Issue on combined locoregional and systemic treatment for hepatocellular carcinoma
Abstract
Treatment for hepatocellular carcinoma (HCC) has been challenging as most patients present with late, advanced disease, where curative options are limited. For years, locoregional therapy (LRT) has been the first-line therapy for intermediate-stage HCC and sorafenib for advanced HCC. However, these treatments are often palliative since they are plagued by tumor recurrence or progression. Therefore, there is growing interest in combined therapy to utilize their respective strengths to produce synergistic effects. This review outlines past and current research on the efficacy and safety of combined LRT and systemic therapy.
Keywords
INTRODUCTION
Primary liver cancer is the seventh most occurring cancer and the second most common cause of cancer mortality worldwide[1]. Hepatocellular carcinoma (HCC) accounts for 75% of all liver cancers and is often diagnosed at an advanced stage associated with a poor prognosis and high mortality rate[2].
Liver transplantation (LT) is the best treatment option and is potentially curative, but it is only recommended in early-stage HCC. Surgical resection and radiofrequency ablation (RFA) are also options for early-stage HCC but require either a single nodule or small tumor sizes, respectively. However, for those who are not surgical candidates, treatment with RFA has shown to be just as effective and less invasive[3].
Unfortunately, HCC often presents in advanced stages not amendable for transplantation or resection. Current guidelines for the treatment of unresectable HCC recommend either locoregional therapy (LRT) or systemic therapy. The recommendations do not specify one treatment over the other due to inadequate evidence regarding the balance of benefits vs. harm[4,5]. Trans-arterial chemoembolization (TACE), a form of LRT, is the standard treatment for intermediate-stage HCC up to Child-Pugh class B without vascular invasion or extrahepatic spread. TACE has been shown to significantly delay tumor progression and improve median survival compared to best supportive care[6]. However, multiple sessions of TACE increase the risk of systemic toxicity[7].
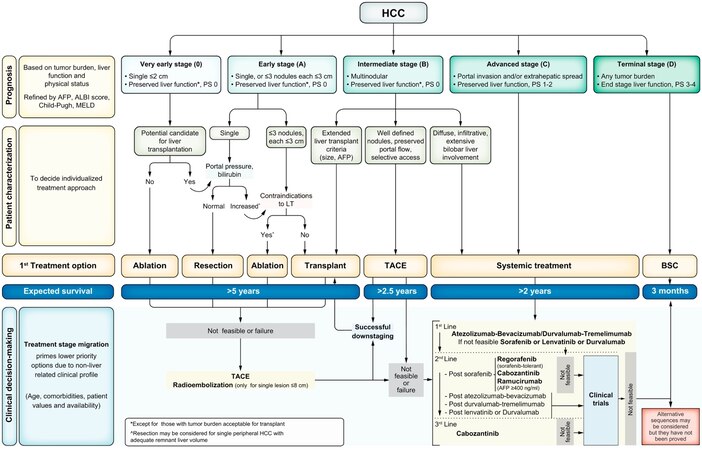
Historically, there was no effective therapy for the treatment of advanced HCC in patients who have failed local therapies until sorafenib, which was the first systemic agent to show an increase in survival when compared to a placebo[8,9]. Although sorafenib was the standard of care and first-line agent for advanced HCC for over a decade [Figure 1], interest in other systemic agents such as immune checkpoint inhibitors and immunotherapy has grown rapidly -either as a single or a combined agent- which have become standard therapy in advanced HCC. Immunotherapy enhances the immune system response against tumor-associated antigens (TAA) and has become the first-line agent in advanced unresectable HCC. For instance, the combination of atezolizumab plus bevacizumab has demonstrated improved overall survival (OS) and progression-free survival (PFS) compared to sorafenib[11]. Tremelimumab and durvalumab combination has also demonstrated superior outcomes compared to Soreafinib in the HIMALAYA trial[12].
Figure 1. Barcelona Clinic Liver Cancer (BCLC) staging. PS: Performance status; BSC: best supportive care. Adapted with permission from Reig et al. (Journal of Hepatology 2022)[10].
In addition to monotherapy, there have also been efforts in clinical trials to evaluate the efficacy of combined systemic therapy and LRT therapies to take advantage of their synergistic effects. Although LRT is the standard therapy for early and intermediate-stage HCC, clinical trials have shown its use is associated with a brief upregulation of vascular endothelial growth factor (VEGF), which is vital in HCC pathogenesis. The upregulation of VEGF is likely from the ischemic injury to the surrounding arteries following LRT treatment, and higher levels of VEGF have been shown to correlate with poorer outcomes after treatment[13,14]. It could be for this reason that high rates of tumor recurrence occur after LRT treatment for HCC. Therefore, antiangiogenic agents, such as sorafenib, were proposed as an adjuvant treatment to LRTs to combat its angiogenic properties and inhibit tumor growth post-treatment. Furthermore, LRT has also been used with immunotherapy to boost the body’s immune response against TAAs. Although the mechanism is unclear, supposedly, the post-LRT necrotic area releases tumor antigens that induce dendritic cells, inflammatory cytokines, and T-cells to infiltrate and stimulate the antitumor response. Therefore, when done in conjunction with immunotherapy, the synergistic effects strengthen the immune response to residual tumors[15].
Here, we review the available clinical trials assessing the combined therapeutic efficacy of LRTs and systemic therapy.
LOCOREGIONAL THERAPIES
Locoregional therapies include two major types: local ablative techniques and intra-arterial therapies.
Local ablative techniques for HCC involved the percutaneous insertion of a needle into the tumor to deliver chemical or thermal ablation. These methods of percutaneous ablation are often curative treatments for early-stage HCC. The most commonly used ablative therapy is RFA, which is the first-line option for non-transplant, non-surgical candidates with early-stage HCC tumors < 5 cm. The best results are in tumors
In contrast, intra-arterial therapies are largely palliative as they are used in intermediate to advanced HCC unless they are used to bridge to transplant. Intra-arterial therapies encompass TACE and hepatic arterial infusion chemotherapy (HAIC), which utilize tumor hypervascularity and allow for selective delivery of chemotherapeutic agents to the tumor. TACE is conventionally performed by injection of an oil emulsion of a chemotherapeutic drug, such as cisplatin or doxorubicin, followed by embolization. Alternatively, it can be performed by delivery of embolic drug-eluting beads (DEB). Historically, the survival benefit of TACE yielded contradictory survival benefits due to the heterogeneous selection of patients for treatment. A meta-analysis showed an improved 2-year survival of unresectable HCC receiving arterial embolization or chemoembolization compared to conservative treatment[16]. On the contrary, another meta-analysis reported no significant increase in overall survival in patients treated with TACE vs. placebo, sham, or no intervention[6]. TACE seems to offer a tolerable safety profile with low adverse events-related mortality. However, increased TACE sessions lead to an increased risk of hepatic injury[17].
