The role of tumor microenvironment in cholangiocarcinoma
Abstract
Cholangiocarcinoma (CCA) is an extremely aggressive neoplasia, mostly because of diagnostic delay and lack of effective therapies. CCA is typically surrounded by a peculiar microenvironment that includes abundant desmoplastic stroma and various cell types, which support and enhance CCA development. Among the tumor microenvironment (TME) cells, there are tumor infiltrating lymphocytes (TILs), such as CD8+ and CD4+ cells, Tregs, natural killers (NKs) and B lymphocytes. TILs contribute to an immunosuppressive microenvironment that leads to tumor immune escape. Dendritic cells (DCs) may lead to immunotolerance by maturation or antigen-presentation deficiency. Hepatic stellate cells (HSCs) are one of the major precursors of cancer-associated fibroblast (CAFs), which are distinguished in various subpopulations, each with different functions and interactions with other TME cells. CAFs can promote lymphangiogenesis, early lymph-node metastasis and proinflammatory environment, but they can also provide a physical and chemical barrier to protect CCA. Tumor-associated macrophages (TAMs) could be differentiated between two phenotypes, pro- and anti-inflammatory, and they may sustain invasiveness and immunosuppression. Myeloid-derived suppressor cells (MDSCs) impair cytotoxic T lymphocytes (CTLs) function, stimulating tumor proliferation and angiogenesis. Tumor-associated neutrophils (TANs) function is influenced by the TME, leading to tumor-suppressing or tumor-promoting functions. This paper aims to provide an overview of the CCA microenvironment cells, their role in tumor progression and possible correlated diagnostic, therapeutic and prognostic implications.
Keywords
INTRODUCTION
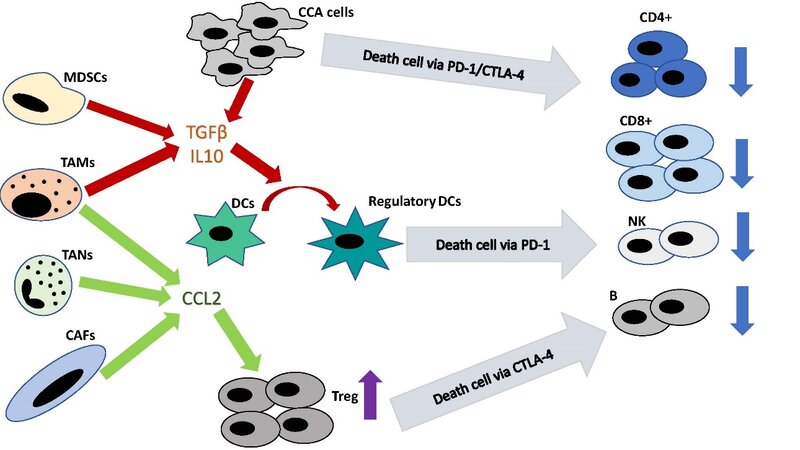
Cholangiocarcinoma (CCA) is a rare but aggressive biliary-derived cancer with few therapeutic options. The tumor microenvironment (TME) has a key role in sustaining tumor progression. In fact, CCA has an abundant desmoplastic stroma and it is surrounded by many cell types, such as hepatic stellate cells (HSCs), cancer-associated fibroblasts (CAFs), tumor-infiltrating lymphocytes (TILs), tumor-associated neutrophils (TANs), tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), dendritic cells (DCs) and extracellular matrix (ECM), which have intense crosstalk between themselves and cancer cells [Figure 1]. This interplay provides a good environment for tumor growth, metastasis, chemoresistance, and tumor-specific immune tolerance, which may be a target for new immunotherapy approaches. Here, we will describe the main cell types of CCA TME, and their related pathways, that have been shown to influence tumor development, prognosis or response to current or future treatments.
Figure 1. Tumor microenvironment influence on effector lymphocytes. CCA: cholangiocarcinoma; PD-1: programmed cell death;
TUMOR-INFILTRATING LYMPHOCYTES
TILs are involved in immune response against tumor cells, detection of cancer antigens and killing of neoplastic cells[1]. In CCA, TILs consist mainly of CD8+ and CD4+ T lymphocytes but also, to a lesser degree, of NKs and B lymphocytes[2]. Interestingly, TILs from resected CCA differ from their counterparts in tumor-free liver. In fact, in CCA, cytotoxic T cells and natural killer cells (NKs) are reduced while regulatory T cells (Tregs) are increased[3]. In TME, indeed, CAFs, TANs and TAMs produce C-C motif chemokine ligand 2 (CCL2) enrolling Tregs[4-6], while MDSCs and TAMs secrete interleukin-10 (IL10) and transforming growth factor β (TGF-β), which also convert DCs into regulatory DCs. Tregs and regulatory DCs perpetuate this vicious cycle attracting more immunosuppressive immune cells and weakening antitumor defenses[7,8]. However, TGF-β and IL10 production is not dependent only on TME cells but also on CCA cells[4] [Figure 1]. Furthermore, extrahepatic cholangiocarcinoma (eCCA) cells seem to produce prostaglandin E2 and adenosine, reducing T cell activity[9-13] and the expression of CXCL12 by CAFs disrupt T-cells migration into tumors[14].
TILs subpopulations have different localizations. In fact, CD8+ T cells and CD4+ T (Foxp3-) cells are in cancer margins, while Tregs (Foxp3+) infiltrate the core[11]. This setup demonstrates the relegation of the effectors in CCA[15].
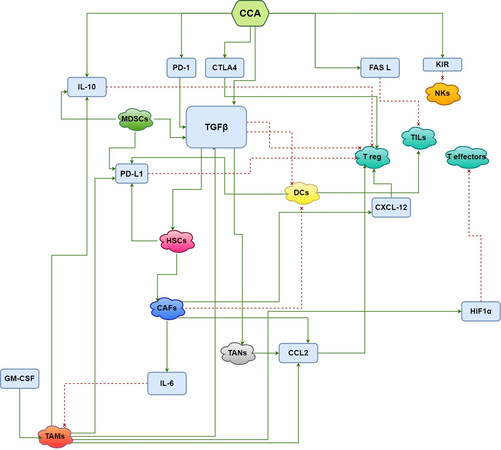
Various molecular mechanisms underlying TILs regulation have been described. Intrahepatic cholangiocarcinoma (iCCA) cells induce T and NK lymphocyte death via Fas/Fas ligand (Fas L) high expression levels[16]. 67-kDa laminin receptor induces FasL expression in human CCA cells with subsequent activation of the FasL promoter via the extracellular signal-regulated kinase (ERK) pathway, which is a possible target of specific mitogen-activated protein kinase (MAPK)-ERK cascade inhibitor[17]. Killer cell immunoglobulin-like receptors (KIRs) regulate NK cells function and KIR genes were found altered in CCA, possibly affecting NK cell tumor surveillance[18,19]. Moreover, Wingless and Int-1(Wnt)/-catenin and TGF-signaling pathways are correlated to a reduced number of tissue-resident memory-like CD8+ TILs, which are involved in immune response against tumor cells[20]. The expression of B7-H1 and its receptor programmed death 1 (PD-1) in iCCA leads to immune escape due to CD8+ TILs apoptosis[21] [Figure 2]. The atypical protein kinase C-iota (aPKC-i)/Ser59-phosphorylated specificity protein 1 (P-Sp1)/Snail signaling stimulates the differentiation in T regulatory-like cluster of CD25- cells which have an immunosuppressive function in CCA[22].
