Cholangiocarcinoma: early detection and screening in high-risk population
Abstract
Cholangiocarcinoma (CCA) is a highly lethal malignancy that comprises approximately 15% of all the primary liver tumors and 3% of gastrointestinal cancers. Diagnosis is often done when the disease is already at advanced stages, resulting in poor outcomes. Prevention of risk factors and early diagnosis are the cornerstones for improving survival. Early diagnosis is feasible in the setting of surveillance programs in patients at high risk of CCA such as patients with primary sclerosing cholangitis. Regrettably, surveillance of CCA in this population is hampered by the low diagnostic accuracy of current tumor markers at earlier stages, the difficulties of imaging techniques for the differential diagnosis between benign and malignant biliary strictures, and the need for invasive procedures for diagnostic confirmation. In this review we discuss the rationale for surveillance of CCA in high-risk populations, particularly patients with primary sclerosing cholangitis, the recommended tools for surveillance and diagnostic work-up, and future perspectives.
Keywords
INTRODUCTION
Cholangiocarcinoma (CCA) is the second most common primary hepatic malignancy after hepatocellular carcinoma (HCC), comprising approximately 15% of all primary liver tumors and 3% of gastrointestinal cancers. Its incidence has been increasing in the past decades worldwide, and despite significant advancements in the knowledge of CCA mechanisms, diagnosis, and management, survival has not substantially improved in the past decade[1,2].
Since CCAs are usually asymptomatic in early stages, the diagnosis is established when the disease is already at advanced stages[3,4], which highly compromises treatment options, resulting in a dismal outcome. Therefore, prevention and early diagnosis remain the cornerstone for improving the survival of this devasting disease. Furthermore, identifying preventable risk factors and patients at risk of CCA are keys to decreasing the disease-related mortality of this highly lethal neoplasia[2]. In this review, we provide a comprehensive and critical overview of the current knowledge and future directions for the early diagnosis of CCA.
SURVEILLANCE IN CANCER: ANY CHANCE FOR CCA?
Surveillance in cancer is defined as the repeated application of a test over time with the aim of reducing mortality from a disease[5,6]. It is critical to tease apart between mortality (measured as the number of deaths per unit of time) and survival (duration of life after the diagnosis of the disease). Decreasing cancer-related mortality should be the sole objective of surveillance programs since survival is a surrogate endpoint that is subject to multiple biases that do not impact mortality[7]. The most common biases in studies evaluating the efficacy of cancer surveillance are lead-time (refers to the phenomenon where early diagnosis of a disease falsely makes it look like people are surviving longer), length bias (surveillance is more likely to detect slow-growing cancers than rapidly growing cancers), and healthy-volunteer bias (those individuals willing to participate in early detection efforts may be more attuned to health messages, more predisposed to adhere to health providers’ recommendations and they may also be from a higher socioeconomic group and have better access to quality healthcare). The ability of surveillance to detect the disease at an earlier stage is a required outcome of surveillance programs, but the mere finding of early-stage disease is not sufficient as proof of efficacy. In addition, treatment at an early stage should impact on survival in most patients. Otherwise, its effect on mortality will not be evident.
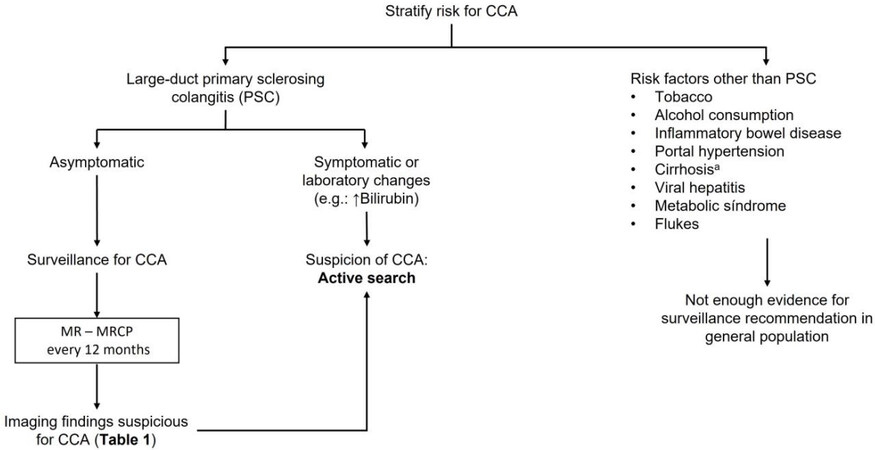
The World Health Organization (WHO) has suggested some principles for recommending surveillance in cancer[5,8,9]: The condition should be an important health problem with high mortality and/or morbidity, there should be an accepted treatment for patients with recognized disease, treatment should be better at an earlier stage, facilities for diagnosis and treatment should be available, there should be a recognizable latent or early symptomatic stage, a suitable test or examination acceptable for the population should be available, the natural history of the condition, including development from latent to declared disease, should be adequately understood, the target population for surveillance should be clearly identified, the cost of case-finding (including diagnosis and treatment of patients diagnosed) should be economically balanced, and finally, case-finding should be a continuous process. Unfortunately, most of these criteria are not met in CCA. Although several risk factors are linked to CCA, most CCA cases remain sporadic, without any identifiable risk factor, limiting the applicability of any surveillance program. Only patients with primary sclerosing cholangitis (PSC), the most well-known risk factor for CCA, can be identified as a target population for surveillance since the cumulative incidence of CCA at 20 years is 20%-25%[10], and its occurrence constitutes one of the most important causes of death in this population[11]. Furthermore, the natural history of the disease, including development from latent to declared disease, is not adequately understood despite recent advancements in the knowledge of CCA mechanisms. In addition, recommended surveillance tests are expensive (which clearly impacts on cost-efficacy of any surveillance program proposal), not widely available, and there is not a validated recall diagnostic strategy upon the suspicion of CCA. Finally, only a few proportion of patients may benefit from potential curative therapies[1], which calls into question the efficacy of any surveillance program.