A relatively more recent method is selective internal radiation therapy (SIRT) or trans-arterial radioembolization (TARE). Instead of causing ischemia to the tumor, TARE delivers smaller particles to deliver radiation to the tumor, most popularly Y90 b-emitting isotope. Radioembolization is advantageous due to lower toxicity and being an option in patients with portal vein thrombus. A comparative analysis showed a longer time to progression (TTP) with 13.3 months vs. 8.4 months, respectively. However, median survival times were not different[18]. Further evolution of TARE has led to radiation lobectomy (RL) and radiation segmentectomy (RS) for earlier-stage disease[18]. Because surgical intervention is often limited by insufficient future liver remnant (FLR), RL was developed as a means to apply neoadjuvant TARE to the hepatic lobe containing the tumor, which causes atrophy but also causes hypertrophy in contralateral liver parenchyma as a compensatory mechanism. This effectively increases FLR prior to hepatectomy and is safe with a low incidence of post-hepatectomy liver failure[19]. RS delivers high doses of Y90 to particular hepatic segments to minimize the risk of damage to surrounding parenchyma. Results for TTP, median OS, and survival probability were promising in early-stage HCC[20]. Finally, HAIC infuses high levels of chemotherapeutic agents, such as 5-fluorouracil (5-FU) and cisplatin, into the hepatic artery in hopes of direct drug delivery to the tumor while reducing the risk of systemic effects. Historically, large studies and randomized controlled trials were lacking in demonstrating the role of HAIC[21]. However, given recent developments, HAIC has been an effective and safe therapy for locally advanced HCC when compared to sorafenib[22,23].
As LRT is used more widely for bridging to transplant or palliative treatment for advanced HCC, it is plagued by the risk of progression and recurrence due to the release of angiogenic factors and the risk of liver injury with repeated treatments. Therefore, it becomes increasingly relevant to explore the adjunct of systemic therapy with the hopes of increasing its efficacy and decreasing the risk of tumor recurrence.
SYSTEMIC THERAPIES
For patients with advanced HCC, systemic therapy remains the only option. Sorafenib was the first systemic therapy recognized as the first-line agent for advanced HCC based on the SHARP and Asia-Pacific trials[9,11]. Since then, other systemic therapeutic agents have shown survival benefits and even superiority, such as atezolizumab plus bevacizumab.
Systemic therapies can be divided into two categories: molecularly targeted agents (MTAs) and immunotherapy. The MTAs include multikinase inhibitors with antiproliferative and antiangiogenic properties by inhibiting receptors, for instance, platelet-derived growth factor receptor (PDGFR) and vascular endothelial growth factor receptor (VEGFR) -β tyrosine kinases. These agents include sorafenib, lenvatinib, cabozantinib, and regorafenib[24]. In phase III multicenter non-inferiority REFLECT trial, the median survival for lenvatinib of 13.6 months showed non-inferiority to sorafenib as first-line treatment for unresectable HCC. More importantly, secondary endpoints of the trial demonstrated a significant increase in PFS by 3.7 months and TTP by 5.2 months in levatinib compared to sorafenib[25]. Later on, regorafenib was approved as a second-line treatment in Child-Pugh class A patients with HCC that progressed on sorafenib based on the RESORCE study. This phase III trial showed an increase in median overall survival by 2.8 months compared to the placebo[26]. The standard dose of 160 mg was associated with an increase in adverse events-related dose reduction, interruption, and permanent discontinuation; therefore, dose personalization is recommended[27]. Following results from the CELESTIAL trial, cabozantinib was approved for patients with HCC who were given sorafenib in the past. Cabozantinib demonstrated a significant improvement in median OS of 10.2 months compared to the placebo of 8.0 months. In addition, the treatment of cabozantinib also resulted in improved median PFS and objective response rates. It is important to note that the rate of high-grade adverse events was approximately twice that of the placebo group[28].
Other MTAs are the monoclonal antibodies that inhibit the VEGF/VEGFR pathway. Such examples include bevacizumab (anti-VEGF-A) and ramucirumab (anti-VEGFR2)[24]. Ramucirumab was shown to improve median OS in patients with HCC with progression despite treatment with sorafenib and AFP ≥ 400 compared to the best supportive care in the REACH-2 trial[29]. This trial was significant for being the first trial to select a patient population based on biomarkers. Most recently, the combination of atezolizumab-bevacizumab was approved and recommended as first-line treatment for patients with advanced HCC, performance status of 0-1, Child- Pugh A, and lack of active esophageal varices[30]. This was based on a global phase III trial looking at treatment with atezolizumab plus bevacizumab or sorafenib for unresectable HCC without prior treatment. Treatment with atezolizumab-bevacizumab resulted in a significant improvement in OS at 12 months with 67.2% vs. sorafenib with 54.6%. Median PFS was also improved in the atezolizumab-bevacizumab group. The percentage of grade 3 or 4 adverse events was similar in both groups[11].
Lastly, immunotherapy in HCC aims to strengthen the antitumor immune response through different tumor microenvironments (TME) which can be divided into four subclasses (C1-C4). The C1 subclass lacks priming T cells, and C2 is characterized by macrophages, CD4+, and CD8+ T cells, and B cell infiltration. C3 is associated with worse outcomes and displays M2 macrophages and C4 promotes epithelial-mesenchymal transition (EMT)[31]. Currently, immune checkpoint inhibitors (ICI), which serve to boost the existing immune response, are the most commonly used. Agents include PD-L1 antibodies (durvalumab, atezolizumab, and avelumab), anti-PD-1 antibodies (nivolumab, pembrolizumab, and camrelizumab), and anti-CTLA-4 antibodies (ipilimumab and tremelimumab). Nivolumab, a PD-1 inhibitor, is currently approved as a second-line systemic treatment in advanced HCC patients who are ineligible for tyrosine kinase inhibitors. The landmark CheckMate 459 trial showed a better safety profile of Nivolumab monotherapy compared to Sorafenib but no significant OS (16.4 months with nivolumab vs. 14.7 months with sorafenib) (HR, 0.85; 95%CI: 0.72-1.02; P = 0.075)[32]; as a result, the FDA Oncologic Drugs Advisory Committee (ODAC) opposed the accelerated approval of Nivolumab monotherapy for patients with advanced HCC. As mentioned previously, the combination of atezolizumab plus bevacizumab has led to better overall and progression-free survival outcomes than sorafenib, leading the dual MTA and immunotherapy to be a standard first-line therapy in advanced HCC. Finding biomarker predictors for ICI response in HCC is one of the main unanswered questions. To date, several biomarkers have been studied, including PD-L1, Tumor mutational burden (TMB), and Microsatellite instability (MSI) status[33]. Further research in this area is critical to allow for treatment response and disease monitoring.