Figure 2. Interactions of cells involved in cholangiocarcinoma development and tumor microenvironment. The green arrows indicate induction, while the red dotted lines suggest inhibition. CCA: cholangiocarcinoma; PD-1: programmed cell death; CTLA4: cytotoxic T-lymphocyte-associated protein 4; FasL: Fas ligand; KIR: killer cell immunoglobulin-like receptor; NKs: natural killers; IL-10: interleukin-10; TGFβ: transforming growth factor β; MDSCs: myeloid-derived suppressor cells; PD-L1: programmed cell death ligand;
TILs are also important for potential immunotherapy for CCA. Tumor-induced immunological checkpoints control [e.g., PD-1 and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4)] establish an immunosuppressive microenvironment, which leads to tumor immune escape[23,24] [Figure 1]. A combination of anti-CD40 and anti-PD-1 in mice iCCA results in myeloid cells, CD4+ and CD8+ T cells and NK cells activation, with a cancer burden reduction[25]. Pan et al. showed that the development of specific antibodies, which inhibit iCCA growth in rats, may be elicited by CTLA4 - anti-programmed cell death ligand (PD-L1) DNA immunization[26]. Moreover, the combination of gemcitabine with cytotoxic T-lymphocytes (CTLs) increases the cytotoxic activity of effector T cells against chemo-resistant CCA cells in vitro, suggesting a potential benefit by this combination therapy[27]. Cetuximab is found to stimulate the activity of cultured cytokine-activated (with a high dose of IL-2 and anti-CD3 monoclonal antibodies) killing cells against CCA[28]. Conversely, Kirsten rat sarcoma (KRAS) alteration is associated, in PD-1/PD-L1 blockade-treated patients, with resistance to immunotherapy. This seems to be related to a low TILs density in the TME of KRAS-altered neoplasia, suggesting a correlation between KRAS and low immunogenicity in iCCA[29]. Furthermore, high-level microsatellite instability (MSIH) in CCA predicts response to immune checkpoint blockade, in particular to anti-PD-1 or PD-L1 therapy[30]. MSIH is also associated with a longer overall survival (OS) and the presence of a more numerous population of CD8+ T cells, FOXP3+ regulatory T cells, and CD20+ B cells[31]. Moreover, MSIH correlates with a higher level of BRCA-mutated iCCA[32].Immune checkpoint inhibitors may represent a promising strategy for CCA treatment. Durvalumab (anti-PD-L1), which is now under investigation for advanced biliary tract cancer, showed interesting results with a 36-month OS rate of 30.7% and a manageable safety profile as second-line in monotherapy and in combination with other molecules[33].
Other than for position, CD8+ and CD4+ T cells and Tregs differ for prognostic value. The former and CD20+ B cells are related to a better prognosis; the latter, when abundant, are associated with worse overall survival (OS)[2,3,20,34-40]. A recent systematic review showed that high levels of CD8+ and CD4+ T cells are associated with better prognosis in CCA, regardless of their position. Recently, Alvisi et al. demonstrated that in iCCA CD4+ Tregs are hyperactivated in comparison with CD8+ T cells, suggesting that CD8+ effector functions are reduced and CD4+ Tregs immunosuppressive functions are amplified[41]. This evidence comes from the hyperexpression of mesenchyme homeobox 1 (MEOX1) by Tregs and the consequent evolution of circulating Tregs in tumor-infiltrating Tregs. Therefore, these finding correlate with a poor prognosis due to immunosuppression[41]. However, the prognostic value of Foxp3+ T cells is still not clear and requires further research[2,34-39,42-46]. Instead, B cells seem to be associated with an improved prognosis[2,15]. Finally, high C-X-C motif ligand 9 (CXCL9) expression, which in animal models stimulates NK cell recruitment, enhancing antitumor immunity, is correlated with better survival after resection[6].
DENDRITIC CELLS
Tumor-infiltrating DCs are abundant in TME and are characterized by an elevated expression of CD40 on their surface[25,47]. They may contribute to immunotolerance by maturation or antigen-presentation deficiency, which leads to an inhibition of CD8+ and CD4+ T-cell priming[7,8]. Moreover, DCs produce PD-L1[8] and attract Tregs which express (CTLA-4) that sustains the regulatory phenotype of DCs[7,8]. In CCA, CAFs can attract DCs and reduce the expression of human leukocyte antigen (HLA) molecules, weakening the activation of TILs[48]. Interestingly, Martìn-Sierra et al. found that in CCA patients, the immunomodulation may not be relegated only to the peritumoral area[49]. In fact, in these patients, there are low levels of circulating classical dendritic cells and monocytes positive for the Fc fragment of IgE high affinity I receptor (FceRI), which are suspected to eventually differentiate into classical dendritic cells and tumor necrosis factor α(TNF-α) -producing proinflammatory DCs[49,50].
IL-10 and TGFβ, produced by CCA cells, have an immunosuppressive effect on dendritic cells: they depress antigen presentation and the activation of effector T lymphocytes, leading to tumor evasion of immune surveillance[51-53] [Figure 2]. Thepmalee et al. demonstrated that the blockage of IL-10 and TGF-β receptors on DCs by specific neutralizing antibodies enhances cytolytic activity of effector T-cells against CCA and increases the level of interferon-γ (IFN-γ)[54].
The use of postoperative DCs vaccine plus activated T-cell transfer could prevent recurrence and improve survival in patients affected by iCCA, as demonstrated by a clinical trial. Five years progression-free survival (PFS) and OS were superior in patients treated with DCs vaccine plus activated T-cell transfer than in patients who did not receive that treatment[55].
Intracellular protein kinase CAMP-dependent type I regulatory subunit alpha (PRKAR1A) is overexpressed in CCA. PRKAR1A-presenting self-differentiated monocyte-derived dendritic cells (SD-DC) activates effector T cells killing ability versus the tumor, which is nearly doubled than those stimulated with control DC in vitro[56].
With regards to the prognostic role of DCs, peritumoral plasmacytoid DCs (pDCs) correlate with wider local and distal extension, higher chance of recurrence and shorter OS. Furthermore, larger numbers of pDCs are associated with increased Foxp3+ regulatory T-cell infiltration[57].
CD83+ mature dendritic cells are located primarily on invasive front of CCA, while CD1a+ immature DCs are gathered within the tumor tissue. CD83+ DCs density was associated with a greater number of CD4+ or CD8+ T cells infiltrating the tumor and was correlated with a good prognosis and lower incidence of metastases[50,58].