PRIMARY SCLEROSING CHOLANGITIS AND CCA: AN OPPORTUNITY FOR SURVEILLANCE
PSC is recognized as the most important risk factor for CCA development in Western countries. It is associated with almost a 400-fold increased risk for developing CCA compared to the general population with a 5.65% risk of developing CCA ten years from PSC diagnosis. Approximately 30%-50% of the patients will have CCA at the time of PSC diagnosis or within the first year of diagnosis, and CCA is found to be responsible for almost 30% of PSC-related deaths[12-14]. Anatomically, the majority of CCAs in PSC are perihilar, though distal (below the cystic duct) or intrahepatic CCA (arising beyond second-order IHDs) can also occur.
Available epidemiology data from previous studies regarding the PSC-CCA association are heterogeneous, probably due to: (1) the low frequency of both diseases (PSC prevalence ranges from 0 to 16.2 per 100,000/inhabitants[15] and CCA age‐standardized incidence rate per 100,000 inhabitants in Western countries is 0.5-3[16]); (2) delayed PSC diagnosis as many patients are asymptomatic for a long-term prior to the diagnosis; (3) heterogeneity on the CCA detection time as patients with PSC might develop CCA more than 4 to 6 years from PSC diagnosis[12,13], 50% within the first year after PSC diagnosis and approximately 10% present with CCA at the time of PSC diagnosis[17]; and (4) analysis from previous studies pooling PSC with other inflammatory liver diseases by the generic term of “cholangitis”. PSC is a cholestatic idiopathic liver disease characterized by inflammation and progressive intra and extrahepatic biliary duct fibrosis, so the term is correct, but other biliary tract diseases that mimic PSC (including IgG4-related cholangitis) can be confounders. Accordingly, reliable data regarding the actual incidence of CCA in PSC and the risk factors associated with its occurrence is scarce, which impacts on the design of an effective surveillance strategy.
Furthermore, it is relevant to tease apart CCA surveillance aimed to achieve an early CCA diagnosis in asymptomatic patients with PSC who may be potential candidates for curative treatments, from recall diagnosis of CCA triggered from a change in clinical status, an elevation of biochemical parameters (for example bilirubin or CA 19.9) or upon the detection of a highly suspicious lesion by an imaging technique, events usually related to advanced CCA stages.
The scientific evidence supporting the efficacy of CCA surveillance in PSC is scarce. Although the incidence of CCA is high in patients with PSC, surveillance strategies in asymptomatic patients are not universally endorsed because of several diagnostic and therapeutic limitations. First, the incidence of CCA in asymptomatic PSC patients may not be high enough for recommending surveillance in this population[18]. Second, diagnosis of CCA in PSC is quite challenging because CCA mimics inflammation-related dominant biliary strictures. Third, confirmatory diagnosis requires invasive endoscopic procedures, and conventional cytology or even sophisticated techniques such as fluorescence in situ hybridization (FISH) lack sensitivity. Finally, potentially curative treatment is only feasible in a minority of cases, depending in part on the availability of liver transplantation[2]. Despite these limitations, retrospective studies, some of them monocentric and including a limited number of patients[19], suggest that cancer-related mortality decreases with surveillance[19-22]. While further prospective studies are warranted, despite the lack of evidence, some scientific guidelines recommend surveillance for CCA in patients with PSC[20].
Selecting which patients should be enrolled in the surveillance program and the frequency of the tests is a crucial decision to develop a cost-effective surveillance strategy. Imaging tests with high sensitivity may increase false-positive results and consequently increase exposure to invasive tests like endoscopic retrograde cholangiopancreatography (ERCP). On the contrary, selecting a test imbalanced to a higher specificity would increase the number of false-negative cases, missing early CCA diagnosis[23].
