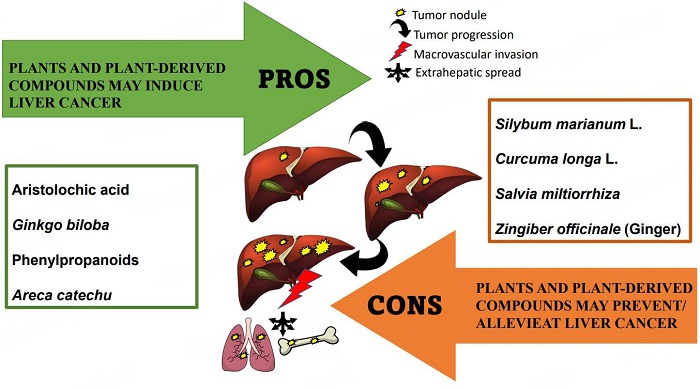
The pros and cons of biological effects of herbs and herb-derived compounds on liver tumorigenesis
Abstract
Consumption of natural products such as herbs, spices, plant-derived compounds, and foods is on the rise globally. The use of these substances is widely recognized as an integral part of culture and tradition, with the philosophy being “no benefit is no harm”. The utility of medicinal plants and extracts is under scrutiny, and the scientific community needs to clarify many conceptual gaps. Medicinal plants are rich in bioactive phytochemicals that produce chemopreventive effects at different levels, including cellular, animal, and clinical. The ultimate translational value is often missing, and some studies suggest that botanicals may contain toxic compounds that cause acute or chronic toxicity. In this regard, the liver is the center, and herbal products can show protective effects or induce hepatotoxicity, thereby promoting liver cancer. In this review article, we examine a range of herbal products implicated in hepatocarcinogenesis and extend the discussion to herbal products that may be potentially involved in the prevention and treatment of liver carcinoma.
Keywords
INTRODUCTION
The liver is the largest internal organ and gland in the human body (approximately 2.5% of an adult’s body weight) and is involved in the regulation of physiological metabolism of carbohydrates (glucose, glycogen, and fructose), lipids (bile acids, cholesterol, fatty acids, triglycerides, and phospholipids), proteins, and vitamins[1]. The liver is also essential for regulating hormonal function and bile secretion, as well as metabolism of xenobiotic substances and drugs, with the capacity of detoxifying endogenous (waste metabolites) and exogenous substances (toxic compounds).
Individuals may use herbal products and dietary supplements that are believed to have health benefits[2]. Although regulations exist regarding the use, indications, safety, and efficacy of specific herbal preparations, compounds also have potential hazards due to several factors such as inaccurate labeling, poor quality, pollution, unclear efficacy and safety, side effects, and interactions with other drugs[3]. The liver becomes the center of the detoxification function of herbal products and metabolites, but it can also become the target of damage from drugs, herbs, and supplements, leading to acute or chronic hepatotoxicity[4]. The effects of herbal products can also interact with mechanisms associated with hepatocellular carcinoma (HCC), the most common type of primary liver cancer[5].
HCC is one of the most prevalent life-threatening solid tumors, with more than one million new cases diagnosed worldwide each year, and it remains the third leading cause of cancer-related death[6].
The causes of liver cancer include viral hepatitis (HBV or HBC) infection and toxin exposure such as alcohol and aflatoxin. In addition, fatty liver diseases and related metabolic disorders such as obesity, diabetes, and chronic liver inflammation are potential risk factors for liver cancer[7,8].
Eating herbs that contain toxic compounds or mutagenic and carcinogenic substances such as aristolochic acid is associated with a higher HCC risk. Compounds from other plant sources may be toxic to the liver, and, in the presence of other risk factors, they may promote the development of HCC.
In contrast, many natural compounds derived from plants are used as anticancer agents and are currently undergoing medical development[9]. Many plants and plant-derived compounds can have beneficial effects against tumors with low toxicity to normal tissues. Some of these compounds are used as anticancer agents against HCC[10]. Generally, the most common phytochemicals in cancer treatments include alkaloids such as vinblastine and vincristine from Catharan roseus for the treatment of lymphomas, leukemias, and solid tumors; terpenes such as taxol from Taxus brevifolia for the treatment of lung, breast, and ovarian cancers; and quinolones such as camptothecin from Camptotheca acuminate for the treatment of leukemia[11]. Recent evidence suggests that plants, such as Silybum marianum, Solanum nigrum, Claviceps purpurea, Nigella sativa, and Thymbra spicata, and isolated natural compounds, such as curcumin, resveratrol, carvacrol, and thymoquinone, alone or in combination with anticancer drugs exert potential antitumor and chemoprotective effects in vitro and in vivo[11-14].
In this review article, we present evidence-based research on herbal and natural products that may cause cancer and damage to the liver, as well as plants with antitumor effects against HCC.
LIVER TUMORIGENESIS: PLANTS AND PLANT-DERIVED COMPOUNDS MAY INDUCE LIVER CANCER
In traditional medicine, herbal preparations are widely used to prevent or treat many diseases and as food supplements. The World Health Organization estimated that approximately 80% of the population relies on complementary and traditional medicines, including herbal medicine, for their primary healthcare[15]. The idea of using herbs and herbal products is appealing, and people prefer using medicinal plants rather than chemical drugs because herbs are considered safe and efficient. However, herb-induced cardiotoxicity, hepatotoxicity, and nephrotoxicity due to long-term use are also observed, which indicates that some herbal formulations are not completely safe. Other plant-derived constituents show genotoxic and/or carcinogenic effects and may raise safety concerns. In this regard, many hepatocarcinogenic compounds have been reported, including aristolochic acid, alkenylbenzenes, and pyrrolizidine alkaloids.
In this section, we report a panel of herbs and herb-derived compounds that have been reported to induce liver cancer. Table 1 presents studies addressing the possible promoting effects of herbs and their compounds on liver tumorigenesis.