CANCER-ASSOCIATED FIBROBLASTS
CAFs are a group of various cells, in which the predominant type is the activated myofibroblasts. CAFs express several phenotypic markers such as α-smooth muscle actin (α-SMA), platelet-derived growth factor receptor β (PDGFRβ), fibroblast specific protein-1 (FSP-1 or S100A4), mucin-like transmembrane glycoprotein podoplanin, and the cell surface metalloprotease cluster of differentiation 10 (CD10). CAFs origin is still partially unclear and controversial; it is likely that CAFs derive from HSCs[59], periductal or portal fibroblasts (PFs)[60], pericytes, mesenchymal stem cells, circulating bone marrow-derived mesenchymal cells and adipocytes[61,62].
Various clusters of CAFs have been described. The first is vascular CAFs, which were found in the tumor core and in the microvascular region. Vascular CAFs are characterized by the presence of microvascular genes and the production of IL-6 and CCL8, implying a possible interaction with cancer[63]. Matrix CAFs express high levels of ECM molecules [e.g., collagen molecules and periostin (POSTN)] and lower levels of α-SMA. Inflammatory CAFs produce low levels of α-SMA and high levels of fibulin 1 (FBLN1), insulin-like growth factor 1 (IGFI), insulin-like growth factor binding protein 6 (IGFBP6), secretory leukocyte peptidase inhibitor (SLPI), serum amyloid A1 (SAA1), C3 and C7, intimating a role in cancer immunity. Myofibroblastic CAFs and mesothelial CAFs coexpress portal fibroblast/mesothelial markers[64]. These different phenotypes are probably involved in the CCA progression through the release of biochemical signals such as TGF-β1, connective tissue growth factor (CTGF), stromal cell-derived factor-1 (SDF-1), ECM components such as POSTN, collagen type I, osteopontin, IL-6 and IL-33, and matrix metalloproteases (1, 2, 3,9)[65,66] [Table 1 and Figure 2].
Different CAFs phenotypic subpopulation, their location in the tumor and roles
| CAFs phenotypic subpopulation | Location | Expression | Role |
| Vascular CAFs | tumor core and microvascular region | production of IL-6 and CCL8 | interaction with malignant cells |
| Matrix CAFs | invasive front of intrahepatic CCA | high levels of ECM molecules, low level of α-SMA | Invasiveness/ECM and collagen fibril organization |
| Inflammatory CAFs | no specific spatial distribution | low levels of α-SMA, high levels of FBLN1, IGFI, IGFBP6, SLPI, SAA1, C3 and C7, | immune modulation |
| Myofibroblastic CAFs | no specific spatial distribution | Express portal fibroblast/mesothelial markers | Promotion of tumor growth |
| Mesothelial CAFs | no specific spatial distribution | Express portal fibroblast/mesothelial markers | Fibrogenic effect |
In iCCA, low tissue expression of osteopontin was associated with lymph node metastasis and worse prognosis, while serum osteopontin levels were elevated in patients with CCA compared to healthy controls and patients with primary sclerosing cholangitis. Moreover, high concentrations of serum osteopontin before and after surgery are associated with poor postoperative survival.
Similarly, high POSTN, produced by α-SMA+ CAFs, can be evaluated to discriminate CCA from normal/cirrhotic liver or hepatocellular carcinoma and is correlated with a shorter 5-year survival in post-resected iCCA.
PDGF D domain (PDGF-DD) produced by CCA cells binds to PDGFRβ and stimulates fibroblasts motility[67] and vascular endothelial growth factor (VEGF)-C and VEGF-A secretion, thus contributing to early metastasis to lymph nodes[68]. Nevertheless, imatinib mesylate (suppressor of the migration on myofibroblast) has shown disappointing preliminary results[69].
CAF-released heparin-binding (HB) EGF, which binds the EGF receptor on CCA cells, activates signal transducers and activators of transcription 3 (STAT3); this promotes the formation of a proinflammatory microenvironment through activation of the IL6/STAT3 axis[70] with subsequent tumor cell migration, motility, and invasion[71].
Another interesting peculiarity about activated CAFs is the enhanced susceptibility to apoptosis. In fact, BH3-only proteins initiate apoptosis by the ignition of Bax and Bak (multidomain proapoptotic Bcl-2 proteins) in activated CAFs. CCA cells widely express antiapoptotic multidomain Bcl-2 proteins, such as Mcl-1, which inhibits this pathway[72]. Navitoclax, a BH3-only protein mimetic, leads to selective apoptosis in α-SMA+ CAFs but not in CCA cells and quiescent fibroblasts. The downregulation of Mcl-1 and the upregulation of Bax protein sensitize activated CAFs to navitoclax-mediated apoptosis, inducing a reduction in neoplastic burden and metastatization, due to a reduced lymphatic vascularization. Thus, navitoclax leads to an improvement in survival in animal models[68,73].
Moreover, CCA cells overexpress CXC chemokine receptor-4 (CXCR4)- SDF-1, the cognate receptor of SDF-1, which is widely expressed by CAFs in the peritumoral stroma. The binding between CXCR4 and SDF-1 stimulates the antiapoptotic protein Bcl-2 and activates ERK1/2 and PI3K/Akt pathways, permitting CCA cells survival and invasiveness and enhancing HSCs differentiation, supporting further CAFs enrichment[67]. Furthermore, Okamoto et al. demonstrated that the wide expression of SDF-1 is correlated with cancer fibrogenesis and epithelial-to-mesenchymal transition (EMT), predicting poor prognosis[74].
Interestingly, CAFs could also inhibit CCA progression. It has been demonstrated that high IL-33 content in CAFs and cancer cells is associated with a better prognosis. Therefore, IL-33 may be considered as a valuable prognostic marker and a potential future treatment target[75].
Finally, high expression of α-SMA is correlated to larger tumor size, lymph node metastasis, higher histological grade and a worse 5-year survival rate (6% vs. 29%)[67]. Nintedanib (a tyrosine kinase inhibitor of PDGFR, VEGFR, and FGFR) seems to be a promising treatment in refractory iCCA by inhibiting activation, proliferation and αSMA expression in CAFs and reducing cancer-promoting cytokines, such as IL-6 and
HEPATIC STELLATE CELLS
HSCs have an established role in liver tumor carcinogenesis[79] and activated HSC/myofibroblasts are relevant in TME development. The activation of HSCs into tumor‐promoting myofibroblasts is induced by TGFβ through the binding with its receptors TGFβR1/TGFβR2 and the subsequent nuclear translocation of small mothers against decapentaplegic homolog (SMAD)[80]. Furthermore, TGFβ induces HSCs expression of α‐SMA, fibronectin and CTGF, markers of HSC activation and paracrine factors, that enhance liver progression and metastatization[81,82]. Recently, Sun et al. showed how PD-L1, produced by HSCs, stabilizes TGFβR2 and TGFβR1 supporting TGF-β-stimulated activation of HSCs into myofibroblasts[83]. Interestingly, an extracellular domain of PD-L1 halts the lysosomal degradation of TGFβR2 protein and the RNA exosome complex degradation of TGFβR1 mRNA [Figure 2]. Moreover, PD-L1 is a possible target for suppressing HSC activation in iCCA microenvironment due to its role in CAFs differentiation[83]. Furthermore, focal adhesion kinase (FAK) drives TGFβR2 to the HSCs membrane which protects the receptor from degradation, perpetuating HSC activation. Thus, targeting FAK could have a role in the suppression of HSC activation[84].
