Hepatocellular carcinoma surveillance in non-alcoholic fatty liver disease patients
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is the leading cause of chronic liver disease worldwide and is an umbrella term for liver disease encompassing non-alcoholic steatohepatitis (NASH), advanced fibrosis, cirrhosis, and/or hepatocellular carcinoma (HCC)[1]. The burden of NAFLD is rapidly mounting alongside rising rates of metabolic syndrome and obesity, and NAFLD is projected to become the leading cause of HCC in the United States[2]. Among the NAFLD’s global burden, HCC surveillance in patients with NAFLD is challenging given the drawbacks of specific screening modalities and the well-recognized potential for HCC development in those without cirrhosis and even in those with lean NAFLD[3].
THE PREVALENCE OF HCC IN NAFLD
Using the National Veterans Affairs system, a retrospective cohort study of 296,707 NAFLD patients found that the overall mean risk of HCC in NAFLD was 1.06% annually in the United States[4]. More specifically, HCC incidence in NAFLD was 0.03 and 3.78 per 100 person-years, respectively, for those without and with cirrhosis, according to a meta-analysis that included 470,404 patients from 18 studies[5]. According to the United Network for Organ Sharing registry, NAFLD is the most rapidly growing etiology of HCC-related liver transplant, and the number of NAFLD patients undergoing liver transplantation for HCC nearly quadrupled from 2002 to 2012 based on a study of 61,868 liver transplant patients, including 10,061 patients with HCC, in the United States[6-8].
The incidence of HCC in NAFLD patients with cirrhosis is ~1 per 100 person-years[9,10]. Although HCC incidence in NAFLD cirrhosis is lower or comparable compared to that of hepatitis C virus (HCV) or alcoholic cirrhosis, respectively, NAFLD and its high prevalence contribute to a greater global burden of HCC compared to other chronic liver disease etiologies[10].
Up to 40% of HCC cases can develop in NAFLD patients without cirrhosis, and NAFLD represents the most common cause of HCC in those without cirrhosis, accounting for 26.3% of 605 HCC cases without cirrhosis compared to 13.4% of 4539 of HCC cases with cirrhosis[11-13]. In the NAFLD spectrum, HCC incidence in patients with uncomplicated steatosis is estimated to be approximately 0.8-6.2 per 100 person-years, but is poorly reported in NASH due to its invasive histological nature[14-16]. However, it is reasonable to approximate the incidence of HCC in NASH to the median prevalence of simple steatosis and cirrhosis[13]. The pathophysiology of NAFLD may therefore independently contribute to the development of HCC regardless of fibrosis stage and brings up key challenges regarding screening for HCC[17].
Furthermore, NAFLD has been shown to develop in approximately 10%-20% of non-obese
HCC SURVEILLANCE IN PATIENTS WITH NAFLD
Targeted Patient Populations for HCC Surveillance
Surveillance for HCC remains suboptimal in NAFLD, as 51.5% of NASH cirrhotic fail to undergo any screening before the diagnosis of HCC, compared with 25.9% of HCV cirrhotics[19]. This may be due in part to the fact that NAFLD and HCC can often be clinically silent, especially in the early stages, and patients may therefore never consult the doctor. NASH cirrhotics with complete HCC screening had smaller tumors (P = 0.006) at diagnosis but no differences in treatment outcomes (P = 0.281) or mortality (P = 0.468) in comparison with NASH cirrhotics with incomplete or no screening[19]. Similarly, compared with 13.3% of HCV-associated HCC cases (P < 0.01) and 40.2% of alcohol-associated HCC cases (P < 0.01), 56.7% of NAFLD-associated HCC cases did not follow recommended surveillance for HCC in the 3 years before diagnosis based on a United States national cohort of 1500 veterans who developed HCC from 2005-2010[20].
Currently, the European Association for the Study of the Liver (EASL) and American Association for the Study of Liver Diseases (AASLD) recommend surveillance for HCC for NAFLD patients with cirrhosis regardless of compensation or decompensation, which is cost-effective given that the predicted HCC incidence remains ≥ 1.5% annually[21,22].