COMBINATION OF LOCOREGIONAL AND SYSTEMIC THERAPIES
RFA and molecular targeting agents
Combined RFA and sorafenib was initially demonstrated in animal models to increase volumes of coagulation with a significant decrease in tumor size after treatment[15]. Since then, few studies have been performed that have demonstrated some efficacy of RFA and sorafenib compared to RFA alone. Small retrospective studies have shown that sorafenib-RFA treatment had a significantly larger ablated area, lower incidence of recurrence, and better OS after treatment than RFA alone in early to intermediate stage HCC, barcelona clinic liver cancer (BCLC) 0-B[34,35]. Furthermore, a randomized controlled trial looked at the TTP and recurrence rate in medium-sized single HCC (3.1-5.0 cm) in hepatitis B virus (HBV) infected patients treated with sorafenib-RFA and RFA alone. Results showed that the combined group with sorafenib significantly decreased recurrence rates (56.7% vs. 87.5%) and increased TTP (17 months vs. 6.1 months) compared to RFA alone. In addition, the combination therapy was relatively safe, and the addition of RFA did not increase the risk of adverse effects compared to using sorafenib alone[36]. However, the phase III STORM trial demonstrated that there was no difference in the median recurrence-free survival between adjuvant sorafenib and placebo after response with local ablation[37]. A recent 2021 meta-analysis looking into physical thermal ablation, including RFA and microwave ablation (MWA), combined with sorafenib showed that the RFA + sorafenib group showed longer OS at one, two, and three years, lower two-year recurrence rates, and higher overall efficacy [defined by the World Health Organization as (complete remission + partial remission)/total number × 100%] than RFA-alone[38]. These studies show that combined sorafenib and RFA has the potential to increase the treatment area of the tumor in intermediate-stage HCC with improved overall survival and decreased recurrence rates.
Lenvatinib is a relatively newer MTA that became available in 2018. Because of recent studies that have shown potential success in RFA and lenvatinib in the treatment of intermediate HCC, Wang et al. conducted a retrospective study that enrolled patients to be treated with lenvatinib with sequential RFA vs. lenvatinib alone[39]. Enrolled patients must have intermediate-stage HCC, Child-Pugh class A, heavy tumor burden, and not have received any prior systemic treatment. Compared to lenvatinib alone, the combination group had longer PFS and OS without a significant increase in adverse events[39]. Therefore, this combination treatment may be a viable option in patients with high tumor burden but good liver function.
RFA and immunotherapy
Currently, there are few clinical trials involving combined RFA with other molecular targeting agents or immunotherapy. A comparative study looked at combined RFA-CIT and RFA alone in patients with primary HCC who received RFA with curative intent. Results showed a significant increase in PFS in the RFA-CIT compared to RFA alone[40]. There was a randomized trial that looked at one and two-year recurrence rates and median time to overall tumor recurrence in BCLC 0-B HCC patients treated with combined RFA-[(131)I] metuximab and RFA alone. Results demonstrated that the combined group had a greater anti-recurrence benefit than RFA alone, with overall tumor recurrence at 17 months vs. 10 months, respectively[41]. A phase III randomized controlled trial conducted in Korea showed that patients who underwent curative treatment for HCC (surgery, RFA, or percutaneous ethanol injection) had a longer recurrence-free and overall survival with adjuvant immunotherapy with activated CIK cells (CD3 +/CD56 + and CD3 +/CD56 - T cells and CD3-/CD56 + natural killer cells) than without[42]. The same institution then followed up with a retrospective study to look at the efficacy of this combination treatment in real-world clinical practice. During a median follow-up of 28 months, adjuvant immunotherapy in patients who have had curative treatment (surgery or RFA) had a significantly longer RFS[43]. Both of these studies demonstrated a good safety profile. This combination of immunotherapy after RFA was also seen in a retrospective study looking at patients with established recurrent HCC. Patients were included in the study if they were diagnosed with recurrent HCC that was previously treated with hepatic resection or RFA. Recurrence had to be a solitary tumor or no more than three nodules, each < 3 cm in size. They were treated with either RFA alone or RFA with anti-PD-1 therapy. The 1-year recurrence-free survival rate was significantly higher in the anti-PD-1 plus RFA group than in RFA alone, 32.5% and 10.0%, respectively, after propensity score matching[44]. Overall, these studies imply that the safety and efficacy of immunotherapy after RFA treatment may reduce the recurrence of HCC more than RFA therapy alone. However, most of these studies are small and lacking in a large multicenter randomized controlled trial.
SIRT/TARE and systemic therapy
There are few studies examining combined SIRT and systemic therapy. The SORAMIC randomized controlled trial compared the efficacy and safety of SIRT with sorafenib vs. sorafenib alone in patients with advanced HCC. The combination of SIRT and sorafenib did not result in a significantly higher median OS than sorafenib alone. However, a subgroup analysis showed that there was a survival benefit in certain groups: non-cirrhotics, non-alcoholic cirrhotics, and patients less than 65 years of age[45]. A prospective phase II trial evaluated the safety and efficacy of Child-Pugh class A patients with advanced or metastatic HCC treated with sorafenib followed by Y90 glass microspheres. Median PFS was 10.3 months and OS was 13.2 months. Safety analysis showed that the therapy was well tolerated[46]. Further studies may look into this combined therapy in specific subgroups.