All these considerations thicken the plot, where the clinician will have to decide if the findings justify exposing the patient to invasive procedures for histopathological confirmation.
Imaging techniques for CCA surveillance in PSC
The ideal imaging test for surveillance should offer an adequate diagnostic accuracy balancing a very high sensitivity to detect an early-stage CCA on asymptomatic patients with an acceptable specificity for avoiding unacceptable false-positive results. On top of that, the test should be acceptable by the population and widely available. More importantly, an appropriate and validated recall strategy in the case of a positive surveillance test should be available.
There are three non-invasive imagine techniques that could be considered for surveillance: ultrasonography (US), computerized tomography (CT), and magnetic resonance imaging (MRI) with cholangiopancreatography technique (MRCP). US is widely available and relatively cheap, but the sensitivity of US for early-stage CCA detection and the ability to provide a full virtual an reproducible map of the biliary tree is far from optimal and it is inferior to MRI for early-stage CCA detection[22]. CT is associated with radiation exposure and limited image quality of the biliary tree when the biliary ducts are not dilated.
MRI/MRCP is considered the imaging standard for diagnosis and follow-up in patients with PSC[24]. The MRI/MRCP is non-invasive without radiation exposure, and is the best image technique to explore the biliary tree with a pooled sensitivity of 98.9% (95%CI: 98.6-99.3) and specificity close to 100% as reported in a recent meta-analysis, increasing CCA detection sensitivity by combining with contrast agents, without affecting the specificity[23,25]. MRI should be considered as the study of choice as it is superior against US in the detection of early-stage perihilar CCA in patients with PSC, showing better area under the curve (AUC) in the entire cohort (0.87 vs. 0.70) and also in asymptomatic patients (0.81 vs. 0.59)[22].
There are different findings on imaging tests which are considered to be suspicious for CCA[3,26] [Table 1]. The most frequent finding is the presence of an obstructive biliary stricture, but this finding is not a specific as patients with PSC usually have inflammatory/fibrotic obstructive biliary strictures on imaging studies known as dominant strictures (DS), which may mimic malignant strictures. Early CCA has different presentations in imaging tests: dilatation and/or thickening of the biliary duct, tumor infiltration along the biliary tree resulting in ductal narrowing, beading irregularities of the central hepatic ducts, diffuse strictures, or a discrete mass-forming lesion, making the diagnosis between malignant and benign strictures extremely difficult[3,23].
Imaging findings suspicious for CCA in PSC patients
| Intrahepatic ductal dilatations or DS |
| Marked dilatation next to strictures |
| Biliary duct wall thickening |
| Irregular ductal narrowing with shouldered margins |
| Intraductal polypoid lesions (> 1 cm) |
| Focal bile duct thickening with enhancement at MRI |
| Hyperenhancement in more delayed contrast enhanced phases |
| Rapid progression of strictures |
| Focal biliary duct dilatation associated with ipsilateral lobar atrophy |
The presence of a dominant stricture (DS) is the imaging hallmark for CCA diagnosis. Chapman et al. found a correlation between DS and the development of CCA in a retrospective 25-year study of PSC patients[27]. This relationship has been described in further retrospective and prospective studies, showing an increase of risk of 6.2% to 26.3% in patients with PSC with a DS in comparison of patients with PSC without a DS, with CCA being diagnosed over a 6.2- to 9.8-year follow-up period[28,29]. However, the presence of a DS for CCA diagnosis is not mandatory since patients without DS are diagnosed with CCA[29,30]. In addition, the presence of DS is not a synonym of CCA since 50% of patients with PSC will develop focal DS during the course of the disease[31], and in a retrospective analysis of 230 patients with PSC, only 19% of biliary strictures were caused by CCA[32].
Based on the current evidence, the recommended strategy for imaging screening of CCA in PSC patients without other additional risk factors would be an annual contrast-enhanced MRCP [Figure 1][20,33].