Studies of the possible promoting effects of herbs and their compounds on liver tumorigenesis
| Herbal product | Sample | Study type | Mechanism | Ref. |
| Aristolochic acid (AA) | AA | In vitro: HepG2 | ↑DNA migration ↑Frequency of micronuclei ↑NO, 8-OHdG | [16] |
| AA | In vitro: Hepa1-6 cell line | ↑HCC cell migration and invasion ↑Epithelial-to-mesenchymal transition ↑C3a/C3aR axis activation. | [17] | |
| AA | In vivo: mice | ↑HCC in CCl4 -treated or Pten-deficient mice ↑DNA damage ↑AA-DNA adduct | [18] | |
| AA | In vivo: male beagle canines | ↑Hepatic premalignant alterations ↑c-Myc, Lin28B, FOXO1 phosphorylation ↓let-7 miRNAs, miR-23a | [19] | |
| AA | Genome‐wide analysis | ↑AA-associated mutational signature, ↑AA-DNA adduct | [18] | |
| Chinese herbs containing AA | Population-based, cohort study | ↑HCC risk in patients with HBV infection. | [20] | |
| AA | HCC cohort study | ↑AA-dominated mutational signature tumoral ↑PD-L1 expression ↑CD3+ T-cell infiltration ↑CD68+ TAMs, CD66b+ TANs | [21] | |
| Gingko biloba | Extract | In vivo: F344/N rats and B6C3F1/N mice (Gavage studies) | ↑Incidences of HCC and hepatoblastoma | [27] |
| Extract | In vivo: B6C3F1 mice | ↑Hepatocellular necrosis and centrilobular hypertrophy | [28] | |
| Extract | In vivo: mice | ↑Eosinophilic altered foci and adenomas | [29] | |
| Phenylpropanoid (Alkenylbenzene) compounds | Methyleugenol | In vivo: B6C3F1 mice | ↑Genotoxicity | [55] |
| Methyleugenol | In vivo: F344/N rats and B6C3F1/N mice (Gavage studies) | ↑incidences of liver neoplasms | [31] | |
| Methyleugenol | In vivo: B6C3F1 mice | ↑β-catenin mutations | [32] | |
| Isoeugenol | In vivo: F344/N rats and B6C3F1 mice (Gavage studies) | ↑Incidences of hepatocellular adenoma and HCC | [30] | |
| Safrole | In vivo: Sprague-Dawley rats | ↑Oxidative damage in rat hepatic tissue | [56] | |
| Safrole and derivatives | In vivo: preweanling male | ↑Hepatocarcinogenic risk | [41] | |
| Safrole | In vivo-In vitro: hepatocytes of F344 rats | ↑Chromosome aberrations, sister chromatid exchanges, DNA adducts formation | [35] | |
| Safrole | In vivo: Young Balb/c mice | ↑Basophilic and acidophilic foci ↑Neoplastic nodules ↑ GGT | [37] | |
| Safrole | In vivo: male BALB/c mice | ↑Hypertrophy of centrilobular hepatocytes ↑Oval cell proliferation ↑Fatty change in periportal hepatocytes, ↑Hepatocellular adenomas and HCC | [36] | |
| estragole and derivatives | In vivo: preweanling male | ↑Hepatocarcinogenic risk | [41] | |
| Areca catechu | Chewing betel-nuts | Population-based study | ↑Risk of cirrhosis and HCC | [45] |
| Betel quid chewing | Case-control study | ↑Risk of cirrhosis and HCC | [47] [48] | |
| Betel quid chewing | Hospital-based case-control study | ↑Risk of hepatic fibrosis, cirrhosis and HCC | [49] | |
| Betel quid chewing | Community-based cohort study | ↑Risk of HCC | [46] | |
| Arecoline | In vitro: normal rat hepatocytes (Clone-9 cells) | ↑Genotoxicity ↑TGF-β | [53] | |
| Arecoline /arecoline N-oxide | In vitro: normal liver cells | ↑Genotoxicity, mutagenicity | [54] |
Aristolochic acid
Aristolochic acid (AA) is found in different plant families such as Asarum, Bragantia, and Aristolochia. These plants are traditionally used in Chinese herbal medicine. Based on IARC monographs on the identification of carcinogenic hazards to humans, AA and AA-containing plants are classified as Carcinogenic Group 1. The genotoxic effect of AA has been demonstrated in different studies. In a cellular model with the human hepatoma HepG2 cells, AA caused a significant increase of DNA migration and micronuclei formation. The AA-induced DNA and chromosome damage was associated with an increase in nitric oxide (NO) level and the formation of 8-hydroxydeoxyguanosine. This observation confirms that AA could exert a genotoxicity effect, likely through increasing NO and its derivatives[16]. In addition, in Hepa1-6 cells, AA promoted hepatocellular carcinoma invasion and migration and enhanced epithelial-mesenchymal transition via Snail, N-cadherin, and vimentin upregulation and E-cadherin downregulation; those effects were mediated by the C3a/C3AR activation pathway[17]. Furthermore, the liver tumor-promoting effect of AA was studied using animal models. Mice exposed to AA alone or combined with CCl4 (a well-known hepatotoxic agent) developed HCC or combined HCC and intrahepatic cholangiocarcinoma. The study showed that AA caused DNA damage and AA-DNA adduct formation, leading to the initiation of different liver tumors through adenine-to-thymine transversions[18]. In dogs, 10-day oral administration of AA caused hepatic premalignant alterations and tumor progenitor-like cell formation by overexpression of c-Myc oncoprotein and oncofetal RNA-binding protein Lin28B. The activation by phosphorylation of forkhead box O1 also increased upon AA exposure. Furthermore, the c-Myc signaling pathway seems related to microRNAs in the liver, where let-7 miRNAs and miR-23a are significantly dysregulated in animals treated with AA[19].
A human cohort study of HCC Chinese patients has found an AA-associated mutational signature in 25.8% of livers[18]. Another cohort study investigated the association between the use of AA containing Chinese herbs and HCC risk among HBV-infected patients. A significant dose-dependent relationship was observed between herbs containing AA consumption and HCC in patients with HBV, suggesting that aristolochic acid in HBV patients is crucial for HCC pathogenesis[20]. Another HCC cohort study showed that the AA-associated mutational signature is correlated with different tumor-immune markers such as high PD-L1 expression and CD3+ T-cell infiltration[21].
Ginkgo biloba
Since prehistoric times, the Ginkgo biloba tree has been used in Chinese folk medicine for treating several illnesses[22,23]. Moreover, extracts from G. biloba are diffused as natural supplements[24]. Accumulative studies over the last decades explored the possible bioactivity of G. biloba, which includes neuroprotective and cardioprotective effects and anticancer proprieties[25]. Despite this, some components of ginkgo are suspected of possessing mutagenic properties[26]. Indeed, the IARC monographs on the identification of carcinogenic hazards to humans classify Ginkgo biloba extract as Carcinogenic Group 2B. Due to its long-term and high-dose consumption, G. biloba extract (GBE) was studied by the National Cancer Institute for carcinogenic tests in rodents. B6C3F1/N mice and F344/N rats received GBE by gavage for two years. The study found an increased incidence of hepatocellular adenoma in male F344/N rats. Moreover, the study indicated that administration of GBE also caused dose-dependent carcinogenic activity in livers of mice with high incidences of HCC, hepatoblastoma, and hepatocellular adenoma[27].
To better address the mechanism of GBE-induced hepatocarcinoma, Hoenerhoff and colleagues[28] showed that oral GBE exposure to B6C3F1 mice resulted in a dose-dependent increase of centrilobular hypertrophy risk after 90 days and hepatocellular necrosis and hepatocellular tumors by two years. The study showed that, despite the similarity between GBE-exposed and spontaneous HCC at the morphologic level, the hepatocarcinogenesis alterations were different; in particular, high-dose GBE exposure is associated with Ctnnb1 mutation. In addition, GBE exposure is also associated with dysregulation of the WNT pathway. This finding provides a molecular and genetic profile behind GBE-induced hepatocarcinogenesis in mice[28].
The mode of action for GBE hepatocarcinogenesis was further clarified by investigating the involvement of the constitutive androstane receptor (CAR) using CAR-knockout (CARKO) and wild-type mice[29]. The results show that HCC DNA replication increased only in wild-type mice after one-week exposure to GBE; in contrast, no significant increase in HCC DNA replication in CARKO mice was observed. Additionally, long-term GBE exposure (4 or 13 weeks) led to a higher increase in hepatic Cyp2b10 expression and hepatocellular hypertrophy in wild-type mice than in CARKO mice. In addition, using a well-established hepatocarcinogenesis model by diethylnitrosamine treatment, GBE exposure promoted more eosinophilic foci and hepatocellular adenomas in wild-type mice than in CARKO mice. These findings indicate that the constitutive androstane receptor is essential for GBE-mediated hepatocarcinogenesis.