Given the synergistic immunomodulatory effects of SIRT and immunotherapy, there have been a couple of recent clinical trials to test the efficacy and safety of this combined therapy. Two small retrospective trials examined patients with advanced HCC but preserved liver function (Child-Pugh class A-B7) who had received nivolumab and SIRT. Both of these studies documented that the most common adverse event was hepatobiliary toxicity; otherwise, the therapy appears to be safe[47,48]. Zhan et al noted that PFS was 5.7 months and median OS after the first immunotherapy and first radioembolization was 17.2 and 16.5 months, respectively[47]. Furthermore, a phase II single-arm trial evaluated the objective response rate (based on the RECIST 1.1 criteria) of Child-Pugh class A cirrhotics with advanced HCC not amenable to resection, who were treated with nivolumab and Y90SIRT. The objective response rate was 30.6%. Five out of 40 patients had serious adverse events, including Steven-Johnson syndrome, hepatitis E infection, fever, liver abscesses, and ascites[49]. There are more ongoing clinical trials evaluating SIRT and immunotherapy, including two-arm prospective trials.
TACE and sorafenib
The promise of adding sorafenib to prior TACE treatment was first suggested by a subgroup analysis of the SHARP trial. In this subanalysis, sorafenib treatment in patients with prior TACE resulted in longer median OS and TTP than placebo[50]. However, many clinical trials since then have attempted to demonstrate the efficacy of this combined treatment without success.
The phase III post-TACE trial took Japanese and Korean Child-Pugh class A cirrhotics with unresectable HCC, who had > 25% tumor shrinkage with TACE 1-3 months prior, and randomized them to sorafenib or placebo. The primary endpoint looked at median TTP in the sorafenib and placebo groups, which was 5.4 and 3.7 months, respectively, P = 0.252[51]. Therefore, the addition of sorafenib was not shown to prolong TTP. Although it was thought that the results could be due to the delay in sorafenib administration after TACE, a smaller single-center randomized controlled trial demonstrated a significantly longer TTP in Child-Pugh class A intermediate-stage chronic hepatitis C virus (HCV) HCC, who received sorafenib 30 days after TACE compared to placebo[52]. Both studies did not have any unexpected adverse events.
The phase II SPACE trial randomized patients with intermediate-stage multinodular HCC without macrovascular invasion or extrahepatic spread to DEB-TACE plus sorafenib or placebo. Sorafenib was administered continuously, starting about one week prior to the first TACE. The median TTP in DEB-TACE plus sorafenib was 169 days vs. 166 days in the placebo group, and the median OS was not significantly prolonged in the group with sorafenib[53]. Another similar study, phase III TACE 2, also looked at the efficacy of continuous sorafenib plus DEB-TACE and placebo plus DEB-TACE in Child-Pugh class A unresectable, liver-confined HCC. The primary endpoint was PFS, which was not significantly different between the sorafenib and placebo groups[54]. Therefore, studies evaluating continuous administration of sorafenib with TACE in intermediate-stage HCC amenable to TACE have not shown any increased efficacy compared to TACE alone.
Furthermore, some studies also looked at interrupted administration of sorafenib in combination with TACE. Interrupted schedule focused on briefly holding sorafenib around the time of TACE therapy to minimize the combined toxicity of the two drugs. The phase II SOCRATES trial showed TPP of 16.4 months and median OS of 20.1 months in locally advanced unresectable HCC treated with sorafenib-TACE. Overall, there did not seem to be any additional toxicity with the combined therapy compared to sorafenib or TACE alone[55]. Another phase II trial, START, evaluated interrupted dosing of sorafenib with TACE in intermediate unresectable HCC. Median PFS was 384 days and TTP was 415 days. The estimated 3-year OS was 86.1%. Only 8.1% of patients discontinued the trial due to adverse events (AEs), and although 52 of 192 patients experienced serious AEs, only four were considered to be related to sorafenib[56]. Similar conclusions were also reached by the single-arm phase II study showing promising efficacy and tolerable safety profile with treatment with sorafenib-TACE[57]. Although these results suggest the interrupted administration of sorafenib plus TACE is efficacious with a relatively tolerable safety profile, randomized comparative studies were lacking until the recent TACTICS trial.
So far, the larger randomized controlled trials mentioned previously (post-TACE, SPACE, TACE-2) have all been negative trials, showing no benefit of the addition of sorafenib to TACE. The multicenter randomized controlled TACTICS trial examined the efficacy and safety of TACE plus sorafenib compared with TACE alone in unresectable HCC and is the first positive trial, demonstrating the efficacy of the combined treatment. Sorafenib was given 2-3 weeks prior to the first TACE session in an interrupted administration. The primary endpoint was met with PFS significantly higher in sorafenib-TACE (25.6 months) than in TACE alone (13.5 months) with a hazard ratio (HR) of 0.59. In addition, time to cancer progression and spread was also significantly higher in the combined therapy group than in TACE alone. The reasons why the TACTICS trial was able to demonstrate improved clinical outcomes stem largely from two concepts. First, it was shown in exploratory analyses of the post-TACE and SPACE trial that a longer duration of sorafenib treatment was associated with significantly favorable TTP than shorter durations. The shorter duration of treatment was due to utilizing the RECIST1.1 or modified RECIST (mRECIST) criteria for tumor progression, which resulted in earlier termination of therapy even though it is thought that patients meeting these criteria still have disease amendable to further treatment of TACE. Therefore, the TACTICS trial used the Response Evaluation Criteria in Cancer of the Liver (RECICL), which resulted in a longer duration of treatment (38.7 weeks) compared to previous trials (17-21 weeks). Second, although there is no good data currently out on the preferred timing of sorafenib administration prior to and after TACE, the TACTICS trial started sorafenib considerably earlier than previous trials, 2-3 weeks and 3-7 days, respectively. The earlier pe-treatment with sorafenib likely allowed for reduced vascular ischemic injury and reduced upregulation of VEGF[58].
So far, the studies of TACE plus sorafenib have largely included intermediate-stage HCC that qualify for locoregional therapy. Although, the phase III STAH trial, a large Korean multicenter study, included patients with advanced HCC indicated for palliative systemic therapy (including those refractory to TACE, with vascular invasion, or with extrahepatic spread). Patients were randomized to receive sorafenib alone or in combination with TACE. Results showed no significant improvement in OS in sorafenib-TACE vs. sorafenib alone, 12.8 vs. 10.8 months, respectively (HR 0.91; P = 0.290). However, sorafenib-TACE did significantly improve TTP, PFS, and tumor response rates compared to sorafenib alone[59].