Biomarkers for CCA surveillance in PSC
The advantages of using biomarkers for screening are the worldwide availability and reproducibility. The most widely used biomarker in CCA is carbohydrate antigen 19.9 (CA 19-9). Unfortunately, its diagnostic performance is not optimal since it is common to find elevated levels in the context of cholestasis. A preliminary study from the Mayo Clinic reported an AUC of 0.949 (95%CI: 0.895-1.000), with a 78.6% sensitivity and 98.5% specificity for CA 19-9 level > 129 IU/mL. Unfortunately, all patients had an advanced-stage CCA at diagnosis[34]. In a later study at the same center including 73 patients with PSC and evaluating the same cut-off value (CA19-9 values > 129 IU/L), 37% of the patients did not present CCA after an exhaustive study and a median follow-up of 30 months[35]. Similar results were reported in Sweden[36]. In a retrospective analysis evaluating 230 patients followed from 2000 to 2006, 23 developed CCA (15 stage I-II and 8 stages III-IV). By ROC analysis, the cut-off point with the highest diagnostic yield was 20 IU/mL, which was associated with a sensitivity, specificity, positive predictive value, and negative predictive value of 78%, 67%, 23%, and 96% respectively[32]. Joint use of CA19-9 and CEA has been explored. Although a study that included 74 patients with PSC showed a diagnostic yield of 86%[37], subsequent studies indicate that this combination does not offer an acceptable diagnostic yield[36,38]. The combination of serum tumor markers and imaging is associated with an improvement in sensitivity, including MRI/MRCP plus CA19-9 with a cut-off value of 20 U/mL (sensitivity, 100%; specificity, 38%; diagnostic average, 89%) and US plus CA19-9 with a cut-off value of 20 U/mL (sensitivity, 91%; specificity, 62%; diagnostic average, 93%)[32]. Interestingly, when both tests were negative (CA 19.9 less than 20 UI/mL and lack of evidence of biliary tract dilatation/bile duct wall thickening by imaging), the negative predictive value was very high which suggests that physicians can confidently use negative test results to rule out cancer.
According to the available studies, there is not enough evidence for recommending the use of CA 19-9 for surveillance purposes[39].
Role of the endoscopic tests
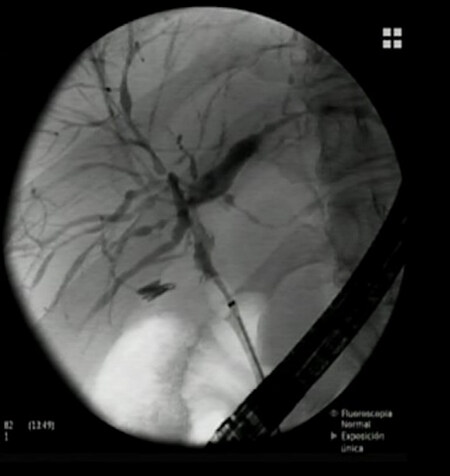
Biliary strictures are one of the main problems of PSC as DS can be difficult to characterize. The definition of DS is based on ERCP and is not considered applicable to MRI given the insufficient spatial resolution and lack of hydrostatic pressure. The ESGE/EASL guidelines define a DS as stenosis of the common bile duct and right and left confluence of the hepatic ducts with a diameter of < 1.5 mm in the common bile duct and/or < 1.0 mm in a hepatic duct within 2 cm of the main hepatic confluence [Figure 2][40]. It is noteworthy to point out that these thresholds were arbitrary and are not consistent in every PSC study to define DS. Half of the patients with PSC will develop focal DS during the course of the disease, as reported by Gotthardt et al. in a prospective 20-year study including 170 patients with PSC during a median follow-up of 7 years[41]. The risk of cholangiocarcinoma in DS is around 5%[40,41]. However, there are no specific imaging features in ERCP that can distinguish benign from malignant strictures. Definitive diagnosis always requires additional techniques such as CT/MRI, biliary cytology, or histology.
Figure 2. A 58 year-old man with long lasting primary sclerosing cholangitis and ulcerative cholangitis. A stenosis in the hepatic duct associated with focal bile duct thickening with enhancement at MRI was detected. A diagnostic ERCP was performed, confirming the presence of a noncritical stenosis of 2.5 mm in diameter and 5 mm in length in the hepatic duct, just below the hepatic confluent. Brush cytology and biopsy were obtained for anatomopathological analysis, and no atypia were found.
Available guidelines recommend ERCP in symptomatic PSC patients with clinical symptoms, worsening cholestasis, weight loss, or a new or progressive DS[20,40,42,43]. These patients are candidates for a therapeutic ERCP with stricture dilatation and/or biliary stent depending on the DS characteristics and clinical specific indication. Any stricture must always be evaluated for malignancy during the procedure, with brush cytology with or without FISH analysis, fluoroscopy guided biopsy and/or biopsy by cholangioscopy[20].
The reliability of bile duct brushing for CCA diagnosis is variable, and sensitivity can be as low as 8 %[32]. A meta-analysis reported a pooled sensitivity of 43% (95%CI: 35-52%) and specificity of 97% (95%CI: 95%-98%) with a pooled diagnostic odds ratio of 20.23
Cholangioscopy is a promising option for patients with PSC and high suspicion of malignancy by imaging but with indeterminate histopathological results and is most useful when there is a high pre-test probability for CCA (high CA 19-9 levels, jaundice, and weight loss). A meta-analysis found that single-operator cholangioscopy was a highly accurate diagnostic modality for CCA diagnosis with a pooled sensitivity and specificity of 65% (95%CI: 35%-87%) and 97% (95%CI: 87%-99%), respectively[46]. It should state that a negative biopsy does not exclude CCA owing to the possibility of sampling error.
