang S, Lohia N, et al. Extracellular matrix dynamics in hepatocarcinogenesis: a comparative proteomics study of PDGFC transgenic and Pten null mouse models. PLoS Genet 2011;7:e1002147.
34. Gelse K, Pöschl E, Aigner T. Collagens--structure, function, and biosynthesis. Adv Drug Deliv Rev 2003;55:1531-46.
35. Exposito JY, Valcourt U, Cluzel C, Lethias C. The fibrillar collagen family. Int J Mol Sci 2010;11:407-26.
36. Okamura N, Yoshida M, Shibuya A, et al. Cellular and stromal characteristics in the scirrhous hepatocellular carcinoma: comparison with hepatocellular carcinomas and intrahepatic cholangiocarcinomas. Pathol Int 2005;55:724-31.
37. Linsenmayer TF, Gibney E, Igoe F, et al. Type V collagen: molecular structure and fibrillar organization of the chicken alpha 1(V) NH2-terminal domain, a putative regulator of corneal fibrillogenesis. J Cell Biol 1993;121:1181-9.
38. Von Der Mark K. Structure, biosynthesis and gene regulation of collagens in cartilage and bone. dynamics of bone and cartilage metabolism. Elsevier; 2006. pp. 3-40.
39. Yu X, Zou Y, Li Q, et al. Decorin-mediated inhibition of cholangiocarcinoma cell growth and migration and promotion of apoptosis are associated with E-cadherin in vitro. Tumour Biol 2014;35:3103-12.
40. Mak KM, Mei R. Basement membrane type IV collagen and laminin: an overview of their biology and value as fibrosis biomarkers of liver disease. Anat Rec 2017;300:1371-90.
41. Terada T, Nakanuma Y. Expression of tenascin, type IV collagen and laminin during human intrahepatic bile duct development and in intrahepatic cholangiocarcinoma. Histopathology 1994;25:143-50.
42. Brivio S, Cadamuro M, Fabris L, Strazzabosco M. Molecular mechanisms driving cholangiocarcinoma invasiveness: an overview. Gene Expr 2018;18:31-50.
44. Keene DR, Engvall E, Glanville RW. Ultrastructure of type VI collagen in human skin and cartilage suggests an anchoring function for this filamentous network. J Cell Biol 1988;107:1995-2006.
45. Takahara T, Sollberg S, Muona P, Uitto J. Type VI collagen gene expression in experimental liver fibrosis: quantitation and spatial distribution of mRNAs, and immunodetection of the protein. Liver 1995;15:78-86.
46. Baiocchini A, Montaldo C, Conigliaro A, et al. Extracellular matrix molecular remodeling in human liver fibrosis evolution. PLoS One 2016;11:e0151736.
47. Chen P, Cescon M, Bonaldo P. Collagen VI in cancer and its biological mechanisms. Trends Mol Med 2013;19:410-7.
48. Trueb B, Odermatt BF. Loss of type VI collagen in experimental and most spontaneous human fibrosarcomas. Int J Cancer 2000;86:331-6.
49. Izzi V, Heljasvaara R, Heikkinen A, Karppinen SM, Koivunen J, Pihlajaniemi T. Exploring the roles of MACIT and multiplexin collagens in stem cells and cancer. Semin Cancer Biol 2020;62:134-48.
50. Abdollahi A, Hahnfeldt P, Maercker C, et al. Endostatin’s antiangiogenic signaling network. Mol Cell 2004;13:649-63.
51. Musso O, Theret N, Heljasvaara R, et al. Tumor hepatocytes and basement membrane-Producing cells specifically express two different forms of the endostatin precursor, collagen XVIII, in human liver cancers. Hepatology 2001;33:868-76.
52. Saarela J, Ylikärppä R, Rehn M, Purmonen S, Pihlajaniemi T. Complete primary structure of two variant forms of human type XVIII collagen and tissue-specific differences in the expression of the corresponding transcripts. Matrix Biol 1998;16:319-28.
53. Cox TR, Bird D, Baker AM, et al. LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Res 2013;73:1721-32.
54. Lin HY, Li CJ, Yang YL, et al. Roles of lysyl oxidase family members in the tumor microenvironment and progression of liver cancer. Int J Mol Sci 2020;21:9751.
55. Bergeat D, Fautrel A, Turlin B, et al. Impact of stroma LOXL2 overexpression on the prognosis of intrahepatic cholangiocarcinoma. J Surg Res 2016;203:441-50.
56. Vihinen P, Kähäri VM. Matrix metalloproteinases in cancer: prognostic markers and therapeutic targets. Int J Cancer 2002;99:157-66.
57. Terada T, Okada Y, Nakanuma Y. Expression of immunoreactive matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in human normal livers and primary liver tumors. Hepatology 1996;23:1341-4.
58. Jones CE, Sharick JT, Colbert SE, et al. Pten regulates collagen fibrillogenesis by fibroblasts through SPARC. PLoS One 2021;16:e0245653.
59. Deng S, Zhang L, Li J, Jin Y, Wang J. Activation of the PI3K-AKT signaling pathway by SPARC contributes to the malignant phenotype of cholangiocarcinoma cells. Tissue Cell 2022;76:101756.