TACE with bevacizumab
Bevacizumab is a human monoclonal anti-VEGF alpha antibody that is FDA-approved for use in multiple metastatic tumors such as renal cancer, lung cancer, and colon cancer[60]. Bevacizumab was associated with strong angiogenesis inhibition in pre-clinical HCC models.[61]
There have been several data on the use of Bevacizumab alone in unresectable HCC. A systematic review of phase II clinical trials involving bevacizumab for the treatment of advanced HCC showed promising results. 8 trials (n = 300 patients) were included in this review which showed overall median progression-free survival and overall survival (5.3-9.0) months and (5.9-13.7) months, respectively[62].Bevacizumab plus TACE combination was examined in a pilot study of IV bevacizumab (intravenous) plus TACE, and there was a statistically significant improvement in progression-free survival at 16 weeks [0.19 in TACE group vs. 0.79 in the combination group (P = < 0.05)]. Overall, bevacizumab was well tolerated, without significant bleeding risk. The Authors concluded that the addition of bevacizumab to TACE was a feasible and safe option in selected HCC patients[63].
Unfortunately, the AVATACE-1 trial (ClinicalTrials.gov Identifier: NCT00280007), which compared TACE with Bevacizumab vs. TACE alone, was terminated early due to safety concerns in the treatment arm, but the sample size was only 32 participants. However, a large clinical trial combining Bevacizumab with Atezolizumab was compared to sorafenib and showed improved overall survival at 12 months 67.2% (95%CI: 61.3-73.1) with atezolizumab-bevacizumab vs. 54.6% (95%CI: 45.2-64.0) with sorafenib[11]. Currently, Bevacizumab is being studied in combination with Atezolizumab following TACE (ClinicalTrials.gov: NCT04224636)
TACE with lenvatinib
In a retrospective study involving 120 patients, the overall survival rates were significantly higher in the TACE + lenvatinib arm (88.4% and 79.8%) than in the TACE arm alone (79.2% and 49.2%, P = 0.047)[64].
An Open-label, Single-arm, Phase 2 Trial (Thalen) will analyze the safety and efficacy of TACE plus Lenvatinib for patients with unresectable HCC; the study is currently recruiting new subjects. (ClinicalTrials.gov Identifier NCT04531228). Data from the Phase Ib study of lenvatinib + Pembrolizumab showed promising results with a median Progression-free survival of 9.3 months, and median Overall survival of 22.0 months[65].
TACE with axitinib
Axitinib is an oral multi-targeted receptor TKI, binding vascular endothelial growth factor receptor (VEGF), PDGFR, and c-KITR. Axitinib is FDA-approved for use as a second-line age for advanced renal cell carcinoma[66]. A phase II trial examined the efficacy of the TACE plus axitinib combination in the treatment of unresectable HCC. The 2-year overall survival rate was 43.7%, and the median OS was 18.8 months which is better than the average overall survival in the TACE monotherapy (31%)[67]. Although the overall survival was not different from the placebo, Axitinib showed improvement in progression-free survival and time to tumor progression[68].
TACE with other molecular agents
Multiple trials that combined TACE with other agents have been done. There are several that did not show survival benefits, including TACE, BRISK-TA, SPACE, ORIENTAL, and TACE-2. The BRISK-TA compared TACE with placebo vs. TACE with Brivanib - a fibroblast growth factor receptor and VEGF receptor inhibitor- the endpoint was overall survival which was not different among the two arms[69].
The ORIENTAL trial examined orantinib- a VEGF and PDGF inhibitor- compared to a placebo. The overall survival was not significantly different among both groups. OS was 31.1 and 32.3 months in the orantinib and placebo groups, respectively (HR: 1.090; 95%CI: 0.878-1.352; P = 0.435)[70].
TACE with immunotherapy
In recent years, the combination of TACE and immunotherapy has emerged as a promising therapy as it has demonstrated a favorable antitumor immune response. A study looked at the efficacy and safety of tremelimumab (anti-CTLA4) combined with TACE in BCLC stage B patients with HCC. Median TTP was 7.4 months median OS was 12.3 months during a follow-up of 18.8 months (this included an additional BCLC stage C group treated with thermal ablation instead of TACE)[71]. A more recent study by Ji et al. evaluated the efficacy and safety of RFA and TACE combined with postoperative cytokine-induced killer (CIK) cell immunotherapy in Child-Pugh class A or B cirrhotics with HCC meeting Milan’s criteria[72]. The median OS of patients was 42.1 ± 5.6 months and 37.8 ± 4.8 months and the 5-year OS rate was 29.3% and 13.8% in RFA + TACE + CIK vs. RFA + TACE, respectively[72]. The study found that RFA and TACE combined with postoperative autologous CIK cell reinfusion have significant efficacy in the treatment of primary HCC, enhancing the immune function, improving the postoperative quality of life, and raising the patient’s survival rate with tolerable adverse reactions[72]. Multiple studies to assess the efficacy of TACE and other immunotherapies are underway. Two trials are set to study the combined effects of TACE and Nivolumab (NCT03143270 and NCT03572582). Another trial (NCT03397654) is exploring the sequential use of TACE and Pembrolizumab[73]. The combination of 2 immunotherapies (Durvalumab and Bevacizumab) with TACE is considered in another emerging study (NCT03937830). Using the combination of LRTs and immune checkpoint inhibitors may provide promising options for patients with HCC.
HAIC with sorafenib
A couple of studies were conducted to evaluate the effect of adding HAIC to sorafenib therapy in advanced HCC. A recent single-arm phase II study demonstrated favorable outcomes of OS and PFS in HAIC with 5-FU and sorafenib in HCC ineligible for curative treatment[74]. In a phase II trial by Ikeda, patients with advanced HCC were randomized to receive sorafenib with HAIC with cisplatin or sorafenib alone[75]. There was a significant improvement in OS with HR 0.6. Both studies had tolerable adverse events. More randomized trials need to be conducted to further evaluate the efficacy of this combined method.