ERCP is not exempt of side effects, and therapeutic ERCP in patients with PSC carries a higher complication profile due to the multifocal nature of the disease and intrahepatic bile duct obstruction[47].
SURVEILLANCE IN OTHER HIGH-RISK PATIENTS
There are multiple risk factors for CCA. The common characteristic they share is that they are associated with chronic inflammation of the biliary epithelium and bile stasis[1,2]. For instance high alcohol consumption, presence and longer duration of inflammatory bowel
Infections with specific trematodes such as Opisthorchis viverrini and Clonorchis sinensis are a leading cause of CCA in East and Southeast Asia[51]. Infection can cause recurrent inflammation, leading to disorders of the biliary system, including cholangitis, obstructive jaundice, hepatomegaly, fibrosis of the periportal system, cholecystitis, and cholelithiasis[52]. Given the high prevalence of liver fluke infection in those regions, large-scale screening with stool examination for fluke ova supplemented with serological assay for the diagnosis of opisthorchiasis and clonorchiasis coupled with abdominal ultrasonography or other radiological imaging is performed[53,54]. Retrospective studies indicate that US-screening is an effective tool for detecting early-stage, operable CCA in high incidence areas[53,54], and when associated with prevention of infection by educational campaigns and the use of medications (praziquantel) to treat liver fluke infection, both measures can decerase incidence and mortality of CCA in highly prevalent areas[51,52].
FUTURE PERSPECTIVES
Major efforts should be directed in identifying those patients at high risk of CCA. Although there are several risk factors, most CCA cases remain sporadic[55]. Only patients with PSC can be identified as a target population for surveillance, but the actual risk of CCA development is not well-known, hindering the recognition of those patients in whom the expected risk of CCA supports the surveillance recommendation. In addition, major improvements need to be implemented in the current diagnostic tools. Although MRI/MRCP is considered the best diagnostic imaging modality, distinguishing between malignant and benign strictures is challenging. In recent years, artificial intelligence (AI) techniques applied to healthcare are evolving and are used in many biomedical areas, in particular oncology[56]. In the field of radiology, the implementation of AI through machine-learning techniques has allowed the development of radiomics as a new field of medical research. That said, radiomic studies in CCA are scarce and most of them include a relatively small number of patients and lack from external validation. In addition, there has been little standardization and generalization of radiomic findings, which limit the use of this methodology into the clinical practice[57,58]. Finally, the differential diagnosis of DS is frequently challenging, and the current techniques are invasive and impaired by low sensitivity. Liquid biopsy may become a reliable tool for improving the diagnostic accuracy. Recently, a prospective study including patients with suspicious biliary strictures (n = 68) showed that the mutational analysis of bile cell-free DNA (cfDNA) by next-generation sequencing (NGS) in bile showed a sensitivity and specificity of 96.4% and 69.2%, respectively. More interestingly, 22 out of 35 patients initially categorized as having a benign/indeterminate stricture were finally diagnosed of malignancy during the follow-up and in them, the NGS assay showed a 100% sensitivity for malignancy diagnosis[59]. Validation studies including patients with PSC are eagerly awaited. Finally, refinements in the selection of patients in whom liver transplantation may be effective[60] and improvement in the current medical therapy, with the implementation of targeted therapies[61,62] or immunotherapy[63] will change the outcome of this lethal disease.
DECLARATIONS
Authors’ contributionsContributed on the conception of the manuscript, the data management organization, wrote the manuscript, and gave the final approval of the manuscript: Forner A
Substantially contributed to the writing of the manuscript and gave the final approval before submission: Muñoz-Martínez S, Rimola J, Londoño MC, Cárdenas A
Availability of data and materialsNot applicable.
Financial support and sponsorshipMaría Londoño is funded by the Instituto de Salud Carlos III (grant No PI17/00955 and PI21/00080); Andrés Cárdenas is funded by the Instituto de Salud Carlos III and Plan Estatal de Investigación Ciéntifica y Técnica y de Innovación - Grant No PI19/00752 and has received funding for this work by “Fundación Marta Balust”. Alejandro Forner is funded by the Instituto de Salud Carlos III (grant No PI18/00542).
Conflicts of interestMuñoz-Martínez S: speaker fees from Bayer and travel funding from Bayer and Eisai. He received grant support from Bristol Myers Squibb and Celgene. Rimola J: has received speaker fees and travel grants from Bayer, BTG and Terumo, and consultancy fees from Roche and COR2ED. Londoño MC: Lecture fees from Intercept, Cárdenas A: is a consultant for Mallinckrodt Pharmaceuticals, Boston Scientific Corp, and has participated on Advisory Boards for Mallinckrodt Pharmaceuticals, and has received grant support by Mallinckrodt and Boston Scientific Corp. Forner A: lecture fees from Bayer, Gilead, Roche, Boston Scientific and MSD; consultancy fees from Bayer, AstraZeneca, Roche, SIRTEX, AB Exact Science and Guerbert.