TUMOR-ASSOCIATED MACROPHAGES
Environmental stimuli, such as IFN-γ, TNF-α, granulocyte-macrophage colony-stimulating factor (GM-CSF) or bacterial endotoxin, may enhance the differentiation of macrophages towards the M1 inflammatory subtype. In contrast, the anti-inflammatory M2 macrophage phenotype is triggered by IL-4, IL-10, IL-13
TAMs are induced by costimulation by toll-like receptor (TLR) ligands and A2 adenosine receptor (A2R) agonists or by IL‐6. TAMs highly express IL‐10, TGF‐β, VEGF and angiopoietins, increasing tumor aggressivity[91], while they express low levels of IL‐12, TNF‐α, and IL‐1β. These molecules trigger cholangiocytes proliferation, fibrogenesis, angiogenesis and biliary carcinogenesis[92-95]. In fact, TAMs are negatively correlated with prognosis in CCA[96].
TAMs have multiple functions due to their proinflammatory activity, such as invasiveness, adhesion, and immunosuppression. For instance, TAMs activate Wnt/β-catenin pathway due to Wnt3a and Wnt7b, which contributes to CCA proliferation[97,98]. Moreover, TAMs may suppress T cells effector antitumor activity via hypoxia-inducible factor-1 (HIF-1α) expression, which is expressed in about 66% of CCA and modulates the production of VEGF-A through hypoxia and other autophagy modulators such as PI3KC3, which is highly expressed in CCA and correlates with worse prognosis. Hypoxia-associated autophagy, in fact, is associated with CCA metastasis and a worse prognosis[99,100]. Interestingly, in a recent study, Ruffolo et al. demonstrated that anti-GM-CSF antibodies stimulate the repolarisation of immunosuppressive TAMs and MDSCs, promoting anti-tumoral T cell immunity and depressing inflammatory networks[87]. Indeed, a lower expression of GM-CSF in CCA is associated with improved overall survival after tumor resection[78,87].
Liver macrophages express TNF-like weak inducer of apoptosis (TWEAK). In case of damage, Fn14 modulates TWEAK, which supports the proliferation, migration, and polarization of both macrophages and CAFs. Furthermore, TWEAK builds up a proinflammatory environment in CCA, and it is hypothesized to induce NF-kB-driven mitogen, which stimulates neoplastic proliferation[101].
TNFα is widely produced by Kupffer cells surrounding CCA. TNFα stimulates cholangiocyte proliferation, differentiation and carcinogenesis through the activation of c-Jun N-terminal kinase (JNK) signaling. In fact, mitochondrial dysfunction and oxidative stress, due to reactive oxygen species (ROS) secretion from Kupffer cells, are demonstrated to trigger cholangiocellular growth. Thus, ROS/Tnf/JNK axis may be a possible target of therapy in iCCA[102]. Another interesting pathway under study is PCAT6/miR-326/RohA, which has a role in M2 polarization of TAMs since prostate cancer-associated transcript 6 (PCAT6) is an oncogene highly expressed by macrophages in CCA patients[103].
Finally, exosome Circ_0020256 has been recently described in TAM-secreted exosomes and is involved in neoplastic progression in vivo[104]. Tumor-derived exosomal miR-183-5p stimulates, through the miR-183-5p/PTEN/AKT/PD-L1 pathway, the expression of macrophage PDL-1, of which major source are TAMs. Thereby exosomal miR-183-5p may be a target against immunotolerance in CCA[105].
MYELOID-DERIVED SUPPRESSOR CELLS
MDSCs derive from bone marrow and are divided into two groups: polymorphonuclear MDSCs (PMN-MDSCs) and monocytes (M-MDSCs). The first group includes differentiated neutrophils, basophils, eosinophils and mast cells. The second group is composed of macrophages and DCs[106]. Specifically,
TUMOR-ASSOCIATED NEUTROPHILS
Neutrophils are part of the innate immune system and play an active function in inflammation. Previous studies have shown that neutrophils seem also involved in the development of cancer as tumor-associated neutrophils (TANs)[109]. Their function in cholangiocarcinogenesis has not been deeply understood and investigated. They seem able to have both tumor-suppressing and tumor-promoting functions. TANs may have two different phenotypes, depending on the signals coming from the TME. In mouse models of different cancers, Fridlender et al. showed that type 1 (“N1 phenotype”) is induced by IFNs and has an antitumor role, while type 2 (“N2 phenotype”) is stimulated by TGFβwith tumor-promoting features[110]. In iCCA, both cancer and stromal cells produce CXCL5, which strongly attracts TANs to tumor and promote metastatization as a result of the PI3K-AKT and ERK1/2 pathways activation[5]. TANs expressing CCL2 and CCL17 foster immunosuppression by the recruitment of TAMs and Tregs[4] [Figure 2]. TANs and TAMs interactions favor iCCA development through OSM/IL-11/STAT3 signaling pathway activation. TANs and TAMs interaction has been abolished by STAT3 knockdown or STAT3 inhibitors, as demonstrated in vitro and in vivo[111].
Conversely, N1 may have an antitumor function. In fact, Gao et al. studied the effect of the administration, via percutaneous transhepatic biliary drainage, of tumor-cell-derived microparticles loaded with methotrexate into the bile-duct lumen above biliary obstruction from eCCA[112]. Tumor-cell-derived microparticles may serve as a carrier of chemotherapeutic drugs and simultaneously act as an immune modulator. Mobilization and activation of neutrophils and relief of biliary obstruction were observed in 25% of cases. Neutrophils showed an N1 phenotype, and they were able to attack and kill eCCA cells[78,112].
The prognostic role of TANs has been evaluated in various studies. Kitano et al. analyzed eCCA microenvironment and found that TANs were directly correlated with FOXP3+ (T-regs) and inversely with CD8+ T cells[45]. Moreover, a high number of TANs and T-regs are significantly related to poor OS. Mao et al. studied neutrophils in CCA and adjacent tissues, using CD15 as their marker[113]. They found that patients with deep neutrophils infiltration (high CD15 expression) had a reduced disease-free survival time and OS[99,113]. Interestingly, a systematic review showed a correlation between neutrophil to lymphocyte ratio (NLR) and OS: a high NLR is associated with significantly poorer OS in CCA[114,115]. Finally, epithelial expression of CXCL15 in CCA was found to correlate with TANs recruitment and α-SMA expression and it is related to a worse prognosis due to shorter survival after resection[116-118].
CONCLUSION
Growing evidence shows how CCA microenvironment plays a key role in multiple aspects of tumor progression. Although a reduction of lymphocyte effector cells leads to immune escape, the development of an immunotolerant environment is the final step of non-tumoral and tumoral cell crosstalk. Specifically, TILs are switched to an immunoregulatory phenotype, DCs have their antigen-presenting activity reduced, CAFs provide both a physical and a chemical barrier to protect CCA, TAMs differentiate themselves in a pro- and anti-inflammatory phenotype and MDSCs impair the cytotoxic activity [Figure 1]. Moreover, CAFs and TAMs support lymphangiogenesis and angiogenesis through VEGF, IL10 and TGFβ production.