Recommendations for HCC surveillance in those without cirrhosis remain controversial. Both EASL guidelines and the American Gastroenterological Association (AGA) Clinical Practice Update recommend surveilling for HCC in those with advanced fibrosis, defined as fibrosis stage 3 or higher (F ≥ 3)[22,23]. Additionally, AASLD guidance advises against screening for HCC in those with advanced fibrosis, considering the need for additional cost-effectiveness studies[21,24]. In the absence of advanced fibrosis, AASLD and AGA clinical practice guidance recommend against routine HCC screening, whereas EASL states that this remains unclear given the known possibility of HCC occurrence in NAFLD patients without advanced fibrosis[21-23].
Factors such as genetics may play a role in the pathophysiology of HCC in NAFLD without advanced fibrosis or cirrhosis. Impacting 40% of the European population, the patatin-like phospholipase
Staging for NAFLD
Staging fibrosis is a priority in NAFLD not only because HCC surveillance is based on fibrosis stage, but also because Angulo et al. have shown that fibrosis stage is independently associated with long-term overall mortality, liver transplantation, and major adverse liver events[30]. Liver biopsy is the gold standard but is unsuitable as the initial staging method due to its invasive nature, risk of complications, high expense, and potential for sampling error[31-33].
Aside from liver biopsy, other modalities for staging include non-invasive serum biomarkers, imaging [i.e., vibration-controlled transient elastography (VCTE, cirrhosis cutoff 16.1 kPa) and magnetic resonance elastography (MRE, cirrhosis cutoff 5 kPa)], and/or risk stratifying algorithms[33-36]. However, compared to the gold standard, imaging modalities are often limited by the inability to definitively exclude advanced fibrosis given their low negative predictive values.
Non-invasive algorithms that offer risk stratification for HCC development include the Agile 3+ and 4 scores, Aspartate Aminotransferase-to-Platelet Ratio Index (APRI), Fibrosis-4 score (FIB-4), NAFLD fibrosis score (NFS), Metabolomics-Advanced StEatohepatitis Fibrosis (MASEF), MAST score and MR elastography combined with fibrosis-4 (MEFIB) score[35,37-44]. In NAFLD, a FIB-4 score of ≥ 2.67 is associated with a higher HCC risk of 13.5/1000 person-years in those with cirrhosis and 0.39/1000 person-years in those without cirrhosis, both of which are higher than the HCC risk of 0.04/1000 person-years in those without cirrhosis with a low FIB-4 score[4]. With a median follow-up of 7 years, HFS and NFS have similar performances compared to that of FIB-4 in predicting the development of HCC[45].
Given the drawbacks of percutaneous liver biopsy and the vast array of available non-invasive testing, advanced fibrosis for which HCC surveillance can be considered in NAFLD may be determined via concordance from 2 non-invasive tests (1 serum-based, 1 imaging-based), based on the AGA Clinical Practice Update[23]. However, this guidance is limited in that these NAFLD patients with advanced fibrosis may have a HCC risk that is less than the proposed 1.5% deemed optimal for cost-effectiveness. As such, utilizing higher thresholds with 90% specificity for HCC surveillance is recommended by the AGA[23].
HCC Surveillance
Both EASL and AASLD recommend biannual ultrasonography (US) with or without serum α-fetoprotein (AFP) levels for NAFLD patients meeting the aforementioned recommended eligibility criteria for HCC screening[21,22]. AFP testing alongside US remains much debated. EASL recommends using abdominal US alone, but AASLD supports US with or without AFP, whose combination with US increases HCC detection sensitivity from 45% (only US) to 63% (US + AFP)[21,22,46].