FUTURE CONSIDERATION AND STUDIES
With the emergence of multiple new tyrosine kinase inhibitors along with immunotherapy, the future perspective is focusing on finding combination therapies and the best sequential therapeutic options [Table 1]. The success with Atezolizumab/ Bevacizumab combination therapy has led to new perspectives. Currently, multiple different combination therapies are being studied. For example, a German multicenter phase II trial is evaluating Pembrolizumab and Lenvatinib in patients with advanced HCC who failed Atezolizumab/ Bevacizumab (NCT05101629). Toripalimab with Bevacizumab is being evaluated for first-line therapy for HCC (NCT04605796). The combination of TACE with sorafenib and Tislelizumab is also being studied (NCT04599777). The START-FIT trial is currently examining sequential transarterial chemoembolization and stereotactic radiotherapy with immunotherapy; the study is being done in China and recruiting patients (NCT03817736). Arginine hydrochloride is a new anticancer drug that is being evaluated for the treatment of HCC. Levamisole may play an antitumor role by inhibiting the reverse TCA cycle (NCT03950518). The results of multiple ongoing trials will be unexpected to be revealed in the upcoming years, and it is anticipated to have guidelines-changing outcomes.
Ongoing clinical trials
| Study Title | Aim | Interventions | Locations |
| TACE, Sorafenib and PD-1 Monoclonal Antibody in the Treatment of HCC (NCT04518852) | Prospective study assessing objective response rate and overall survival as primary outcomes | ● Combination Product: TACE combined with sorafenib and PD-1 mAb | Sichuan Cancer Hospital and Research Institute, China |
| DEB-TACE Plus Lenvatinib or Sorafenib or PD-1 Inhibitor for Unresectable Hepatocellular Carcinoma (NCT04229355) | This is a prospective study aiming to investigate the safety and efficacy of DEB-TACE plus sorafenib or lenvatinib or PD-1 Inhibitor for unresectable HCC. | ● Drug: DEB-TACE plus Sorafenib ● Drug: DEB-TACE plus Lenvatinib ● Drug: DEB-TACE plus PD-1 inhibitor | Guangxi Medical University Cancer Hospital, China |
| A Novel Immunotherapy PD-1 Antibody to Suppress Recurrence of HCC Combined With PVTT After Hepatic Resection (NCT03914352) | The use of novel immunotherapy using the PD-1 antibody could suppress postoperative recurrence and prolong HCC patients survival period effectively. | ● Drug: PD-1 antibody ● Procedure: TACE | Affiliated Tumor Hospital of Guangxi Medical University, China |
| IMMULAB - Immunotherapy With Pembrolizumab in Combination With Local Ablation in Hepatocellular Carcinoma (HCC) (NCT03753659) | ● Drug: Pembrolizumab ● Procedure: Radio Frequency Ablation (RFA) ● Procedure: Microwave Ablation (MWA) | Hannover Medical School, Germany | |
| Autologous Immune Cell Therapy in Primary Hepatocellular Carcinoma Patients Following Resection and TACE Therapy (NCT01828762) | To assess the safety of Autologous Immune Cell Therapy in Primary Hepatocellular Carcinoma Patients Following Resection and TACE Therapy | ● Biological: DC-TC + GM-CSF | Shanghai, China |
| Tremelimumab With Chemoembolization or Ablation for Liver Cancer (NCT01853618) | To assess the safety and feasibility of combining Tremelimumab with trans-arterial catheter chemoembolization (TACE) radiofrequency ablation (RFA) or cryoablation in patients with advanced HCC | ● Drug: Tremelimumab ● Procedures: RFA, TACE and cryoablation | National Institutes of Health Clinical Center, Maryland, USA |
CONCLUSION
HCC is a devastating disease with grim outcomes, as it is often diagnosed during an advanced stage. For many decades, the treatment options for patients with HCC who are not transplant or surgical candidates were limited to bridging-to-transplant or palliative therapy. Currently, we have LRT for early to intermediate-stage HCC and sorafenib for advanced HCC. Awareness of the shortcomings of each modality and the potential for increasing their efficacy has prompted the exploration of combined therapy, most notably LRT and systemic treatment. The intent of combining LRT with MTAs is to reduce the post-ischemia upregulation of angiogenic antigens with MTAs. More and larger studies need to be conducted to see if MTA administration after RFA results in improved survival benefits. TACE had probably been the most studied agent in combination with MTAs, mainly sorafenib. Although past studies have largely demonstrated no improvement in OS or PFS in combined therapy, the recent TACTICS trial did show improved efficacy with TACE and sorafenib, which highlights the importance of timing the administration of therapy and evaluation of tumor progression. Further studies may adopt the methods used in the TACTICS trial to determine if there is a further significant improvement. Combining LRT and immunotherapy works to create a synergistic boost in the existing immune response. Given immunotherapy in HCC is relatively new, the combination of these agents with LRT is up and coming as multiple studies are currently underway. Combined therapy is a promising direction in the treatment for HCC and future studies are encouraged to reveal the patient population who will best benefit from these therapies.
DECLARATIONS
Author contributionContributed to the conception and the design of the study, data search and manuscript writing: Wu Y
Made contributions to conception, manuscript writing, and table design: Wakil A
Performed data acquisition and manuscript writing: Solomon F
Supervised the article concept and manuscript review: Pyrsolpoulos N
Availability of Data and MaterialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209-49.
2. McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology 2021;73 Suppl 1:4-13.
3. Livraghi T, Meloni F, Di Stasi M, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology 2008;47:82-9.
4. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358-80.
5. National Comprehensive Cancer Network. Hepatocellular cancer (Version 5.2021). Available from: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf [Last accessed on 10 Mar 2023].
6. Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 2003;37:429-42.
7. Raza A, Sood GK. Hepatocellular carcinoma review: current treatment, and evidence-based medicine. World J Gastroenterol 2014;20:4115-27.
8. Llovet JM, Ricci S, Mazzaferro V, et al. SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90.
9. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34.
10. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol 2022;76:681-93.
11. Finn RS, Qin S, Ikeda M, et al. IMbrave150 Investigators. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020;382:1894-905.
12. Abou-alfa GK, Lau G, Kudo M, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evidence 2022:1.
13. Sergio A, Cristofori C, Cardin R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol 2008;103:914-21.
14. Li X, Feng GS, Zheng CS, Zhuo CK, Liu X. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol 2004;10:2878-82.
15. Chen L, Sun J, Yang X. Radiofrequency ablation-combined multimodel therapies for hepatocellular carcinoma: current status. Cancer Lett 2016;370:78-84.
16. Oliveri RS, Wetterslev J, Gluud C. Transarterial (chemo) embolisation for unresectable hepatocellular carcinoma. Cochrane Database Syst Rev 2011;(3):CD004787.