The late diagnosis of CCA and the frequent struggle to obtain a diagnostic biopsy have stimulated the research of novel diagnostic biomarkers. Liquid biopsy seems a promising tool to achieve this aim. In fact, the serum or bile evaluation of circulating tumor DNA and miRNA could play a future role as minimally invasive screening, diagnostic, prognostic and therapeutic monitoring biomarkers[119].
Other than the evaluation of circulating genetic material, also proteins, cytokines and serum metabolites could have a relevant role in the diagnostic and prognostic assessment of CCA. As listed above, POSTN and osteopontin may discriminate CCA from healthy controls and offer a stratification of post-surgical survival[119-122].
Furthermore, non-tumoral cells and various molecular signaling are associated with different prognostic values. For instance, CD8+ and CD4+ T cells are correlated with a better prognosis in CCA as well as high IL-33 levels in CAFs, while Tregs are correlated with worse OS. High levels of pDCs, TAMs and NLR and the expression of α-SMA and SDF-1 by CAFs are correlated with worse prognosis.
Targeting TME could be a strategy for the development of more effective therapies against CCA. In particular, immunotherapy seems to offer a promising option in clinical practice. Novel biomarkers could assist in a diagnostic and prognostic evaluation of CCA. However, further studies are needed to achieve a better comprehension of the relationship between CCA and TME and to deliver new findings in clinical practice.
DECLARATIONS
Authors’ contributions
Conceptualization and resources: Argenziano ME, Montori M
Methodology: Argenziano ME, Montori M, Maroni L
Software and project administration: Argenziano ME
Data curation, writing-original draft preparation: Argenziano ME, Montori M, Scorzoni C
Writing-review and editing: Argenziano ME, Montori M, Scorzoni C, Marzioni M, Maroni L
Visualization: Argenziano ME, Montori M, Scorzoni C, Benedetti A
Supervision: Benedetti A, Marzioni M, Maroni L
All authors have read and agreed to the published version of the manuscript.
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2023.
REFERENCES
1. Labib PL, Goodchild G, Pereira SP. Molecular pathogenesis of cholangiocarcinoma. BMC Cancer 2019;19:185.
2. Goeppert B, Frauenschuh L, Zucknick M, et al. Prognostic impact of tumour-infiltrating immune cells on biliary tract cancer. Br J Cancer 2013;109:2665-74.
3. Zhou G, Sprengers D, Mancham S, et al. Reduction of immunosuppressive tumor microenvironment in cholangiocarcinoma by ex vivo targeting immune checkpoint molecules. J Hepatol 2019;71:753-62.
4. Fabris L, Perugorria MJ, Mertens J, et al. The tumour microenvironment and immune milieu of cholangiocarcinoma. Liver Int 2019;39 Suppl 1:63-78.
5. Zhou SL, Dai Z, Zhou ZJ, et al. CXCL5 contributes to tumor metastasis and recurrence of intrahepatic cholangiocarcinoma by recruiting infiltrative intratumoral neutrophils. Carcinogenesis 2014;35:597-605.
6. Yang X, Lin Y, Shi Y, et al. FAP promotes immunosuppression by cancer-associated fibroblasts in the tumor microenvironment via STAT3-CCL2 signaling. Cancer Res 2016;76:4124-35.
7. Veglia F, Gabrilovich DI. Dendritic cells in cancer: the role revisited. Curr Opin Immunol 2017;45:43-51.
8. Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer 2005;5:263-74.
9. Miura T, Yoshizawa T, Hirai H, et al. Prognostic impact of CD163+ macrophages in tumor stroma and CD8+ T-Cells in cancer cell nests in invasive extrahepatic bile duct cancer. Anticancer Res 2017;37:183-90.
10. Chariyalertsak S, Sirikulchayanonta V, Mayer D, et al. Aberrant cyclooxygenase isozyme expression in human intrahepatic cholangiocarcinoma. Gut 2001;48:80-6.
11. Sirica AE, Lai GH, Zhang Z. Biliary cancer growth factor pathways, cyclo-oxygenase-2 and potential therapeutic strategies. J Gastroenterol Hepatol 2001;16:363-72.
12. Endo K, Yoon BI, Pairojkul C, Demetris AJ, Sirica AE. ERBB-2 overexpression and cyclooxygenase-2 up-regulation in human cholangiocarcinoma and risk conditions. Hepatology 2002;36:439-50.
13. Hayashi N, Yamamoto H, Hiraoka N, et al. Differential expression of cyclooxygenase-2 (COX-2) in human bile duct epithelial cells and bile duct neoplasm. Hepatology 2001;34:638-50.
14. Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science 2015;348:74-80.
15. Liu D, Heij LR, Czigany Z, et al. The role of tumor-infiltrating lymphocytes in cholangiocarcinoma. J Exp Clin Cancer Res 2022;41:127.
16. Carnevale G, Carpino G, Cardinale V, et al. Activation of Fas/FasL pathway and the role of c-FLIP in primary culture of human cholangiocarcinoma cells. Sci Rep 2017;7:14419.
17. Duan SG, Cheng L, Li DJ, et al. The role of MAPK-ERK pathway in 67-kDa laminin receptor-induced FasL expression in human cholangiocarcinoma cells. Dig Dis Sci 2010;55:2844-52.
18. Parham P, Moffett A. Variable NK cell receptors and their MHC class I ligands in immunity, reproduction and human evolution. Nat Rev Immunol 2013;13:133-44.
19. Cornillet M, Jansson H, Schaffer M, et al. Imbalance of genes encoding natural killer immunoglobulin-like receptors and human leukocyte antigen in patients with biliary cancer. Gastroenterology 2019;157:1067-1080.e9.
20. Kim HD, Jeong S, Park S, et al. Implication of CD69+ CD103+ tissue-resident-like CD8+ T cells as a potential immunotherapeutic target for cholangiocarcinoma. Liver Int 2021;41:764-76.
21. Ye Y, Zhou L, Xie X, Jiang G, Xie H, Zheng S. Interaction of B7-H1 on intrahepatic cholangiocarcinoma cells with PD-1 on tumor-infiltrating T cells as a mechanism of immune evasion. J Surg Oncol 2009;100:500-4.
22. Qian Y, Yao W, Yang T, et al. aPKC-ι/P-Sp1/Snail signaling induces epithelial-mesenchymal transition and immunosuppression in cholangiocarcinoma. Hepatology 2017;66:1165-82.
23. Labani-Motlagh A, Ashja-Mahdavi M, Loskog A. The tumor microenvironment: a milieu hindering and obstructing antitumor immune responses. Front Immunol 2020;11:940.
24. Tormoen GW, Crittenden MR, Gough MJ. Role of the immunosuppressive microenvironment in immunotherapy. Adv Radiat Oncol 2018;3:520-6.