Phenylpropanoid (Alkenylbenzenes) compounds
The IARC monographs on the identification of carcinogenic hazards to humans classify some phenylpropanoid compounds such as methyleugenol and safrole as Carcinogenic Group 2B. Eugenol, isoeugenol, and methyleugenol are produced by different plants such as calamus, savory, basil, and clove. They are used in foods and beverages as flavoring compounds, as well as fragrance compounds in numerous hygiene products, perfumes, creams, and detergents. Due to the large human exposure and their similar chemical structure to safrole, which is known to be a mutagenic compound, methyleugenol and isoeugenol were studied by the National Toxicology Program for a carcinogenic test. In 1999, it was announced by the Committee of Experts on Flavoring Substances of the Council of Europe that methyleugenol exhibits DNA-binding propriety, and it was identified as genotoxic carcinogenic natural. For that purpose, male and female F344/N rats and B6C3F1 mice received pure (≥ 99%) isoeugenol[30] or methyleugenol[31] for two years. The studies reported that isoeugenol exerted a carcinogenic effect on B6C3F1 male mice by increasing HCC and/or hepatocellular adenoma[30]. In addition, the methyleugenol displayed an evident carcinogenic effect in mice by increasing liver neoplasms incidence[31]. Methyleugenol has a mutagenic effect on the β-catenin gene. In detail, Devereux et al.[32] showed that, in B6C3F1 mice, 20 of 29 methyleugenol-induced hepatocellular neoplasms had β-catenin gene point mutations.
Methyleugenol and other alkenylbenzenes represent the main component of the aromatic composition of basil[33] and are also present in basil-containing sauce (e.g., Italian pesto). The daily exposure to alkenylbenzenes by regular pesto consumption may have a carcinogenic effect. The assessment of pesto sauce risk was performed based on the detected level of methyleugenol in different pesto sauces[34]. The study concluded that pesto sauce consumption does not represent a cancerogenic risk; however, a safety concern might be considered upon long-term daily consumption of pesto sauce.
Safrole, another member of the phenylpropanoid family of natural products, is found in sassafras and other plants; it is also found in many Chinese herbal drugs derived from Asarum species. It is considered a “weak” hepatocarcinogen, and its effect is related to stable safrole-DNA adduct formation. Safrole also caused oxidative damage, chromosome aberrations, and sister chromatid exchanges in rat liver, which is linked to hepatic injury and hepatocarcinoma[32,35]. In male BALB/c mice, 16 weeks of exposure to safrole resulted in histopathologic alteration including centrilobular hepatocytes hypertrophy. In contrast, long-term exposure to safrole induced hepatocellular adenomas in 7 of 10 mice[36]. In addition, 24 weeks of safrole exposure was able to induce the formation of hepatocyte basophilic and acidophilic foci, and 36 weeks of exposure led to the formation of neoplastic nodules[37].
Turkey egg genotoxicity assay showed that high doses of alkenylbenzenes caused DNA adduct formation[38,39]. The DNA binding ability of these compounds was also investigated in CD-1 mice[40], showing that methyleugenol, estragole, and safrole had a strong binding ability to liver DNA. Interestingly, the evidence suggests that the metabolic activation of alkenylbenzene compounds is crucial for their genotoxic and carcinogenic properties. In detail, sulfotransferases (enzyme for 1'-hydroxylation of allylbenzenes) inhibition resulted in the binding inhibition of safrole to liver DNA[41].
Areca catechu
Areca catechu (betel palm) is originally native to South and South-East Asia. Betel nuts (A. catechu seeds) have been widely used in herbal medicine practices for their antiparasitic properties. The nuts are also used for the treatment of diarrhea, throat inflammations, and urinary disorders. However, long-term betel nuts consumption is linked to the development of several diseases including cancer and metabolic dysfunction[42,43]. In this regard, in a cohort study, Chou et al.[44] found a positive correlation between nut chewing and liver disease (fibrosis) in persons with nonalcoholic fatty liver disease.
The widespread betel nut chewing habit may represent a risk factor for the development of liver diseases such as cirrhosis and HCC. A population-based study in Taiwan with a high prevalence of hepatitis B/C infection conducted by Wu et al.[45], showed increased risks of cirrhosis and HCC in betel chewers independently from hepatitis B/C infection. Subjects with hepatitis B/C infection had a higher HCC risk[45], and similar results were observed in a community-based cohort study[46] and in case-control studies[47-49]. The pathogenesis of betel-quid-induced liver cirrhosis and HCC is still not clear. Recently, the betel nut chewing cancerogenic effect appears to be mediated by arecoline, a nicotinic acid-based mild parasympathomimetic stimulant. Arecoline, the major alkaloid of betel quid, has been reported as one of the abundant constituents of areca nut, and it can act as an atherogenic[50], hepatotoxic[51], and cancerogenic agent[52]. A study using normal rat hepatocytes showed that arecoline had genotoxic properties and increased the expression of TGF-β[53]. Similarly, Wang et al.[54] showed that arecoline and its metabolite arecoline N-oxide induced DNA damage.
PROTECTION ON LIVER TUMORIGENESIS: PLANTS AND PLANT-DERIVED COMPOUNDS MAY PREVENT/ALLEVIATE LIVER CANCER
Besides their safety profile, some herbs and plant-derived compounds possess multitarget effects, making them optimum preventive and/or therapeutic/adjuvants agents against HCC proliferation, progression, and metastasis. Table 2 depicts the studies on the possible preventive effects of herbs and their compounds on liver tumorigenesis.