17. Paul SB, Sharma H. Role of transcatheter intra-arterial therapies for hepatocellular carcinoma. J Clin Exp Hepatol 2014;4:S112-21.
18. Miller FH, Lopes Vendrami C, Gabr A, et al. Evolution of radioembolization in treatment of hepatocellular carcinoma: a pictorial review. Radiographics 2021;41:1802-18.
19. Ahmed A, Stauffer JA, LeGout JD, et al. The use of neoadjuvant lobar radioembolization prior to major hepatic resection for malignancy results in a low rate of post hepatectomy liver failure. J Gastrointest Oncol 2021;12:751-61.
20. Lewandowski RJ, Gabr A, Abouchaleh N, et al. Radiation segmentectomy: potential curative therapy for early hepatocellular carcinoma. Radiology 2018;287:1050-8.
21. Song MJ. Hepatic artery infusion chemotherapy for advanced hepatocellular carcinoma. World J Gastroenterol 2015;21:3843-9.
22. Li S, Lyu N, Han X, et al. Hepatic artery infusion chemotherapy using fluorouracil, leucovorin, and oxaliplatin versus transarterial chemoembolization as initial treatment for locally advanced hepatocellular carcinoma: a propensity score-matching analysis. J Vasc Interv Radiol 2021;32:1267-1276.e1.
23. Lyu N, Wang X, Li JB, et al. Arterial chemotherapy of oxaliplatin plus fluorouracil versus sorafenib in advanced hepatocellular carcinoma: a biomolecular exploratory, randomized, phase III trial (FOHAIC-1). J Clin Oncol 2022;40:468-80.
24. Bteich F, Di Bisceglie AM. Current and future systemic therapies for hepatocellular carcinoma. Gastroenterol Hepatol (NY) 2019;15:266-72.
25. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163-73.
26. Bruix J, Qin S, Merle P, et al. RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56-66.
27. Rizzo A, Nannini M, Novelli M, Dalia Ricci A, Scioscio VD, Pantaleo MA. Dose reduction and discontinuation of standard-dose regorafenib associated with adverse drug events in cancer patients: a systematic review and meta-analysis. Ther Adv Med Oncol 2020;12:1758835920936932.
28. Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med 2018;379:54-63.
29. Zhu AX, Kang YK, Yen CJ, et al. REACH-2 study investigators. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019;20:282-96.
30. Gordan JD, Kennedy EB, Abou-Alfa GK, et al. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J Clin Oncol 2020;38:4317-45.
31. Gao X, Huang H, Wang Y, et al. Tumor immune microenvironment characterization in hepatocellular carcinoma identifies four prognostic and immunotherapeutically relevant subclasses. Front Oncol 2020;10:610513.
32. Yau T, Park JW, Finn RS, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol 2022;23:77-90.
33. Rizzo A, Ricci AD, Di Federico A, et al. Predictive biomarkers for checkpoint inhibitor-based immunotherapy in hepatocellular carcinoma: Where do we stand? Front Oncol 2021;11:803133.
34. Fukuda H, Numata K, Moriya S, et al. Hepatocellular carcinoma: concomitant sorafenib promotes necrosis after radiofrequency ablation-propensity score matching analysis. Radiology 2014;272:598-604.
35. Feng X, Xu R, Du X, et al. Combination therapy with sorafenib and radiofrequency ablation for BCLC Stage 0-B1 hepatocellular carcinoma: a multicenter retrospective cohort study. Am J Gastroenterol 2014;109:1891-9.
36. Kan X, Jing Y, Wan QY, Pan JC, Han M, Yang Y, Zhu M, Wang Q, Liu KH. Sorafenib combined with percutaneous radiofrequency ablation for the treatment of medium-sized hepatocellular carcinoma. Eur Rev Med Pharmacol Sci 2015;19:247-55.
37. Bruix J, Takayama T, Mazzaferro V, et al. STORM investigators. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol 2015;16:1344-54.
38. Jin M, Yu Q, Liu Y, Xu W, Fu X, Ji B. Safety and efficacy of physical thermal ablation combined sorafenib for hepatocellular carcinoma: a meta-analysis. J Clin Transl Hepatol 2021;9:149-159.
39. Wang F, Numata K, Komiyama S, et al. Combination therapy with lenvatinib and radiofrequency ablation for patients with intermediate-stage hepatocellular carcinoma beyond up-to-seven criteria and child-pugh class a liver function: a pilot study. Front Oncol 2022;12:843680.
40. Cui J, Wang N, Zhao H, et al. Combination of radiofrequency ablation and sequential cellular immunotherapy improves progression-free survival for patients with hepatocellular carcinoma. Int J Cancer 2014;134:342-51.
41. Bian H, Zheng JS, Nan G, et al. Randomized trial of [131I] metuximab in treatment of hepatocellular carcinoma after percutaneous radiofrequency ablation. J Natl Cancer Inst 2014;106:dju239.
42. Lee JH, Lee JH, Lim YS, et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology 2015;148:1383-91.e6.
43. Lee JH, Lee JH, Lim YS, et al. Sustained efficacy of adjuvant immunotherapy with cytokine-induced killer cells for hepatocellular carcinoma: an extended 5-year follow-up. Cancer Immunol Immunother 2019;68:23-32.
44. Wang X, Liu G, Chen S, et al. Combination therapy with PD-1 blockade and radiofrequency ablation for recurrent hepatocellular carcinoma: a propensity score matching analysis. Int J Hyperthermia 2021;38:1519-28.
45. Ricke J, Klümpen HJ, Amthauer H, et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J Hepatol 2019;71:1164-74.
46. Kaseb AO, Kappadath SC, Lee SS, et al. A Prospective phase II study of safety and efficacy of sorafenib followed by 90Y glass microspheres for patients with advanced or metastatic hepatocellular carcinoma. J Hepatocell Carcinoma 2021;8:1129-45.
47. Zhan C, Ruohoniemi D, Shanbhogue KP, et al. Safety of combined Yttrium-90 radioembolization and immune checkpoint inhibitor immunotherapy for hepatocellular carcinoma. J Vasc Interv Radiol 2020;31:25-34.
48. Fenton SE, Kircher SM, Mulcahy MF, et al. A phase I study of nivolumab (NIVO) in combination with TheraSphere (Yttrium-90) in patients with advanced hepatocellular cancer. JCO 2021;39:e16183-e16183.