25. Diggs LP, Ruf B, Ma C, et al. CD40-mediated immune cell activation enhances response to anti-PD-1 in murine intrahepatic cholangiocarcinoma. J Hepatol 2021;74:1145-54.
26. Pan YR, Wu CE, Chen MH, et al. Comprehensive evaluation of immune-checkpoint dna cancer vaccines in a rat cholangiocarcinoma model. Vaccines (Basel) 2020;8:703.
27. Sawasdee N, Thepmalee C, Sujjitjoon J, et al. Gemcitabine enhances cytotoxic activity of effector T-lymphocytes against chemo-resistant cholangiocarcinoma cells. Int Immunopharmacol 2020;78:106006.
28. Morisaki T, Umebayashi M, Kiyota A, et al. Combining cetuximab with killer lymphocytes synergistically inhibits human cholangiocarcinoma cells in vitro. Anticancer Res ;2012,32:2249-56.
29. Yoon JG, Kim MH, Jang M, et al. Molecular characterization of biliary tract cancer predicts chemotherapy and programmed death 1/programmed death-ligand 1 blockade responses. Hepatology 2021;74:1914-31.
30. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409-13.
31. Goeppert B, Roessler S, Renner M, et al. Mismatch repair deficiency is a rare but putative therapeutically relevant finding in non-liver fluke associated cholangiocarcinoma. Br J Cancer 2019;120:109-14.
32. Spizzo G, Puccini A, Xiu J, et al. Molecular profile of BRCA-mutated biliary tract cancers. ESMO Open 2020;5:e000682.
33. Rizzo A, Ricci AD, Brandi G. Durvalumab: an investigational anti-PD-L1 antibody for the treatment of biliary tract cancer. Expert Opin Investig Drugs 2021;30:343-50.
34. Asahi Y, Hatanaka KC, Hatanaka Y, et al. Prognostic impact of CD8+ T cell distribution and its association with the HLA class I expression in intrahepatic cholangiocarcinoma. Surg Today 2020;50:931-40.
35. Tian L, Ma J, Ma L, et al. PD-1/PD-L1 expression profiles within intrahepatic cholangiocarcinoma predict clinical outcome. World J Surg Oncol 2020;18:303.
36. Wu H, Wei Y, Jian M, et al. Clinicopathological and prognostic significance of immunoscore and PD-L1 in intrahepatic cholangiocarcinoma. Onco Targets Ther 2021;14:39-51.
37. Xu YP, Zhou YQ, Zhao YJ, et al. High level of CD73 predicts poor prognosis of intrahepatic cholangiocarcinoma. J Cancer 2021;12:4655-60.
38. Lu JC, Zeng HY, Sun QM, et al. Distinct PD-L1/PD1 profiles and clinical implications in intrahepatic cholangiocarcinoma patients with different risk factors. Theranostics 2019;9:4678-87.
39. Ueno T, Tsuchikawa T, Hatanaka KC, et al. Prognostic impact of programmed cell death ligand 1 (PD-L1) expression and its association with epithelial-mesenchymal transition in extrahepatic cholangiocarcinoma. Oncotarget 2018;9:20034-47.
40. Kasper HU, Drebber U, Stippel DL, Dienes HP, Gillessen A. Liver tumor infiltrating lymphocytes: comparison of hepatocellular and cholangiolar carcinoma. World J Gastroenterol 2009;15:5053-7.
41. Alvisi G, Termanini A, Soldani C, et al. Multimodal single-cell profiling of intrahepatic cholangiocarcinoma defines hyperactivated Tregs as a potential therapeutic target. J Hepatol 2022;77:1359-72.
42. Vigano L, Soldani C, Franceschini B, et al. Tumor-infiltrating lymphocytes and macrophages in intrahepatic cholangiocellular carcinoma. impact on prognosis after complete surgery. J Gastrointest Surg 2019;23:2216-24.
43. Kim HD, Kim JH, Ryu YM, et al. Spatial distribution and prognostic implications of tumor-infiltrating FoxP3- CD4+ T cells in biliary tract cancer. Cancer Res Treat 2021;53:162-71.
44. Hasita H, Komohara Y, Okabe H, et al. Significance of alternatively activated macrophages in patients with intrahepatic cholangiocarcinoma. Cancer Sci 2010;101:1913-9.
45. Kitano Y, Okabe H, Yamashita YI, et al. Tumour-infiltrating inflammatory and immune cells in patients with extrahepatic cholangiocarcinoma. Br J Cancer 2018;118:171-80.
46. Oshikiri T, Miyamoto M, Shichinohe T, et al. Prognostic value of intratumoral CD8+ T lymphocyte in extrahepatic bile duct carcinoma as essential immune response. J Surg Oncol 2003;84:224-8.
47. Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol 2003;3:867-78.
48. Cheng JT, Deng YN, Yi HM, et al. Hepatic carcinoma-associated fibroblasts induce IDO-producing regulatory dendritic cells through IL-6-mediated STAT3 activation. Oncogenesis 2016;5:e198.
49. Martín-Sierra C, Martins R, Laranjeira P, et al. Functional impairment of circulating FcεRI+ monocytes and myeloid dendritic cells in hepatocellular carcinoma and cholangiocarcinoma patients. Cytometry B Clin Cytom 2019;96:490-5.
50. Paillet J, Kroemer G, Pol JG. Immune contexture of cholangiocarcinoma. Curr Opin Gastroenterol 2020;36:70-6.
51. Jiraviriyakul A, Songjang W, Kaewthet P, Tanawatkitichai P, Bayan P, Pongcharoen S. Honokiol-enhanced cytotoxic T lymphocyte activity against cholangiocarcinoma cells mediated by dendritic cells pulsed with damage-associated molecular patterns. World J Gastroenterol 2019;25:3941-55.
52. Changkija B, Konwar R. Role of interleukin-10 in breast cancer. Breast Cancer Res Treat 2012;133:11-21.
53. Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res 2014;2014:149185.
54. Thepmalee C, Panya A, Junking M, Chieochansin T, Yenchitsomanus PT. Inhibition of IL-10 and TGF-β receptors on dendritic cells enhances activation of effector T-cells to kill cholangiocarcinoma cells. Hum Vaccin Immunother 2018;14:1423-31.
55. Shimizu K, Kotera Y, Aruga A, Takeshita N, Takasaki K, Yamamoto M. Clinical utilization of postoperative dendritic cell vaccine plus activated T-cell transfer in patients with intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Sci 2012;19:171-8.
56. Panya A, Thepmalee C, Sawasdee N, et al. Cytotoxic activity of effector T cells against cholangiocarcinoma is enhanced by self-differentiated monocyte-derived dendritic cells. Cancer Immunol Immunother 2018;67:1579-88.
57. Hu ZQ, Zhou ZJ, Luo CB, et al. Peritumoral plasmacytoid dendritic cells predict a poor prognosis for intrahepatic cholangiocarcinoma after curative resection. Cancer Cell Int 2020;20:582.