Studies of the possible preventive effects of herbs and their compounds on liver tumorigenesis
| Herbal product | Sample | Study type | Mechanism | Ref. |
| Silybum marianum | Silibinin | In vitro: HepG2 cells | ↓ROS, lipogenesis, MMP-9, cell invasion. ↓FASN, IL-6, IL-1B, p-Erk. | [60] |
| Silibinin | In vitro: HepG2 and Hep3B cells | ↑Cytotoxicity ↑ Cell cycle arrest ↑Kip1/p27 ↓ Cyclin D1, cyclin D3, cyclin E, cyclin-dependent kinase (CDK)-2, and CDK4 levels ↓CDK2, CDK4, and CDC2 kinase activity | [56] | |
| Doxorubicin-silymarin | In vitro: HepG2 cells | ↓Telomerase activity ↓Cell viability | [59] | |
| Silibinin | In vitro: rat H4IIE hepatoma cell line | ↓cytochrome p4502E1 induction ↓Ethanol metabolism ↓ROS | [57] | |
| Silibinin | In vitro: HCC cell lines In vivo: Mice | ↓Cell proliferation ↑Apoptosis ↓In vivo tumor growth ↓AKT, STAT3, Mcl-1, Bcl-2 ↓HCC stem cells formation and self-renewal | [58] | |
| Silybin | In vitro: HepG2 cells In vivo: Male athymic nude mice | ↓HCC cell viability, cell adhesion, and migration ↑Apoptosis, caspase 3 activity, and ROS ↓NICD, RBP-Jκ, Hes1 ↓Bcl2, survivin, cyclin D1 ↑Bax | [61] | |
| Silymarin | In vivo: male Wistar albino rats | ↓MDA, ALT, AST, GGT ↑GSH, SOD, GPx, GR | [55] | |
| Curcuma longa Linn | extract/ Curcumin | In vitro: HepG2 | ↓Cell growth ↑Apoptosis | [82] |
| Curcumin | In vitro: HCC cells | ↑Cell death ↓NF-κB ↑Cancer stem cells-depletion ↓Tumorigenicity | [83] | |
| Extract | In vivo: HBV X protein (HBx) transgenic mice | ↓Visceral fat ↓HBx-related genes ↑p21, cyclin D, p-p53 | [79] | |
| Extract | In vivo: male Wistar rats | ↓Serum of liver enzymes ↓Carcinogenesis | [80] | |
| Curcumin | In vivo: HCC cells implanted nude mice | ↓tumor neocapillary density ↓COX-2, serum VEGF | [84] [85] | |
| Curcumin | In vitro: H22 cells In vivo: xenograft mice | ↓Cell proliferation ↑Apoptosis ↓Tumor growth ↓VEGF expression, PI3/AKT signaling | [86] | |
| Curcumin | In vivo: mice | ↓Liver enzymes, VGEF, TNF-α, α-fetoprotein, MDA, NF-κB ↑Serum albumin, antioxidant activities | [87] | |
| Curcumin | In vivo: Male Sprague-Dawley rats | ↓α-fetoprotein, serum AST ↑Serum albumin, antioxidant activities ↑Autophagy | [88] | |
| Curcumin | In vivo: BALB/c nude mice In vitro: HepG2 and HCCLM3 cells | ↓HCC growth, cell proliferation ↑Apoptosis ↓miR-21 expression, GF-β1/smad3 signaling pathway ↑TIMP3 expression | [89] | |
| Curcumin | In vivo: Adult female albino rats | ↓α-fetoprotein, proinflammatory cytokines ↑Cx43, UCP-3, LC3 Mito.Q10 expression ↓Liver enzymes | [90] | |
| Curcumin/ Curcumin-cisplatin | In vitro: pediatric epithelial liver tumor cell lines In vivo: xenograft mice | ↓NF-κB, beta-catenin, cyclin D ↓α-fetoprotein | [91] | |
| Salvia miltiorrhiza | Extract | In vitro: HepG2 cells | ↓Cell proliferation ↑Apoptosis ↑DNA fragmentation ↓ GSH ↓Intracellular thiol ↑ ROS ↑Mitochondrial dysfunction ↑ PARP cleavage | [92] [93] |
| Extract | In vitro: HepG2 cells | ↓Cell proliferation ↑LDH leakage ↓ATP | [94] | |
| Chi-shen extract (Salvia miltiorrhiza and Paeoniae radix) | In vitro: HepG2 cells | ↓Cell proliferation ↑Apoptosis ↑DNA fragmentation ↓Bcl-2 ↑Bax, p53, caspases-3 and -9 activation | [95] | |
| Tanshinones | In vitro: HepG2, Hep3B, PLC/PRF/5 cells | ↓Doxorubicin efflux ↑synergism with doxorubicin ↑Apoptosis | [104] | |
| Tanshinone I | In vitro: HepG2 cells | ↓Cell proliferation ↑LC3-II, P62 ↑p-AMPKα, ULK1 | [106] | |
| Cryptotansinone | In vitro: HA22T cells | ↓Cell viability ↑Apoptosis ↓E-type prostaglandin 2, 4 ↓p-PI3K, p-Akt, β-catenin ↑E-cadherin, GSK3-β | [107] | |
| Tanshinone IIA | In vitro: HepG2 cells | ↑Apoptosis, necroptosis | [109] | |
| 15,16-dihydrotanshinone I | In vitro: SK-HEP-1 cells | ↓Cell viability ↑Cell cycle arrest ↓cyclin D1, cyclin A, cyclin E, CDK4, CDK2, c-Myc, p-Rb ↑p21, AMPK ↓Akt/mTOR, MAPK | [112] | |
| Tanshinone IIA | In vitro: BEL-7402 cells | ↓Cell viability ↑Cell cycle arrest ↑Apoptosis ↑Intracellular calcium ↓MMP | [111] | |
| Dihydrotanshinone | In vitro: HepG2 cells | ↑ROS ↓Cell viability ↑Apoptosis ↑p38 MAPK activation | [105] | |
| "Songyou Yin” Herbal extract (S. miltiorrhiza | In vitro: MHCC97H cells In vivo: Male athymic BALB/c nu/nu mice | ↓Cell proliferation ↑Apoptosis ↑Caspases-3 activation ↓Tumor growth ↓VEGF ↓MMP2 | [96] | |
| S. miltiorrhiza extract and Astragalus compound | In vitro: HepG2 cells In vivo: Rats | ↓Cell proliferation ↓Cell migration and invasion ↓Smad 2/3 phosphorylation ↑Smad 7 ↓HCC development ↓Bilirubin ↓Liver enzymes ↓GST-P, α-SMA ↑p-p38 ↓p-ERK, p-JNK, PAI-1 ↓TGF-β1, TβRI, TβRII ↑miR-145 ↓miR-21 | [97,98] [99] [100] [101] [102] | |
| Polysaccharide from S. miltiorrhiza | In vitro: H22 cells In vivo: Male BALB/c mice | ↓Cell proliferation ↓Tumor growth ↑SOD, CAT, GPx, TNF-α | [103] | |
| Tanshinol | In vitro: HCC cell lines In vivo: athymic nude mice | ↓Cell viability, colony formation, cell migration and invasion ↑Apoptosis ↓Tumor growth and metastasis ↓p-PI3K, p-Akt | [108] | |
| Tanshinone IIA | In vitro: HepG2, SMMC-7721 cells In vivo: Male athymic BALB/c nu/nu mice | ↓Cell migration and invasion ↓in vivo metastasis ↓MMP2, MMP9, NF-κB activation | [110] | |
| Cryptotanshinone | In vitro: Hepa1-6 cells In vivo: nude mice | ↓Cell viability ↑Apoptosis ↓Tumor growth ↑Tumor-infiltrating macrophages and dendritic cells, ↑Antitumor T cell response, effector/memory CD8 T cells. | [113] | |
| Miltirone | In vitro: HepG2, Hepa1-6 cells In vivo: nude mice | ↓Cell viability ↓ Gasdermin E cleavage ↓Caspase 3 cleavage ↑Pyroptosis ↑ROS ↓Tumor growth | [114] | |
| S. miltiorrhiza | Population-based cohort study | ↓Incidence of HCC in patients with diabetes mellitus | [115] | |
| Zingiber officinale | Extract | In vitro: HEp-2 cell | ↓Cell viability ↓DNA fragmentation ↑Superoxide production ↓NO, GSH | [116] |
| Flavonoid extract | In vitro: HepG2 cells | ↓Cell viability ↑Apoptosis ↑DNA degradation, comet tail formation, ROS ↓GSH, MMP ↑Caspase 3/9 activation ↑Bax/Bcl-2 ratio, p53 and p21 expression ↓cyclin D1, CDK4 | [117] | |
| Polysaccharides from Z. officinale | In vitro: HepG2 | ↓Cell viability ↑Apoptosis ↑ROS, cell cycle arrest ↑Bax, Fas, FasL, caspase-3, p21 and p53 ↓Bcl-2 | [118] | |
| 6-shogaol /6-gingerol. | In vitro: HepG2, Hep3B | ↓Cell migration and invasion ↓MMP-9, uPA ↑TIMP-1, PAI-1 ↓MAPK, PI3K/Akt signaling, NF-κB and STAT3 activation | [119] [120] | |
| 6-shogaol | In vitro: Huh7, Hep3B, HepG2, SMMC-7721 cells | ↓Cell viability ↑Cell cycle arrest, apoptosis ↑TRAIL-mediated cell death ↑ ROS, p53 ↓MMP ↑p62, microtubule-associated proteins 1A/1B light chain 3B-II ↓AMPK, Akt ↑Caspase activation, endoplasmic reticulum (ER) stress | [121] [122] [123] | |
| 6-dehydrogingerdione | In vitro: HepG2 cells | ↑Apoptosis ↑TRAIL-mediated cell death ↑ROS, p53, DR5 ↓MMP | [124] | |
| 6-Gingerol | In vitro: HepG2 cells | ↑DNA strand breaks, chromosome damage, apoptosis ↓Lysosomal membrane stability, MMP ↑GSH, ROS ↓Cathepsin D | [126] [125] | |
| Extract | In vivo: male albino rats | ↓α-fetoprotein ↑GSH ↓Liver enzymes ↓Oxidative stress and inflammatory biomarkers ↑Nrf2 | [131] | |
| Extract | In vivo: male Wistar albino rats | ↑Hepatic metallothionein and endostatin ↓α-fetoprotein, carcinoembryonic antigen | [129] | |
| Extract | In vivo: male Wistar rats | ↓Liver tumour, SOD activity, MDA level ↑CAT | [130] | |
| Extract | In vivo: Wistar rats | ↓Hepatic dyschromatic nodules ↓Positive focal areas ↓ GST ↓Oxidative stress ↓Cox-2, NFkB p65 | [127] | |
| Extract | In vivo: Male Wistar rats | ↓NF-κB, TNF-α | [128] |
Silybum marianum L.