Other biomarkers have been investigated for HCC surveillance. Some promising, risk-stratifying HCC biomarkers include lens culinaris agglutinin-reactive AFP (AFP-L3), des-gamma-carboxyprothrombin (DCP), methylated DNA markers, circulating tumor DNA (ctDNA), and circulating tumor cells (CTCs). However, robust phase 3 clinical trials are necessary before clinical use, and several phase 2 clinical trials have proven the insufficiency of sole DCP or AFP-L3 use[47-49]. In light of these findings, biomarker-based algorithms such as the GALAD score have been developed to predict the development of HCC[47,50]. Utilizing gender, age, DCP, AFP, and AFP-L3, the GALAD score is more accurate in detecting HCC than US (GALAD: AUROC 0.95, 95%CI: 0.93-0.97, US: AUROC 0.82, P < 0.01)[50]. In addition, GALAD used in conjunction with US (GALADUS) has AUROC of 0.98 (95%CI: 0.96-0.99), specificity of 91%, and sensitivity of 95%[50].
US for HCC screening has previously been shown to be inadequate[51,52]. According to a retrospective cohort study of 941 patients, 20% and over 33% of USs, respectively, are insufficient for excluding HCC in cirrhotic patients overall and with BMI > 35 kg/m2[52]. Sonographic surveillance failure in overweight or obese patients results from heterogeneity in the parenchyma, focal fatty infiltration, and suboptimal sonographic attenuation, all of which prevent the identification of smaller cancerous nodules[53]. Quantitatively, suboptimal sonographic quality has been associated with increased BMI [OR = 1.67, (95%CI: 1.45-1.93)], male gender [OR = 1.68, (95%CI: 1.14-2.48)], NAFLD cirrhosis [OR = 2.87, (95%CI: 1.71-4.80)], and
According to the AGA, the quality of US in detecting mass lesions in the liver parenchyma should be documented in order to identify those with suboptimal US screening who should instead undergo computed tomography (CT) or magnetic resonance imaging (MRI) in the future[21,23,56]. Taking into account the extent to which the entire liver is visualized, beam attenuation, and echostructural heterogeneity, the 2017 Liver Imaging Reporting and Data System (LI-RADS) divides US quality into three categories: (1) no or negligible limitations that will not meaningfully impact sensitivity; (2) moderate limitations that may cause obscuration of smaller masses; or (3) severe limitations that significantly decrease the sensitivity for focal liver masses[57]. Though the AGA states that CT or MRI should be utilized for patients with B or C visualization scores, additional guidance is necessary to determine the utility of concomitant AFP alongside CT or MRI and the appropriate intervals for surveillance[23,46].
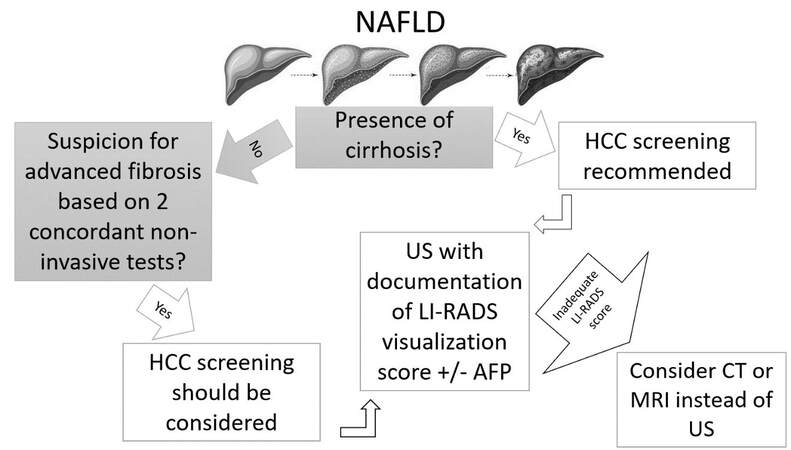
Figure 1 shows a summary of societal guidance recommendations for HCC surveillance in NAFLD.
CONCLUSION
As NAFLD remains the most rapidly growing cause of HCC in the United States, HCC surveillance in NAFLD is essential but plagued by questions surrounding the identification of those without advanced fibrosis or cirrhosis who may warrant HCC screening and the best screening modalities that balance cost-effectiveness and comprehensiveness in detecting HCC lesions. US remains the gold standard for screening for HCC but is often inadequate in NAFLD patients who are overweight or obese. Novel non-invasive tests are undergoing investigation for HCC risk stratification, but additional studies are needed for validation.