Ethical approval and consent to participateNot applicable.
Consent for publicationWritten informed consent for publication was obtained (Figure 2).
Copyright© The Author(s) 2022.
REFERENCES
1. Banales JM, Marin JJG, Lamarca A, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol 2020;17:557-88.
3. Forner A, Vidili G, Rengo M, Bujanda L, Ponz-Sarvisé M, Lamarca A. Clinical presentation, diagnosis and staging of cholangiocarcinoma. Liver Int 2019;39 Suppl 1:98-107.
4. Izquierdo-Sanchez L, Lamarca A, La Casta A, et al. Cholangiocarcinoma landscape in Europe: Diagnostic, prognostic and therapeutic insights from the ENSCCA Registry. J Hepatol 2022;76:1109-21.
5. Díaz-González Á, Forner A. Surveillance for hepatocellular carcinoma. Best Pract Res Clin Gastroenterol 2016;30:1001-10.
7. Sherman M. Surveillance for hepatocellular carcinoma. Best Pract Res Clin Gastroenterol 2014;28:783-93.
8. Wilson JMG, Jungner G, Organization WH. Principles and practice of screening for disease. 1968.
9. Shieh Y, Eklund M, Sawaya GF, Black WC, Kramer BS, Esserman LJ. Population-based screening for cancer: hope and hype. Nat Rev Clin Oncol 2016;13:550-65.
10. Weismüller TJ, Trivedi PJ, Bergquist A, et al. International PSC Study Group. Patient Age, Sex, and Inflammatory Bowel Disease Phenotype Associate With Course of Primary Sclerosing Cholangitis. Gastroenterology 2017;152:1975-1984.e8.
11. Fung BM, Tabibian JH. Cholangiocarcinoma in patients with primary sclerosing cholangitis. Curr Opin Gastroenterol 2020;36:77-84.
12. Boonstra K, Weersma RK, van Erpecum KJ, et al. EpiPSCPBC Study Group. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology 2013;58:2045-55.
13. Burak K, Angulo P, Pasha TM, Egan K, Petz J, Lindor KD. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am J Gastroenterol 2004;99:523-6.
14. Fevery J, Henckaerts L, Van Oirbeek R, et al. Malignancies and mortality in 200 patients with primary sclerosering cholangitis: a long-term single-centre study. Liver Int 2012;32:214-22.
15. Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol 2012;56:1181-8.
16. Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int 2019;39 Suppl 1:19-31.
17. Boberg KM, Bergquist A, Mitchell S, et al. Cholangiocarcinoma in primary sclerosing cholangitis: risk factors and clinical presentation. Scand J Gastroenterol 2002;37:1205-11.
18. Villard C, Friis-Liby I, Nilsson E, et al. Population-based prospective surveillance of patients with primary sclerosing cholangitis (PSC) for early detection of cholangiocarcinoma. Hepatology 2021;74:91A-92A.
19. Ali AH, Tabibian JH, Nasser-Ghodsi N, et al. Surveillance for hepatobiliary cancers in patients with primary sclerosing cholangitis. Hepatology 2018;67:2338-51.
20. Bowlus CL, Lim JK, Lindor KD. AGA Clinical Practice Update on Surveillance for Hepatobiliary Cancers in Patients With Primary Sclerosing Cholangitis: Expert Review. Clin Gastroenterol Hepatol 2019;17:2416-22.
21. Rizvi S, Eaton JE, Gores GJ. Primary Sclerosing Cholangitis as a Premalignant Biliary Tract Disease: Surveillance and Management. Clin Gastroenterol Hepatol 2015;13:2152-65.
22. Eaton JE, Welle CL, Bakhshi Z, et al. Early Cholangiocarcinoma Detection With Magnetic Resonance Imaging Versus Ultrasound in Primary Sclerosing Cholangitis. Hepatology 2021;73:1868-81.
23. Satiya J, Mousa OY, Gupta K, et al. Diagnostic yield of magnetic resonance imaging for cholangiocarcinoma in primary sclerosing cholangitis: a meta-analysis. Clin Exp Hepatol 2020;6:35-41.
24. Venkatesh SK, Welle CL, Miller FH, et al. IPSCSG. Reporting standards for primary sclerosing cholangitis using MRI and MR cholangiopancreatography: guidelines from MR Working Group of the International Primary Sclerosing Cholangitis Study Group. Eur Radiol 2022;32:923-37.
25. Schramm C, Eaton J, Ringe KI, Venkatesh S, Yamamura J. MRI working group of the IPSCSG. Recommendations on the use of magnetic resonance imaging in PSC-A position statement from the International PSC Study Group. Hepatology 2017;66:1675-88.