Silybum marianum, a flowering plant from the Mediterranean basin, is commonly known as milk thistle (or blessed milk thistle) and is used in folk herbal medicine. Silymarin, the common name for the mixture extract of S. marianum, has been found to exert marked antioxidant, anti-inflammatory, and hepatoprotective activities; it is mainly consumed by patients with chronic liver disorders such as fatty liver, cirrhosis, and HCC.
The potential chemopreventive activities of silymarin against N-nitrosodiethylamine-induced HCC in rats were evaluated[55]. In this study, silymarin pre- and post-treatment showed a significant attenuation of oxidative stress by decreasing the MDA level and increasing GSH and liver antioxidant enzymes SOD, GPx, and GR. Interestingly, silymarin was also able to reduce the increased levels of GGT, AST, ALT, and VEGF.
Silibinin, a major bioactive flavanone in silymarin, is one of the most known hepatoprotective agents; its protective effect is found in many liver injuries such as fatty liver, viral hepatitis, bile duct inflammation, and cirrhosis. Silibinin showed a repressive effect on HepG2 and Hep3B cell proliferation, which was associated with apoptosis induction and cell cycle arrest by decreasing cyclin (D1, D3, and E) as well as cyclin-dependent kinase (CDK-2/4) levels[56]. In addition, silibinin inhibits cytochrome p4502E1 induction and ROS generation in ethanol-insulted HCC cells in vitro, which suggests that silibinin is beneficially effective against ethanol-dependent increases in HCC cell proliferation in culture[57]. In vivo, silibinin showed a weak antitumor activity in an HCC cell transplanted animal model. Therefore, the anti-HCC activity of co-treatment with silibinin and sorafenib (standard HCC chemo-drug) has been investigated as a potential therapeutic strategy for HCC[58]. In detail, the combination of silibinin and sorafenib markedly inhibited the cell proliferation of different HCC cells and exhibited significant proapoptotic activity. In addition, in an animal model of HCC subcutaneous transplantation, the co-treatment suppressed the in vivo tumor growth. These effects were accompanied by AKT and STAT3 phosphorylation inhibition, as well as downregulation of antiapoptotic protein expressions such as Mcl-1 and Bcl-2. Using HCC stem cells, silibinin and sorafenib co-treatment reduced the expression of stemness-related proteins, resulting in the reduction of the self-renewal of HCC stem cells[58]. Silymarin, in combination with doxorubicin, also markedly reduced the telomerase activity of HepG2 cells[59].
To evaluate the effect of silibinin on obesity-induced liver cancer, HepG2 cells were exposed to sera from normal weight, overweight, and obese males with or without silibinin. The results show that silibinin attenuated obesity-induced cell proliferation and invasion. At molecular levels, silibinin decreased inflammatory cytokines (IL-6/1B) and reduced Erk phosphorylation[60].
Silybin is a bioactive flavonolignan component of silymarin extract with reported hepatoprotective and chemopreventive proprieties. Silybin reduced HCC cell proliferation and migration and induced caspase 3- and ROS-dependent apoptosis. Furthermore, silybin treatment decreased the Notch signaling pathway in HCC[61].
Curcuma longa L.
Curcuma longa has been used for centuries for culinary and medicinal purposes. Several studies investigated the chemopreventive and antitumor effects of turmeric (C. longa L.) and its main polyphenolic compound, curcumin. A panel of in vitro cellular studies of curcumin alone or in combination with commonly used chemotherapeutic drugs explored its potent anti-hepatocarcinoma effects. These studies showed that curcumin decreased HCC cell proliferation and metastasis and induced apoptotic cell death[62] through different mechanisms including decreasing the hypoxia-induced hypoxia-inducible factor-1α protein level[63]; downregulation of the expression of Snail via suppressing Smad2 pathway and TGF-β1[64]; the mitochondria-dependent pathway related to intracellular accumulation of calcium[65], miR-21-5p, and SOX6[66]; inhibition of the WNT signaling pathway[67-70]; inhibition of metalloproteinases (MMP-2/9); reduced AKT, p38, and STAT3 phosphorylation[71,72]; activation of AMPK signaling pathway[73]; reduction of DJ-1 expression[74]; increasing apoptosis and pyroptosis[75]; inducing endoplasmic reticulum stress and mitochondrial dysfunction[76]; downregulating SREBF1[77]; and induction of FasL-related apoptosis through p38[78]. Moreover, a bioinformatics analysis investigating the crucial genes, transcription factors, and microRNAs associated with the effects of curcumin against HCC showed that curcumin might downregulate LEF1 and CTGF, regulated by miR-19A, and upregulate CDKN1A expression[62].
The chemopreventive effect of C. longa was investigated in an in vivo animal study. Administration of C. longa extract to HBV mice for three months reduced visceral fat and resulted in a smaller and delayed liver disease progression. The study indicated that C. longa could have beneficial effects on preventing and/or delaying liver carcinogenesis[79]. The preventive effect of C. longa was also shown in DENA-induced liver carcinogenesis, where dietary supplementation of C. longa decreased the elevated serum level of liver enzymes and improved hepatic damage[80].
The effects of C. longa constituents and phytochemicals on liver cancer have also been investigated. For example, the aromatic turmerone, a volatile turmeric oil isolated from C. longa, induced ROS-triggered intrinsic and extrinsic apoptosis pathways in HepG2 cells[81].