58. Takagi S, Miyagawa S, Ichikawa E, et al. Dendritic cells, T-cell infiltration, and Grp94 expression in cholangiocellular carcinoma. Hum Pathol 2004;35:881-6.
59. Okabe H, Beppu T, Hayashi H, et al. Hepatic stellate cells may relate to progression of intrahepatic cholangiocarcinoma. Ann Surg Oncol 2009;16:2555-64.
60. Dranoff JA, Wells RG. Portal fibroblasts: underappreciated mediators of biliary fibrosis. Hepatology 2010;51:1438-44.
61. Shimoda M, Mellody KT, Orimo A. Carcinoma-associated fibroblasts are a rate-limiting determinant for tumour progression. Semin Cell Dev Biol 2010;21:19-25.
62. Franco OE, Shaw AK, Strand DW, Hayward SW. Cancer associated fibroblasts in cancer pathogenesis. Semin Cell Dev Biol 2010;21:33-9.
63. Zhang M, Yang H, Wan L, et al. Single-cell transcriptomic architecture and intercellular crosstalk of human intrahepatic cholangiocarcinoma. J Hepatol 2020;73:1118-30.
64. Affo S, Nair A, Brundu F, et al. Promotion of cholangiocarcinoma growth by diverse cancer-associated fibroblast subpopulations. Cancer Cell 2021;39:883.
65. Sirica AE. The role of cancer-associated myofibroblasts in intrahepatic cholangiocarcinoma. Nat Rev Gastroenterol Hepatol 2011;9:44-54.
66. Sirica AE, Campbell DJ, Dumur CI. Cancer-associated fibroblasts in intrahepatic cholangiocarcinoma. Curr Opin Gastroenterol 2011;27:276-84.
67. Brivio S, Cadamuro M, Strazzabosco M, Fabris L. Tumor reactive stroma in cholangiocarcinoma: the fuel behind cancer aggressiveness. World J Hepatol 2017;9:455-68.
68. Cadamuro M, Brivio S, Mertens J, et al. Platelet-derived growth factor-D enables liver myofibroblasts to promote tumor lymphangiogenesis in cholangiocarcinoma. J Hepatol 2019;70:700-9.
69. Sirica AE, Gores GJ. Desmoplastic stroma and cholangiocarcinoma: clinical implications and therapeutic targeting. Hepatology 2014;59:2397-402.
70. Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell 2010;17:135-47.
71. Clapéron A, Mergey M, Aoudjehane L, et al. Hepatic myofibroblasts promote the progression of human cholangiocarcinoma through activation of epidermal growth factor receptor. Hepatology 2013;58:2001-11.
72. Mertens JC, Fingas CD, Christensen JD, et al. Therapeutic effects of deleting cancer-associated fibroblasts in cholangiocarcinoma. Cancer Res 2013;73:897-907.
73. Montori M, Scorzoni C, Argenziano ME, et al. Cancer-associated fibroblasts in cholangiocarcinoma: current knowledge and possible implications for therapy. J Clin Med 2022;11:6498.
74. Okamoto K, Tajima H, Nakanuma S, et al. Angiotensin II enhances epithelial-to-mesenchymal transition through the interaction between activated hepatic stellate cells and the stromal cell-derived factor-1/CXCR4 axis in intrahepatic cholangiocarcinoma. Int J Oncol 2012;41:573-82.
75. Yangngam S, Thongchot S, Pongpaibul A, et al. High level of interleukin-33 in cancer cells and cancer-associated fibroblasts correlates with good prognosis and suppressed migration in cholangiocarcinoma. J Cancer 2020;11:6571-81.
76. Sha M, Jeong S, Qiu BJ, et al. Isolation of cancer-associated fibroblasts and its promotion to the progression of intrahepatic cholangiocarcinoma. Cancer Med 2018;7:4665-77.
77. Yamanaka T, Harimoto N, Yokobori T, et al. Nintedanib inhibits intrahepatic cholangiocarcinoma aggressiveness via suppression of cytokines extracted from activated cancer-associated fibroblasts. Br J Cancer 2020;122:986-94.
78. Cao H, Huang T, Dai M, et al. Tumor microenvironment and its implications for antitumor immunity in cholangiocarcinoma: future perspectives for novel therapies. Int J Biol Sci 2022;18:5369-90.
79. Shiraha H, Iwamuro M, Okada H. Hepatic stellate cells in liver tumor. Adv Exp Med Biol 2020;1234:43-56.
80. Shi Y, Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003;113:685-700.
81. Liu C, Billadeau DD, Abdelhakim H, et al. IQGAP1 suppresses TβRII-mediated myofibroblastic activation and metastatic growth in liver. J Clin Invest 2013;123:1138-56.
82. Tu K, Li J, Verma VK, et al. Vasodilator-stimulated phosphoprotein promotes activation of hepatic stellate cells by regulating Rab11-dependent plasma membrane targeting of transforming growth factor beta receptors. Hepatology 2015;61:361-74.
83. Sun L, Wang Y, Wang X, et al. PD-L1 promotes myofibroblastic activation of hepatic stellate cells by distinct mechanisms selective for TGF-β receptor I versus II. Cell Rep 2022;38:110349.
84. Chen Y, Li Q, Tu K, et al. Focal adhesion kinase promotes hepatic stellate cell activation by regulating plasma membrane localization of TGFβ receptor 2. Hepatol Commun 2020;4:268-83.
85. Raggi C, Correnti M, Sica A, et al. Cholangiocarcinoma stem-like subset shapes tumor-initiating niche by educating associated macrophages. J Hepatol 2017;66:102-15.
86. Wang LX, Zhang SX, Wu HJ, Rong XL, Guo J. M2b macrophage polarization and its roles in diseases. J Leukoc Biol 2019;106:345-58.
87. Ruffolo LI, Jackson KM, Kuhlers PC, et al. GM-CSF drives myelopoiesis, recruitment and polarisation of tumour-associated macrophages in cholangiocarcinoma and systemic blockade facilitates antitumour immunity. Gut 2022;71:1386-98.
88. Zhang Y, Chen S, Li J, Dai W, Qian Y. Immune infiltrating cells in cholangiocarcinoma may become clinical diagnostic markers: based on bioinformatics analysis. World J Surg Oncol 2021;19:59.
89. Thanee M, Loilome W, Techasen A, et al. Quantitative changes in tumor-associated M2 macrophages characterize cholangiocarcinoma and their association with metastasis. Asian Pac J Cancer Prev 2015;16:3043-50.
90. Sun D, Luo T, Dong P, et al. M2-polarized tumor-associated macrophages promote epithelial-mesenchymal transition via activation of the AKT3/PRAS40 signaling pathway in intrahepatic cholangiocarcinoma. J Cell Biochem 2020;121:2828-38.
91. Roy S, Glaser S, Chakraborty S. Inflammation and progression of cholangiocarcinoma: role of angiogenic and lymphangiogenic mechanisms. Front Med (Lausanne) 2019;6:293.
92. Wang Q, Ni H, Lan L, Wei X, Xiang R, Wang Y. Fra-1 protooncogene regulates IL-6 expression in macrophages and promotes the generation of M2d macrophages. Cell Res 2010;20:701-12.