26. Khoshpouri P, Habibabadi RR, Hazhirkarzar B, et al. Imaging Features of Primary Sclerosing Cholangitis: From Diagnosis to Liver Transplant Follow-up. Radiographics 2019;39:1938-64.
27. Chapman MH, Webster GJ, Bannoo S, Johnson GJ, Wittmann J, Pereira SP. Cholangiocarcinoma and dominant strictures in patients with primary sclerosing cholangitis: a 25-year single-centre experience. Eur J Gastroenterol Hepatol 2012;24:1051-8.
28. Rudolph G, Gotthardt D, Klöters-Plachky P, Kulaksiz H, Rost D, Stiehl A. Influence of dominant bile duct stenoses and biliary infections on outcome in primary sclerosing cholangitis. J Hepatol 2009;51:149-55.
29. Janse M, Lamberts LE, Verdonk RC, Weersma RK. IBD is associated with an increase in carcinoma in PSC irrespective of the presence of dominant bile duct stenosis. J Hepatol 2012;57:473-4; author reply 475.
30. Eaton JE, Barr Fritcher EG, Gores GJ, et al. Biliary multifocal chromosomal polysomy and cholangiocarcinoma in primary sclerosing cholangitis. Am J Gastroenterol 2015;110:299-309.
31. Hilscher MB, Tabibian JH, Carey EJ, Gostout CJ, Lindor KD. Dominant strictures in primary sclerosing cholangitis: A multicenter survey of clinical definitions and practices. Hepatol Commun 2018;2:836-44.
32. Charatcharoenwitthaya P, Enders FB, Halling KC, Lindor KD. Utility of serum tumor markers, imaging, and biliary cytology for detecting cholangiocarcinoma in primary sclerosing cholangitis. Hepatology 2008;48:1106-17.
33. Lindor KD, Kowdley KV, Harrison ME. American College of Gastroenterology. ACG Clinical Guideline: Primary Sclerosing Cholangitis. Am J Gastroenterol 2015;110:646-59; quiz 660.
34. Levy C, Lymp J, Angulo P, Gores GJ, Larusso N, Lindor KD. The value of serum CA 19-9 in predicting cholangiocarcinomas in patients with primary sclerosing cholangitis. Dig Dis Sci 2005;50:1734-40.
35. Sinakos E, Saenger AK, Keach J, Kim WR, Lindor KD. Many patients with primary sclerosing cholangitis and increased serum levels of carbohydrate antigen 19-9 do not have cholangiocarcinoma. Clin Gastroenterol Hepatol 2011;9:434-9.e1.
36. Björnsson E, Kilander A, Olsson R. CA 19-9 and CEA are unreliable markers for cholangiocarcinoma in patients with primary sclerosing cholangitis. Liver 1999;19:501-8.
37. Ramage JK, Donaghy A, Farrant J, Iorns R, Williams R. Serum tumor markers for the diagnosis of cholangiocarcinoma in primary sclerosing cholangitis. Gastroenterology 1995;108:865-9.
38. Hultcrantz R, Olsson R, Danielsson Å, et al. A 3-year prospective study on serum tumor markers used for detecting cholangiocarcinoma in patients with primary sclerosing cholangitis. Journal of Hepatology 1999;30:669-73.
39. Association for the Study of the Liver. Electronic address: jhepatology@easloffice.eu., European Association for the Study of the Liver. EASL Clinical Practice Guidelines on Sclerosing Cholangitis. J Hepatol ;2022:S0168-8278(22)00326.
40. Aabakken L, Karlsen TH, Albert J, et al. Role of endoscopy in primary sclerosing cholangitis: European Society of Gastrointestinal Endoscopy (ESGE) and European Association for the Study of the Liver (EASL) Clinical Guideline. Endoscopy 2017;49:588-608.
41. Gotthardt DN, Rudolph G, Klöters-Plachky P, Kulaksiz H, Stiehl A. Endoscopic dilation of dominant stenoses in primary sclerosing cholangitis: outcome after long-term treatment. Gastrointest Endosc 2010;71:527-34.
42. Chapman MH, Thorburn D, Hirschfield GM, et al. British Society of Gastroenterology and UK-PSC guidelines for the diagnosis and management of primary sclerosing cholangitis. Gut 2019;68:1356-78.
43. Bhat P, Aabakken L. Role of Endoscopy in Primary Sclerosing Cholangitis. Clin Endosc 2021;54:193-201.
44. Trikudanathan G, Navaneethan U, Njei B, Vargo JJ, Parsi MA. Diagnostic yield of bile duct brushings for cholangiocarcinoma in primary sclerosing cholangitis: a systematic review and meta-analysis. Gastrointest Endosc 2014;79:783-9.