Curcumin, one of the most abundant polyphenols in C. longa, with multiple pharmacological activities including antitumor effects, showed a potent anti-HCC property in vitro and in vivo. The antiproliferative and proapoptotic activities of curcumin as a single agent in HepG2 cells were more evident than those of C. longa methanolic extract[82]. In addition, curcumin-mediated cell death was observed to be related to the inhibition of NF-κB activation pathway. Furthermore, curcumin inhibited liver cancer stem cells and suppressed tumorigenicity[83]. In HepG2 cells, treatment with curcumin reduced the tumor neocapillary density[84] and decreased COX-2 and serum VEGF[85]. A reduction of VEGF expression and PI3K/AKT signaling was also observed upon curcumin treatment in vitro and in vivo[86].
In an in vivo animal model, nanoparticulate curcumin oral supplementation decreased the elevated liver enzymes, α-fetoprotein, and VEGF and reduced inflammatory markers such as TNF-α, MDA, and NF-κB[87]. Similarly, curcumin decreased α-fetoprotein and AST concentration and increased serum albumin concentration in rats[88].
At the molecular level, curcumin treatment downregulated miR-21 expression, upregulated tissue inhibitor metalloproteinase protein 3 (TIMP) expression, and inhibited the TGF-β1/smad3 signaling pathway in HepG2 and HCCLM3 cells[89]. In vivo, using DENA and CCl4-induced HCC in adult female albino rats, curcumin reduced α-fetoprotein and proinflammatory cytokines and decreased the elevated liver enzymes. In addition, curcumin treatment led to the upregulation of the expressions of Cx43, UCP-3, LC3, and Mito.Q10[90].
The possible effect of curcumin co-treatment with cisplatin on liver epithelial tumor cells was also investigated. Curcumin led to NF-κβ and β-catenin inhibition and decreased cyclin D expression. In animal studies, using a mouse xenograft model, curcumin and cisplatin significantly decreased α-fetoprotein[91].
Salvia miltiorrhiza
Salvia miltiorrhiza is widely used in Chinese herbal medicine for liver diseases. Several studies indicate that S. miltiorrhiza exhibits different biological and pharmaceutical activities including antitumor activity.
In HepG2 cells, Liu et al.[92] showed that S. miltiorrhiza extract inhibited cell proliferation and induced apoptotic cell death. The proapoptotic effect seems to be mediated by GSH depletion and reduction of mitochondrial membrane potential[92]. In addition, they demonstrated in another study that the proapoptotic effects of S. miltiorrhiza extract are correlated with a rapid decline of GSH and protein thiol content, mitochondrial dysfunction, and overproduction of ROS in HepG2 cells[93]. Furthermore, HepG2 cells treated with S. miltiorrhiza showed an increase in LDH leakage, decrease in ATP level, and induction of apoptotic-like cell morphology change, which also might be involved in the cytotoxic and apoptotic effects[94].
Accumulating evidence suggests that S. miltiorrhiza, in combination with other medicinal herbs, displays an appreciated effect against HCC. In this regard, combinations such as “Chi-Shen” extract (S. miltiorrhiza and Paeoniae radix)[95], “Songyou Yin” (containing S. miltiorrhiza and four other herbs)[96], and CASE (compound astragalus and S. miltiorrhiza extract[97-102]) have shown in vitro and in vivo anti-HCC effects mainly by cell proliferation inhibition and apoptosis induction through the modulation of Bcl-2 family and caspase-3 and -9 activation, in addition to inhibition of tumor invasion via downregulation of MMP-2, PAI-1 transcriptional activity, TGF-β/TβR and Imp7/8 protein expression, MAPK, and MAPK-dependent phosphorylation of Smad2/3 and Smad4.
Additionally, fractions or single compounds from S. miltiorrhiza have also shown potent anti-HCC effects. In a cellular model using H22 cells, the polysaccharide fraction from S. miltiorrhiza exerted an antiproliferative effect. Moreover, in an animal model, inhibition of tumor growth and TNF-α level was observed in mice treated with the polysaccharide fraction[103]. Tanshinones from S. miltiorrhiza, such as tanshinone I, tanshinone IIA, cryptotanshinone, and dihydrotanshinone, have been largely studied for their bioactivities including antitumor effect. In vitro, using doxorubicin-resistant human liver cancer cells, cryptotanshinone exerted a repressive effect on doxorubicin efflux, and tanshinone IIA provided a great synergism with doxorubicin[104]. In addition, in HepG2 cells, tanshinones inhibited cell growth and induced caspase-dependent apoptosis accompanied with an increase of ROS[105]. Tanshinone I showed a regulatory effect on autophagic cancer cell death by activation of AMP-activated protein kinase[106]. In HA22T cells, cryptotansinone inhibited cell viability and induced apoptosis. At the molecular level, cryptotansinone inhibited EP-2/4 expression and PI3K and Akt phosphorylation in HA22T[107].
Zhu et al.[108] tested the effect of tanshinol, an aqueous polyphenol isolated from S. miltiorrhiza, and observed inhibition of HCC cell proliferation, migration, invasion, and colony formation. In addition, when tested in vivo, tanshinol treatment reduced tumor growth and metastasis. The in vitro and in vivo antitumor effects were associated with the reduction of PI3K and AKT[108]. Tanshinone IIA also caused apoptotic and necroptotic HepG2 cell death through Nec-1 inhibition and FLIPS regulation[109]. In addition, tanshinone IIA showed a repressive effect on HCC invasion and metastasis in vitro and in vivo by inhibiting MMP-2/9 activity[110]. The proapoptotic effect of tanshinone IIA was also related to calcium-dependent apoptosis and MT 1A upregulation[111]. 15,16-dihydrotanshinone I also suppressed SK-HEP-1 proliferation and induced cell cycle arrest at G0/G1 phase and regulated AMPK/Akt/mTOR and MAPK signaling pathways[112].
Cryptotanshinone inhibited the proliferation of Hepa1-6 cells and induced apoptosis. In addition, cryptotanshinone exerted an immunotherapeutic effect, as shown in tumor-bearing mice, where cryptotanshinone promoted the activation of immune cells against tumor tissue[113]. In the same cell line (Hepa1-6) and HepG2, miltirone, a bioactive compound from S. miltiorrhiza, inhibited cell proliferation and induced gasdermin E and caspase 3 protein cleavage. Miltirone also induced ROS production and decreased MEK and ERK1/2 phosphorylation. Moreover, in a mouse HCC syngeneic model, miltirone exerted a significant tumor growth inhibition[114].
A population-based cohort study showed that the use of S. miltiorrhiza as traditional Chinese herbal medicine reduces the incidence of HCC in diabetes mellitus patients[115].
Zingiber officinale (Ginger)
Ginger root is widely used as a spice and in folk medicine. The anticancer effect of ginger is documented by in vitro cellular and in vivo animal studies.
In vitro, using HEp-2 cells, the ginger extract showed a dose-dependent inhibition of cell proliferation and caused marked apoptosis-like morphological and nuclear changes including cell shrinkage and chromosomes condensation. Those effects were accompanied by an increase in ROS production and a decrease in GSH content[116]. Similarly, in HepG2 cells, treatment with ginger extracts decreased cell viability and induced apoptotic cell death mediated by DNA degradation, comet tail formation, ROS production, GSH depletion, mitochondrial membrane potential alteration, caspase 3/9 activation, and increasing of the Bax/Bcl-2 ratio[117]. Cell cycle arrest was also observed in ginger-treated HCC[118].
Ginger extract and bioactive compounds such as 6-gingerol and 6-shogaol exerted an anti-migration ability in vitro by decreasing MMP-9 activity and increasing TIMP-1 activity[119]. Similarly, 6-gingerol and 6-shogaol strongly reduced HCC invasion and metastasis abilities through MAPK, PI3k/Akt, NF-κB, and STAT3 pathways[120].