49. Tai D, Loke K, Gogna A, et al. Radioembolisation with Y90-resin microspheres followed by nivolumab for advanced hepatocellular carcinoma (CA 209-678): a single arm, single centre, phase 2 trial. Lancet Gastroenterol Hepatol 2021;6:1025-35.
50. Bruix J, Raoul JL, Sherman M, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol 2012;57:821-9.
51. Kudo M, Imanaka K, Chida N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer 2011;47:2117-27.
52. Sansonno D, Lauletta G, Russi S, Conteduca V, Sansonno L, Dammacco F. Transarterial chemoembolization plus sorafenib: a sequential therapeutic scheme for HCV-related intermediate-stage hepatocellular carcinoma: a randomized clinical trial. Oncologist 2012;17:359-66.
53. Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: the SPACE trial. J Hepatol 2016;64:1090-8.
54. Meyer T, Fox R, Ma YT, et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol 2017;2:565-75.
55. Erhardt A, Kolligs F, Dollinger M, et al. TACE plus sorafenib for the treatment of hepatocellular carcinoma: results of the multicenter, phase II SOCRATES trial. Cancer Chemother Pharmacol 2014;74:947-54.
56. Chao Y, Chung YH, Han G, et al. The combination of transcatheter arterial chemoembolization and sorafenib is well tolerated and effective in Asian patients with hepatocellular carcinoma: final results of the START trial. Int J Cancer 2015;136:1458-67.
57. Park JW, Koh YH, Kim HB, et al. Phase II study of concurrent transarterial chemoembolization and sorafenib in patients with unresectable hepatocellular carcinoma. J Hepatol 2012;56:1336-42.
58. Kudo M, Ueshima K, Ikeda M on behalf of the TACTICS study group, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut 2020;69:1492-1501.
59. Park JW, Kim YJ, Kim DY, et al. Sorafenib with or without concurrent transarterial chemoembolization in patients with advanced hepatocellular carcinoma: the phase III STAH trial. J Hepatol 2019;70:684-91.
60. Bonifazi M, Rossi M, Moja L, et al. Bevacizumab in clinical practice: prescribing appropriateness relative to national indications and safety. Oncologist 2012;17:117-24.
61. Finn RS, Bentley G, Britten CD, Amado R, Busuttil RW. Targeting vascular endothelial growth factor with the monoclonal antibody bevacizumab inhibits human hepatocellular carcinoma cells growing in an orthotopic mouse model. Liver Int 2009;29:284-90.
62. Fang P, Hu JH, Cheng ZG, Liu ZF, Wang JL, Jiao SC. Efficacy and safety of bevacizumab for the treatment of advanced hepatocellular carcinoma: a systematic review of phase II trials. PLoS One 2012;7:e49717.
63. Britten CD, Gomes AS, Wainberg ZA, et al. Transarterial chemoembolization plus or minus intravenous bevacizumab in the treatment of hepatocellular cancer: a pilot study. BMC Cancer 2012;12:16.
64. Fu Z, Li X, Zhong J, et al. Lenvatinib in combination with transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma (uHCC): a retrospective controlled study. Hepatol Int 2021;15:663-75.
65. Finn RS, Ikeda M, Zhu AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol 2020;38:2960-70.
66. Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 2011;378:1931-9.
67. Chan SL, Yeo W, Mo F, et al. A phase 2 study of the efficacy and biomarker on the combination of transarterial chemoembolization and axitinib in the treatment of inoperable hepatocellular carcinoma. Cancer 2017;123:3977-85.
68. Kang YK, Yau T, Park JW, et al. Randomized phase II study of axitinib versus placebo plus best supportive care in second-line treatment of advanced hepatocellular carcinoma. Ann Oncol 2015;26:2457-63.
69. Kudo M, Han G, Finn RS, et al. Brivanib as adjuvant therapy to transarterial chemoembolization in patients with hepatocellular carcinoma: a randomized phase III trial. Hepatology 2014;60:1697-707.
70. Kudo M, Cheng AL, Park JW, et al. Orantinib versus placebo combined with transcatheter arterial chemoembolisation in patients with unresectable hepatocellular carcinoma (ORIENTAL): a randomised, double-blind, placebo-controlled, multicentre, phase 3 study. Lancet Gastroenterol Hepatol 2018;3:37-46.
71. Duffy AG, Ulahannan SV, Makorova-Rusher O, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol 2017;66:545-51.
72. Ji Q, Fu Y, Zhu X, Wang L, Ling C. Effect of RFA and TACE combined with postoperative cytokine-induced killer cell immunotherapy in primary hepatocellular carcinoma. J BUON 2021;26:235-242.
73. Viveiros P, Riaz A, Lewandowski RJ, Mahalingam D. Current state of liver-directed therapies and combinatory approaches with systemic therapy in hepatocellular carcinoma (HCC). Cancers (Basel) 2019;11:1085.
74. Ikeda M, Shimizu S, Sato T, et al. Sorafenib plus hepatic arterial infusion chemotherapy with cisplatin versus sorafenib for advanced hepatocellular carcinoma: randomized phase II trial. Ann Oncol 2016;27:2090-6.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Wu YC, Wakil A, Salomon F, Pyrsopoulos N. Issue on combined locoregional and systemic treatment for hepatocellular carcinoma. Hepatoma Res 2023;9:6. http://dx.doi.org/10.20517/2394-5079.2022.37
AMA Style
Wu YC, Wakil A, Salomon F, Pyrsopoulos N. Issue on combined locoregional and systemic treatment for hepatocellular carcinoma. Hepatoma Research. 2023; 9: 6. http://dx.doi.org/10.20517/2394-5079.2022.37
Chicago/Turabian Style
Wu, Yi-Chia (Jasmine), Ali Wakil, Fayssa Salomon, Nikolaos Pyrsopoulos. 2023. "Issue on combined locoregional and systemic treatment for hepatocellular carcinoma" Hepatoma Research. 9: 6. http://dx.doi.org/10.20517/2394-5079.2022.37
ACS Style
Wu, Y.C.; Wakil A.; Salomon F.; Pyrsopoulos N. Issue on combined locoregional and systemic treatment for hepatocellular carcinoma. Hepatoma. Res. 2023, 9, 6. http://dx.doi.org/10.20517/2394-5079.2022.37
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 28 clicks
Cite This Article 28 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.