93. Duluc D, Delneste Y, Tan F, et al. Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood 2007;110:4319-30.
94. Shapouri-Moghaddam A, Mohammadian S, Vazini H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol 2018;233:6425-40.
95. Sato K, Meng F, Giang T, Glaser S, Alpini G. Mechanisms of cholangiocyte responses to injury. Biochim Biophys Acta Mol Basis Dis 2018;1864:1262-9.
96. Subimerb C, Pinlaor S, Khuntikeo N, et al. Tissue invasive macrophage density is correlated with prognosis in cholangiocarcinoma. Mol Med Rep 2010;3:597-605.
97. Boulter L, Guest RV, Kendall TJ, et al. WNT signaling drives cholangiocarcinoma growth and can be pharmacologically inhibited. J Clin Invest 2015;125:1269-85.
98. Loilome W, Bungkanjana P, Techasen A, et al. Activated macrophages promote Wnt/β-catenin signaling in cholangiocarcinoma cells. Tumour Biol 2014;35:5357-67.
99. Fabris L, Sato K, Alpini G, Strazzabosco M. The tumor microenvironment in cholangiocarcinoma progression. Hepatology 2021;73 Suppl 1:75-85.
100. Thongchot S, Yongvanit P, Loilome W, et al. High expression of HIF-1α, BNIP3 and PI3KC3: hypoxia-induced autophagy predicts cholangiocarcinoma survival and metastasis. Asian Pac J Cancer Prev 2014;15:5873-8.
101. Dwyer BJ, Jarman EJ, Gogoi-Tiwari J, et al. TWEAK/Fn14 signalling promotes cholangiocarcinoma niche formation and progression. J Hepatol 2021;74:860-72.
102. Yuan D, Huang S, Berger E, et al. Kupffer cell-derived Tnf triggers cholangiocellular tumorigenesis through JNK due to chronic mitochondrial dysfunction and ROS. Cancer Cell 2017;31:771-789.e6.
103. Tu J, Wu F, Chen L, et al. Long non-coding RNA PCAT6 induces M2 polarization of macrophages in cholangiocarcinoma via modulating miR-326 and RhoA-ROCK signaling pathway. Front Oncol 2020;10:605877.
104. Chen S, Chen Z, Li Z, et al. Tumor-associated macrophages promote cholangiocarcinoma progression via exosomal Circ_0020256. Cell Death Dis 2022;13:94.
105. Luo C, Xin H, Zhou Z, et al. Tumor-derived exosomes induce immunosuppressive macrophages to foster intrahepatic cholangiocarcinoma progression. Hepatology 2022;76:982-99.
106. Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol 2018;19:108-19.
107. Eggert T, Wolter K, Ji J, et al. Distinct functions of senescence-associated immune responses in liver tumor surveillance and tumor progression. Cancer Cell 2016;30:533-47.
108. Loeuillard E, Yang J, Buckarma E, et al. Targeting tumor-associated macrophages and granulocytic myeloid-derived suppressor cells augments PD-1 blockade in cholangiocarcinoma. J Clin Invest 2020;130:5380-96.
109. Shaul ME, Fridlender ZG. Tumour-associated neutrophils in patients with cancer. Nat Rev Clin Oncol 2019;16:601-20.
110. Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 2009;16:183-94.
111. Zhou Z, Wang P, Sun R, et al. Tumor-associated neutrophils and macrophages interaction contributes to intrahepatic cholangiocarcinoma progression by activating STAT3. J Immunother Cancer 2021;9:e001946.
112. Gao Y, Zhang H, Zhou N, et al. Methotrexate-loaded tumour-cell-derived microvesicles can relieve biliary obstruction in patients with extrahepatic cholangiocarcinoma. Nat Biomed Eng 2020;4:743-53.
113. Mao ZY, Zhu GQ, Xiong M, Ren L, Bai L. Prognostic value of neutrophil distribution in cholangiocarcinoma. World J Gastroenterol 2015;21:4961-8.
114. Tan DW, Fu Y, Su Q, et al. Prognostic significance of neutrophil to lymphocyte ratio in oncologic outcomes of cholangiocarcinoma: a meta-analysis. Sci Rep 2016;6:33789.
115. Buettner S, Spolverato G, Kimbrough CW, et al. The impact of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio among patients with intrahepatic cholangiocarcinoma. Surgery 2018;164:411-8.
116. Okabe H, Beppu T, Ueda M, et al. Identification of CXCL5/ENA-78 as a factor involved in the interaction between cholangiocarcinoma cells and cancer-associated fibroblasts. Int J Cancer 2012;131:2234-41.
117. Gentilini A, Pastore M, Marra F, Raggi C. The role of stroma in cholangiocarcinoma: the intriguing interplay between fibroblastic component, immune cell subsets and tumor epithelium. Int J Mol Sci 2018;19:2885.
118. Rimassa L, Personeni N, Aghemo A, Lleo A. The immune milieu of cholangiocarcinoma: from molecular pathogenesis to precision medicine. J Autoimmun 2019;100:17-26.
119. Rompianesi G, Di Martino M, Gordon-Weeks A, Montalti R, Troisi R. Liquid biopsy in cholangiocarcinoma: current status and future perspectives. World J Gastrointest Oncol 2021;13:332-50.
120. Wang Y, Yi J, Chen X, Zhang Y, Xu M, Yang Z. The regulation of cancer cell migration by lung cancer cell-derived exosomes through TGF-β and IL-10. Oncol Lett 2016;11:1527-30.
121. Martinez VG, O'Neill S, Salimu J, et al. Resistance to HER2-targeted anti-cancer drugs is associated with immune evasion in cancer cells and their derived extracellular vesicles. Oncoimmunology 2017;6:e1362530.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Argenziano ME, Montori M, Scorzoni C, Benedetti A, Marzioni M, Maroni L. The role of tumor microenvironment in cholangiocarcinoma . Hepatoma Res 2023;9:9. http://dx.doi.org/10.20517/2394-5079.2022.98
AMA Style
Argenziano ME, Montori M, Scorzoni C, Benedetti A, Marzioni M, Maroni L. The role of tumor microenvironment in cholangiocarcinoma . Hepatoma Research. 2023; 9: 9. http://dx.doi.org/10.20517/2394-5079.2022.98
Chicago/Turabian Style
Argenziano, Maria Eva, Michele Montori, Chiara Scorzoni, Antonio Benedetti, Marco Marzioni, Luca Maroni. 2023. "The role of tumor microenvironment in cholangiocarcinoma " Hepatoma Research. 9: 9. http://dx.doi.org/10.20517/2394-5079.2022.98
ACS Style
Argenziano, ME.; Montori M.; Scorzoni C.; Benedetti A.; Marzioni M.; Maroni L. The role of tumor microenvironment in cholangiocarcinoma . Hepatoma. Res. 2023, 9, 9. http://dx.doi.org/10.20517/2394-5079.2022.98
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 2 clicks
Cite This Article 2 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.