60. Sonongbua J, Siritungyong S, Thongchot S, et al. Periostin induces epithelial-to-mesenchymal transition via the integrin α5β1/TWIST-2 axis in cholangiocarcinoma. Oncol Rep 2020;43:1147-58.
61. Provenzano PP, Eliceiri KW, Campbell JM, et al. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med 2006;4:38.
62. Ray A, Slama ZM, Morford RK, Madden SA, Provenzano PP. Enhanced directional migration of cancer stem cells in 3D aligned collagen matrices. Biophys J 2017;112:1023-36.
63. Huang Y, Zhuang Z. Second harmonic microscopic imaging and spectroscopic characterization in prostate pathological tissue. Scanning 2014;36:334-7.
64. Drifka CR, Loeffler AG, Mathewson K, et al. Highly aligned stromal collagen is a negative prognostic factor following pancreatic ductal adenocarcinoma resection. Oncotarget 2016;7:76197-213.
65. Deng B, Zhao Z, Kong W, Han C, Shen X, Zhou C. Biological role of matrix stiffness in tumor growth and treatment. J Transl Med 2022;20:540.
66. Keikhosravi A, Shribak M, Conklin MW, et al. Real-time polarization microscopy of fibrillar collagen in histopathology. Sci Rep 2021;11:19063.
67. Shi R, Zhang Z, Zhu A, et al. Targeting type I collagen for cancer treatment. Int J Cancer 2022;151:665-83.
68. Baldari S, Di Modugno F, Nisticò P, Toietta G. Strategies for efficient targeting of tumor collagen for cancer therapy. cancers 2022;14:4706.
69. Liang H, Li X, Wang B, et al. A collagen-binding EGFR antibody fragment targeting tumors with a collagen-rich extracellular matrix. Sci Rep 2016;6:18205.
70. Momin N, Mehta NK, Bennett NR, et al. Anchoring of intratumorally administered cytokines to collagen safely potentiates systemic cancer immunotherapy. Sci Transl Med 2019:11.
71. Smithen DA, Leung LMH, Challinor M, et al. 2-Aminomethylene-5-sulfonylthiazole inhibitors of lysyl oxidase (LOX) and LOXL2 show significant efficacy in delaying tumor growth. J Med Chem 2020;63:2308-24.
72. Liu YL, Bager CL, Willumsen N, et al. Tetrathiomolybdate (TM)-associated copper depletion influences collagen remodeling and immune response in the pre-metastatic niche of breast cancer. NPJ Breast Cancer 2021;7:108.
73. Peng T, Deng X, Tian F, et al. The interaction of LOXL2 with GATA6 induces VEGFA expression and angiogenesis in cholangiocarcinoma. Int J Oncol 2019;55:657-70.
74. McGaha TL, Phelps RG, Spiera H, Bona C. Halofuginone, an inhibitor of type-I collagen synthesis and skin sclerosis, blocks transforming-growth-factor-beta-mediated Smad3 activation in fibroblasts. J Invest Dermatol 2002;118:461-70.
75. Cortes E, Lachowski D, Rice A, et al. Tamoxifen mechanically deactivates hepatic stellate cells via the G protein-coupled estrogen receptor. Oncogene 2019;38:2910-22.
76. Nicolas-Boluda A, Vaquero J, Vimeux L, et al. Tumor stiffening reversion through collagen crosslinking inhibition improves T cell migration and anti-PD-1 treatment. Elife 2021:10.
77. Ortona E, Maselli A, Delunardo F, Colasanti T, Giovannetti A, Pierdominici M. Relationship between redox status and cell fate in immunity and autoimmunity. Antioxid Redox Signal 2014;21:103-22.
78. Su B, Zhao W, Shi B, et al. Let-7d suppresses growth, metastasis, and tumor macrophage infiltration in renal cell carcinoma by targeting COL3A1 and CCL7. Mol Cancer 2014;13:206.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Colasanti T, Vakifahmetoglu-Norberg H, Mancone C. Expression and function of collagens in intrahepatic cholangiocarcinoma. Hepatoma Res 2023;9:19. http://dx.doi.org/10.20517/2394-5079.2022.94
AMA Style
Colasanti T, Vakifahmetoglu-Norberg H, Mancone C. Expression and function of collagens in intrahepatic cholangiocarcinoma. Hepatoma Research. 2023; 9: 19. http://dx.doi.org/10.20517/2394-5079.2022.94
Chicago/Turabian Style
Colasanti, Tania, Helin Vakifahmetoglu-Norberg, Carmine Mancone. 2023. "Expression and function of collagens in intrahepatic cholangiocarcinoma" Hepatoma Research. 9: 19. http://dx.doi.org/10.20517/2394-5079.2022.94
ACS Style
Colasanti, T.; Vakifahmetoglu-Norberg H.; Mancone C. Expression and function of collagens in intrahepatic cholangiocarcinoma. Hepatoma. Res. 2023, 9, 19. http://dx.doi.org/10.20517/2394-5079.2022.94
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 2 clicks
Cite This Article 2 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.