45. Navaneethan U, Njei B, Venkatesh PG, Vargo JJ, Parsi MA. Fluorescence in situ hybridization for diagnosis of cholangiocarcinoma in primary sclerosing cholangitis: a systematic review and meta-analysis. Gastrointest Endosc 2014;79:943-950.e3.
46. Njei B, McCarty TR, Varadarajulu S, Navaneethan U. Systematic review with meta-analysis: endoscopic retrograde cholangiopancreatography-based modalities for the diagnosis of cholangiocarcinoma in primary sclerosing cholangitis. Aliment Pharmacol Ther 2016;44:1139-51.
47. Keihanian T, Barakat MT, Tejaswi S, et al. Role of Endoscopic Retrograde Cholangiopancreatography in the Diagnosis and Management of Cholestatic Liver Diseases. Clin Liver Dis 2022;26:51-67.
48. Clements O, Eliahoo J, Kim JU, Taylor-Robinson SD, Khan SA. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J Hepatol 2020;72:95-103.
49. Massarweh NN, El-Serag HB. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control 2017;24:1073274817729245.
50. Darnell A, Rimola J, Belmonte E, et al. Evaluation of LI-RADS 3 category by magnetic resonance in US-detected nodules ≤ 2 cm in cirrhotic patients. Eur Radiol 2021;31:4794-803.
51. Sithithaworn P, Yongvanit P, Duenngai K, Kiatsopit N, Pairojkul C. Roles of liver fluke infection as risk factor for cholangiocarcinoma. J Hepatobiliary Pancreat Sci 2014;21:301-8.
52. Khuntikeo N, Loilome W, Thinkhamrop B, Chamadol N, Yongvanit P. A Comprehensive Public Health Conceptual Framework and Strategy to Effectively Combat Cholangiocarcinoma in Thailand. PLoS Negl Trop Dis 2016;10:e0004293.
53. Khuntikeo N, Koonmee S, Sa-Ngiamwibool P, et al. A comparison of the proportion of early stage cholangiocarcinoma found in an ultrasound-screening program compared to walk-in patients. HPB (Oxford) 2020;22:874-83.
54. Sungkasubun P, Siripongsakun S, Akkarachinorate K, et al. Ultrasound screening for cholangiocarcinoma could detect premalignant lesions and early-stage diseases with survival benefits: a population-based prospective study of 4,225 subjects in an endemic area. BMC Cancer 2016;16:346.
56. Yang CM, Shu J. Cholangiocarcinoma Evaluation via Imaging and Artificial Intelligence. Oncology 2021;99:72-83.
57. Granata V, Fusco R, Setola SV, et al. An update on radiomics techniques in primary liver cancers. Infect Agent Cancer 2022;17:6.
58. Macias RIR, Cardinale V, Kendall TJ, et al. Clinical relevance of biomarkers in cholangiocarcinoma: critical revision and future directions. Gut 2022;71:1669-83.
59. Arechederra M, Rullán M, Amat I, et al. Next-generation sequencing of bile cell-free DNA for the early detection of patients with malignant biliary strictures. Gut 2022;71:1141-51.
60. Moeckli B, Ivanics T, Claasen M, Toso C, Sapisochin G. Recent developments and ongoing trials in transplant oncology. Liver Int 2020;40:2326-44.
61. Zhu AX, Macarulla T, Javle MM, et al. Final Overall Survival Efficacy Results of Ivosidenib for Patients With Advanced Cholangiocarcinoma With IDH1 Mutation: The Phase 3 Randomized Clinical ClarIDHy Trial. JAMA Oncol 2021;7:1669-77.
62. Abou-alfa GK, Sahai V, Hollebecque A, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. The Lancet Oncology 2020;21:671-84.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Muñoz-Martínez S, Rimola J, Londoño MC, Cárdenas A, Forner A. Cholangiocarcinoma: early detection and screening in high-risk population. Hepatoma Res 2022;8:30. http://dx.doi.org/10.20517/2394-5079.2022.22
AMA Style
Muñoz-Martínez S, Rimola J, Londoño MC, Cárdenas A, Forner A. Cholangiocarcinoma: early detection and screening in high-risk population. Hepatoma Research. 2022; 8: 30. http://dx.doi.org/10.20517/2394-5079.2022.22
Chicago/Turabian Style
Muñoz-Martínez, Sergio, Jordi Rimola, María Carlota Londoño, Andrés Cárdenas, Alejandro Forner. 2022. "Cholangiocarcinoma: early detection and screening in high-risk population" Hepatoma Research. 8: 30. http://dx.doi.org/10.20517/2394-5079.2022.22
ACS Style
Muñoz-Martínez, S.; Rimola J.; Londoño MC.; Cárdenas A.; Forner A. Cholangiocarcinoma: early detection and screening in high-risk population. Hepatoma. Res. 2022, 8, 30. http://dx.doi.org/10.20517/2394-5079.2022.22
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 16 clicks
Cite This Article 16 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.