6-shogaol also induced TRAIL mediated apoptosis through ROS production, p53 expression, and mitochondrial transmembrane potential alteration; these effects were accompanied by inhibition of autophagy flux by increasing p62 and microtubule-associated proteins 1A/1B light chain 3B-II levels[121]. Furthermore, 6-shogaol induced G2/M cell cycle arrest in HCC cells and modulated cyclin expression. In addition, 6-shogaol was able to reduce cancer survival signaling pathways such as MAPK, AMPK, and Akt[122]. The proapoptotic activity of 6-shogaol is also linked to endoplasmic reticulum stress and caspase activation in vitro. In an animal xenograft mice model as well, 6-shogaol reduced in vivo tumor growth and induced apoptotic death through caspase 3 activation and eIF2α inactivation[123].
Furthermore, 6-dehydrogingerdione, a compound isolated from Z. officinale, showed a proapoptotic effect on HepG2 through mitochondrial- and Fas receptor-mediated pathways and inhibition of p53 nuclear translocation[124].
In HepG2 cells, 6-gingerol exerted anticancer activity by causing DNA and chromosome damage. Additionally, 6-gingerol altered lysosomal membrane stability and mitochondrial membrane potential[125]. Other studies showed that 6-gingerol exerts prooxidant effects by increasing ROS production and inducing apoptosis involving cathepsin D mediator[126].
In vivo, using DEN-induced rat liver cancer, ginger extract displayed chemo-preventative effects by tumor growth inhibition and apoptosis induction[127]. The chemo-preventative effects of ginger could be attributed to its ability to decrease inflammatory mediators such as NF-κB and TNF-α expression in rats’ liver cancer[128]. In Wistar albino rats, ginger administration showed a protective effect against premalignant stages of liver cancer. Moreover, the ginger extract reduced serum α-fetoprotein and carcinoembryonic antigen[129]. In rats with liver cancer induced by a 0.1% ethionine and choline-deficient diet, ginger treatment reduced tumor growth and decreased MDA level[130]. In addition, ginger pre-treatment improved rats’ liver function by restoring GSH levels and decreasing inflammatory and oxidative markers[131].
DISCUSSION AND CONCLUSION
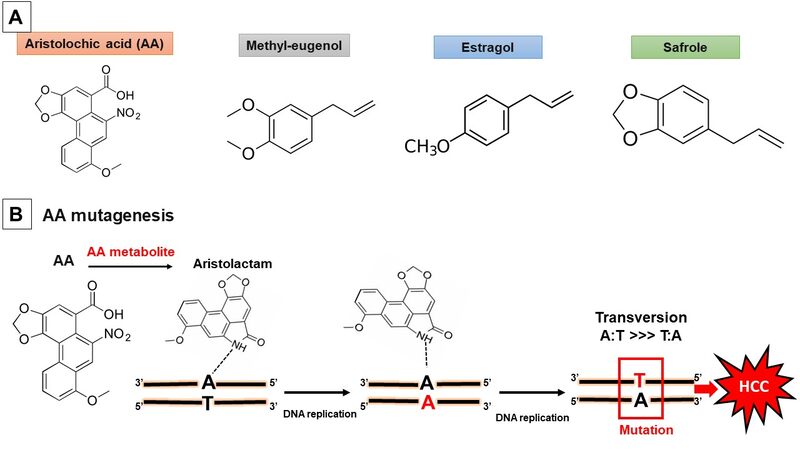
Besides the well-established risk factors for liver cancer, hepatotoxic herbs or herbs-derived compounds could represent an additional liver cancer risk factor. For example, AA is an established human carcinogen and is one of the most potent carcinogens. In particular, AA metabolites bind DNA to form adducts resulting in A > T transversion mutations. Although AA is a main risk factor for urinary tract urothelial carcinoma, we report a different science-based link between AA exposure and liver cancer. Results from in vitro and in vivo studies as well as population-based cohort studies demonstrate the potential hepatocarcinogenic effect of AA. A summary of the properties of AA is presented in Figure 1.
Figure 1. (A) Chemical structural of the aristolochic acid and alkenylbenzenes estragole, methyleugenol, and safrole. (B) Mutagenesis mechanism of aristolochic acid by DNA adduct formation. HCC: hepatocellular carcinoma.
Plants such as Ginkgo biloba and chewing betel-nuts (Areca catechu) as well as plant-derived alkenylbenzene compounds including safrole, methyleugenol, and estragole have been shown to exert hepatocarcinogenic activity in different experimental studies. Here, we summarize several cellular and animal studies concerning their hepatocarcinogenic. In addition, some data from different population-based and case-control studies show marked associations between natural products and HCC development.
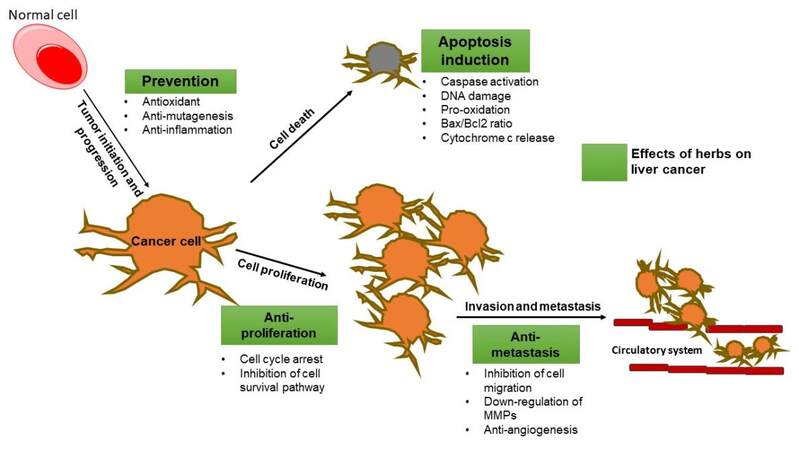
In contrast, many herbal remedies showed a potent anti-liver cancer activity. In fact, several in vitro studies have shown that natural products exhibit strong cytotoxic and proapoptotic activity against HCC, as well as have effects on cell metastasis and invasion [Figure 2].
Figure 2. Possible preventive effects and mechanism of herbs and their compounds on liver tumorigenesis. Bax: Proapoptotic protein; Bcl-2: antiapoptotic protein; MMPs: matrix metalloproteinases. Boxes in green represent the herbal effects on liver cancer.
Hereby, we report evidence from cellular and animal studies that explore the potential anti-liver cancer activity of different herbal products, namely Silybum marianum and its active constituents silymarin, silibinin, and silybin; Curcuma longa with its active compound curcumin; Salvia miltiorrhiza and its principal bioactive compounds tanshinones; and Zingiber officinale and its active polyphenolic fractions, 6-shogaol, and 6-gingerol. In HCC cells, these products inhibited HCC cell proliferation and tumor growth and induced apoptotic cell death. Similarly, in liver cancer animal models, the herbal products reduced tumor growth and metastasis, decreased the elevated level of liver enzymes, and decreased the α-fetoprotein level.