DECLARATIONS
Authors’ contributionsInterpreted the data and drafted the manuscript: Truong E
Critically revised the manuscript for important intellectual content: Noureddin M
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestNoureddin M has been on the advisory board/consultant for 89BIO, Altimmune, Gilead, cohBar, Cytodyn, Intercept, Pfizer, Novo Nordisk, Blade, EchoSens, Fractyl, Madrgial, NorthSea, Prespecturm, Terns, Sami-Sabina group, Siemens and Roche diagnostic; Noureddin M has received research support from Allergan, BMS, Gilead, Galmed, Galectin, Genfit, Conatus, Enanta, Madrigal, Novartis, Pfizer, Shire, Viking and Zydus; Noureddin M is a shareholder or has stocks in Anaetos, Chrownwell, Ciema, Rivus Pharma and Viking.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Vuppalanchi R, Noureddin M, Alkhouri N, Sanyal AJ. Therapeutic pipeline in nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol 2021;18:373-92.
2. Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology 2019;156:477-491.e1.
3. Ioannou GN. Epidemiology and risk-stratification of NAFLD-associated HCC. J Hepatol 2021;75:1476-84.
4. Kanwal F, Kramer JR, Mapakshi S, et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology 2018;155:1828-1837.e2.
5. Orci LA, Sanduzzi-Zamparelli M, Caballol B, et al. Incidence of hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression. Clin Gastroenterol Hepatol 2022;20:283-292.e10.
6. Younossi Z, Stepanova M, Ong JP, et al. Global nonalcoholic steatohepatitis council. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol 2019;17:748-755.e3.
7. Noureddin M, Vipani A, Bresee C, et al. NASH leading cause of liver transplant in women: updated analysis of indications for liver transplant and ethnic and gender variances. Am J Gastroenterol 2018;113:1649-59.
8. Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology 2014;59:2188-95.
9. Yatsuji S, Hashimoto E, Tobari M, Taniai M, Tokushige K, Shiratori K. Clinical features and outcomes of cirrhosis due to non-alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C. J Gastroenterol Hepatol 2009;24:248-54.
10. Ioannou GN, Green P, Lowy E, Mun EJ, Berry K. Differences in hepatocellular carcinoma risk, predictors and trends over time according to etiology of cirrhosis. PLoS One 2018;13:e0204412.
11. Gawrieh S, Dakhoul L, Miller E, et al. Characteristics, aetiologies and trends of hepatocellular carcinoma in patients without cirrhosis: a United States multicentre study. Aliment Pharmacol Ther 2019;50:809-21.
12. Mittal S, El-Serag HB, Sada YH, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2016;14:124-31.e1.
13. Tan DJH, Ng CH, Lin SY, et al. Clinical characteristics, surveillance, treatment allocation, and outcomes of non-alcoholic fatty liver disease-related hepatocellular carcinoma: a systematic review and meta-analysis. Lancet Oncol 2022;23:521-30.
14. Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 2005;129:113-21.
15. White DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol 2012;10:1342-1359.e2.
16. Noureddin M, Rinella ME. Nonalcoholic fatty liver disease, diabetes, obesity, and hepatocellular carcinoma. Clin Liver Dis 2015;19:361-79.
17. Zelber-Sagi S, Noureddin M, Shibolet O. Lifestyle and hepatocellular carcinoma what is the evidence and prevention recommendations. Cancers (Basel) 2021;14:103.
19. Aby E, Phan J, Truong E, Grotts J, Saab S. Inadequate hepatocellular carcinoma screening in patients with nonalcoholic steatohepatitis cirrhosis. J Clin Gastroenterol 2019;53:142-6.
20. Mittal S, Sada YH, El-Serag HB, et al. Temporal trends of nonalcoholic fatty liver disease-related hepatocellular carcinoma in the veteran affairs population. Clin Gastroenterol Hepatol 2015;13:594-601.e1.
21. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology 2018;68:723-50.
22. Association for the Study of the Liver; Electronic address: easloffice@easloffice.eu.; European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182-236.
23. Loomba R, Lim JK, Patton H, El-Serag HB. AGA clinical practice update on screening and surveillance for hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: expert review. Gastroenterology 2020;158:1822-30.
24. Noureddin M, Jones C, Alkhouri N, Gomez EV, Dieterich DT, Rinella ME. NASHNET. Screening for nonalcoholic fatty liver disease in persons with type 2 diabetes in the united states is cost-effective: a comprehensive cost-utility analysis. Gastroenterology 2020;159:1985-1987.e4.
25. Lewis CM, Vassos E. Polygenic risk scores: from research tools to clinical instruments. Genome Med 2020;12:44.
26. Singal AG, Manjunath H, Yopp AC, et al. The effect of PNPLA3 on fibrosis progression and development of hepatocellular carcinoma: a meta-analysis. Am J Gastroenterol 2014;109:325-34.
27. Liu YL, Patman GL, Leathart JB, et al. Carriage of the PNPLA3 rs738409 C >G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J Hepatol 2014;61:75-81.
28. Krawczyk M, Stokes CS, Romeo S, Lammert F. HCC and liver disease risks in homozygous PNPLA3 p.I148M carriers approach monogenic inheritance. J Hepatol 2015;62:980-1.
29. Bianco C, Jamialahmadi O, Pelusi S, et al. Non-invasive stratification of hepatocellular carcinoma risk in non-alcoholic fatty liver using polygenic risk scores. J Hepatol 2021;74:775-82.
30. Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389-97.e10.
31. Tobkes AI, Nord HJ. Liver biopsy: review of methodology and complications. Dig Dis 1995;13:267-74.
32. Saleh HA, Abu-Rashed AH. Liver biopsy remains the gold standard for evaluation of chronic hepatitis and fibrosis. J Gastrointestin Liver Dis 2007;16:425-6.
33. Guha IN, Parkes J, Roderick P, et al. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: validating the European liver fibrosis panel and exploring simple markers. Hepatology 2008;47:455-60.
34. Sanyal AJ. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: findings and recommendations from an American association for the study of liver diseases-U.S. Food and Drug Administration joint workshop. Hepatology 2015;61:1392-405.
35. Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45:846-54.
36. Hsu C, Caussy C, Imajo K, et al. Magnetic resonance vs transient elastography analysis of patients with nonalcoholic fatty liver disease: a systematic review and pooled analysis of individual participants. Clin Gastroenterol Hepatol 2019;17:630-637.e8.
37. Mayo R, Crespo J, Martínez-Arranz I, et al. Metabolomic-based noninvasive serum test to diagnose nonalcoholic steatohepatitis: results from discovery and validation cohorts. Hepatol Commun 2018;2:807-20.
38. Younossi ZM, Harrison SA, Newsome PN, et al. Agile 3+ development and validation: novel fibroscan based score to diagnose advanced fibrosis in non alcoholic fatty liver disease patients. Available from: https://www.postersessiononline.eu/173580348_eu/congresos/NAFLDsummit/aula/-PO_157_NAFLDsummit.pdf [Last accessed on 7 Nov 2022].
39. Younossi ZM, Harrison SA, Newsome PN, et al. LP12-improving diagnosis of cirrhosis in patients with NAFLD by combining liver stiffness measurement by vibration-controlled transient elastography and routine biomarkers: a global derivation and validation study. Available from: https://aasld.confex.com/aasld/2020/meetingapp.cgi/Paper/24427 [Last accessed on 7 Nov 2022].
40. Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology 2017;66:1486-501.
41. Ampuero J, Pais R, Aller R, et al. HEPAmet Registry. Development and validation of hepamet fibrosis scoring system-a simple, noninvasive test to identify patients with nonalcoholic fatty liver disease with advanced fibrosis. Clin Gastroenterol Hepatol 2020;18:216-225.e5.
42. Jung J, Loomba RR, Imajo K, et al. MRE combined with FIB-4 (MEFIB) index in detection of candidates for pharmacological treatment of NASH-related fibrosis. Gut 2021;70:1946-53.
43. Noureddin M, Truong E, Gornbein JA, et al. MRI-based (MAST) score accurately identifies patients with NASH and significant fibrosis. J Hepatol 2022;76:781-7.
44. Younossi ZM, Noureddin M, Bernstein D, et al. Role of noninvasive tests in clinical gastroenterology practices to identify patients with nonalcoholic steatohepatitis at high risk of adverse outcomes: expert panel recommendations. Am J Gastroenterol 2021;116:254-62.
45. Younes R, Caviglia GP, Govaere O, et al. Long-term outcomes and predictive ability of non-invasive scoring systems in patients with non-alcoholic fatty liver disease. J Hepatol 2021;75:786-94.
46. Tzartzeva K, Obi J, Rich NE, et al. Surveillance imaging and Alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta-analysis. Gastroenterology 2018;154:1706-1718.e1.
47. Rich N, Singal AG. Hepatocellular carcinoma tumour markers: current role and expectations. Best Pract Res Clin Gastroenterol 2014;28:843-53.
48. Ye Q, Ling S, Zheng S, Xu X. Liquid biopsy in hepatocellular carcinoma: circulating tumor cells and circulating tumor DNA. Mol Cancer 2019;18:114.
49. Ahn JC, Teng PC, Chen PJ, et al. Detection of circulating tumor cells and their implications as a biomarker for diagnosis, prognostication, and therapeutic monitoring in hepatocellular carcinoma. Hepatology 2021;73:422-36.
50. Yang JD, Addissie BD, Mara KC, et al. GALAD Score for hepatocellular carcinoma detection in comparison with liver ultrasound and proposal of GALADUS score. Cancer Epidemiol Biomarkers Prev 2019;28:531-8.
51. Del Poggio P, Olmi S, Ciccarese F, et al. Italian Liver Cancer (ITA.LI.CA) Group. Factors that affect efficacy of ultrasound surveillance for early stage hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol 2014;12:1927-33.e2.
52. Simmons O, Fetzer DT, Yokoo T, et al. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment Pharmacol Ther 2017;45:169-77.
53. Younes R, Bugianesi E. Should we undertake surveillance for HCC in patients with NAFLD? J Hepatol 2018;68:326-34.
54. Schoenberger H, Chong N, Fetzer DT, et al. Dynamic changes in ultrasound quality for hepatocellular carcinoma screening in patients with cirrhosis. Clin Gastroenterol Hepatol 2022;20:1561-1569.e4.
55. Atiq O, Tiro J, Yopp AC, et al. An assessment of benefits and harms of hepatocellular carcinoma surveillance in patients with cirrhosis. Hepatology 2017;65:1196-205.
56. Morgan TA, Maturen KE, Dahiya N, Sun MRM, Kamaya A, American College of Radiology Ultrasound Liver Imaging and Reporting Data System (US LI-RADS) Working Group. US LI-RADS: ultrasound liver imaging reporting and data system for screening and surveillance of hepatocellular carcinoma. Abdom Radiol (NY) 2018;43:41-55.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Truong E, Noureddin M. Hepatocellular carcinoma surveillance in non-alcoholic fatty liver disease patients. Hepatoma Res 2022;8:40. http://dx.doi.org/10.20517/2394-5079.2022.63
AMA Style
Truong E, Noureddin M. Hepatocellular carcinoma surveillance in non-alcoholic fatty liver disease patients. Hepatoma Research. 2022; 8: 40. http://dx.doi.org/10.20517/2394-5079.2022.63
Chicago/Turabian Style
Truong, Emily, Mazen Noureddin. 2022. "Hepatocellular carcinoma surveillance in non-alcoholic fatty liver disease patients" Hepatoma Research. 8: 40. http://dx.doi.org/10.20517/2394-5079.2022.63
ACS Style
Truong, E.; Noureddin M. Hepatocellular carcinoma surveillance in non-alcoholic fatty liver disease patients. Hepatoma. Res. 2022, 8, 40. http://dx.doi.org/10.20517/2394-5079.2022.63
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 20 clicks
Cite This Article 20 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.