In conclusion, herbs and herb-based preparations may provide beneficial outcomes in human health including hepatoprotective effects. However, they can also contain individual ingredients known to be toxic and even genotoxic and carcinogenic to the liver. Despite the available evidence from cellular and animal studies of herbs promoting or preventing liver tumorigenesis, human studies are still missing. In this regard, clinical trials investigating the safety and toxicity of herbs are highly needed. The mechanism of action involved in liver tumorigenicity or hepatoprotection should be clarified by well-established in vivo experimental models.
DECLARATIONS
Authors’ contributionsConceived the manuscript: Portincasa P
Wrote this manuscript: Khalil M, Portincasa P
Intensive revised the manuscript: Di Ciaula A, Bonfrate L, Calasso M, De Angelis M, Wang DH
Availability of data and materials:Not applicable.
Financial support and sponsorshipThis paper has been partly supported by funding from the European Union’s Horizon 2020 Research and Innovation program under the Marie Skłodowska-Curie Grant Agreement No. 722619 (FOIE GRAS) and Grant Agreement No. 734719 (mtFOIE GRAS).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
2. Rubio-Ruiz ME, El Hafidi M, Pérez-Torres I, Baños G, Guarner V. Medicinal agents and metabolic syndrome. Curr Med Chem 2013;20:2626-40.
4. Navarro VJ, Khan I, Björnsson E, Seeff LB, Serrano J, Hoofnagle JH. Liver injury from herbal and dietary supplements. Hepatology 2017;65:363-73.
5. Zhang HM, Zhao XH, Sun ZH, et al. Recognition of the toxicity of aristolochic acid. J Clin Pharm Ther 2019;44:157-62.
6. Benhammou JN, Lin J, Hussain SK, El-Kabany M. Emerging risk factors for nonalcoholic fatty liver disease associated hepatocellular carcinoma. Hepatoma Res 2020;6:35.
7. Raza S, Rajak S, Anjum B, Sinha RA. Molecular links between non-alcoholic fatty liver disease and hepatocellular carcinoma. Hepatoma Res 2019;5:42.
8. El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007;132:2557-76.
9. Mandlik DS, Mandlik SK. Herbal and natural dietary products: upcoming therapeutic approach for prevention and treatment of hepatocellular carcinoma. Nutr Cancer 2021;73:2130-54.
10. Zhou Y, Li Y, Zhou T, Zheng J, Li S, Li HB. Dietary natural products for prevention and treatment of liver cancer. Nutrients 2016;8:156.
11. Khalil M, Khalifeh H, Baldini F, et al. Antitumor activity of ethanolic extract from thymbra spicata l. aerial parts: effects on cell viability and proliferation, apoptosis induction, STAT3, and NF-κB signaling. Nutr Cancer 2021;73:1193-206.
12. Lin SR, Chang CH, Hsu CF, et al. Natural compounds as potential adjuvants to cancer therapy: preclinical evidence. Br J Pharmacol 2020;177:1409-23.
13. Korak T, Ergül E, Sazci A. Nigella sativa and cancer: a review focusing on breast cancer, inhibition of metastasis and enhancement of natural killer cell cytotoxicity. Curr Pharm Biotechnol 2020;21:1176-85.
14. Ahmad A, Ansari IA. Carvacrol exhibits chemopreventive potential against cervical cancer cells via caspase-dependent apoptosis and abrogation of cell cycle progression. Anticancer Agents Med Chem 2021;21:2224-35.
15. World Health Organization. WHO guidelines on developing consumer information on proper use of traditional, complementary and alternative medicine. Available from: Https://Www.Who.Int/Publications/I/Item/9241591706 [Last accessed on 15 Apr 2022].
16. Wu K, Jiang L, Cao J, Yang G, Geng C, Zhong L. Genotoxic effect and nitrative DNA damage in HepG2 cells exposed to aristolochic acid. Mutat Res 2007;630:97-102.
17. Li Y, Zhu S, Xue M, et al. Aristolochic acid I promotes the invasion and migration of hepatocellular carcinoma cells by activating the C3a/C3aR complement system. Toxicol Lett ;2020:S0378-4274(20)30410.
18. Lu ZN, Luo Q, Zhao LN, et al. The mutational features of aristolochic acid-induced mouse and human liver cancers. Hepatology 2020;71:929-42.
19. Jin K, Su KK, Li T, et al. Hepatic premalignant alterations triggered by human nephrotoxin aristolochic acid I in canines. Cancer Prev Res (Phila) 2016;9:324-34.
20. Chen CJ, Yang YH, Lin MH, et al. Health Data Analysis in Taiwan (hDATa) Research Group. Herbal medicine containing aristolochic acid and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. Int J Cancer 2018;143:1578-87.
21. Hu ZQ, Xin HY, Luo CB, et al. Associations among the mutational landscape, immune microenvironment, and prognosis in Chinese patients with hepatocellular carcinoma. Cancer Immunol Immunother 2021;70:377-89.
23. Beek TA, Montoro P. Chemical analysis and quality control of Ginkgo biloba leaves, extracts, and phytopharmaceuticals. J Chromatogr A 2009;1216:2002-32.
24. Mei N, Guo X, Ren Z, Kobayashi D, Wada K, Guo L. Review of ginkgo biloba-induced toxicity, from experimental studies to human case reports. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 2017;35:1-28.
25. Mahadevan S, Park Y. Multifaceted therapeutic benefits of Ginkgo biloba L.: chemistry, efficacy, safety, and uses. J Food Sci 2008;73:R14-9.
26. Berg K, Braun C, Krug I, Schrenk D. Evaluation of the cytotoxic and mutagenic potential of three ginkgolic acids. Toxicology 2015;327:47-52.
27. National Toxicology Program. Toxicology and carcinogenesis studies of ginkgo biloba extract (Cas No. 90045-36-6) in F344/N rats and B6c3f1/N mice (Gavage Studies). Natl Toxicol Program Tech Rep Ser ;2013:1-183.
28. Hoenerhoff MJ, Pandiri AR, Snyder SA, et al. Hepatocellular carcinomas in B6C3F1 mice treated with Ginkgo biloba extract for two years differ from spontaneous liver tumors in cancer gene mutations and genomic pathways. Toxicol Pathol 2013;41:826-41.
29. Maeda J, Inoue K, Ichimura R, et al. Essential role of constitutive androstane receptor in Ginkgo biloba extract induced liver hypertrophy and hepatocarcinogenesis. Food Chem Toxicol 2015;83:201-9.
30. National Toxicology Program. Toxicology and carcinogenesis studies of isoeugenol (Cas No. 97-54-1) in F344/N rats and B6c3f1 mice (Gavage studies). Natl Toxicol Program Tech Rep Ser ;2010:1-178.
31. National Toxicology Program. Ntp toxicology and carcinogenesis studies of methyleugenol (Cas No. 93-15-2) in F344/N rats and B6c3f1 mice (Gavage studies). Natl Toxicol Program Tech Rep Ser 2000;491:1-412.
32. Devereux TR, Anna CH, Foley JF, White CM, Sills RC, Barrett JC. Mutation of beta-catenin is an early event in chemically induced mouse hepatocellular carcinogenesis. Oncogene 1999;18:4726-33.
33. Miele M, Dondero R, Ciarallo G, Mazzei M. Methyleugenol in ocimum basilicum L. Cv. genovese gigante. J Agric Food Chem 2001;49:517-21.
34. Al-Malahmeh AJ, Al-Ajlouni AM, Wesseling S, Vervoort J, Rietjens IMCM. Determination and risk assessment of naturally occurring genotoxic and carcinogenic alkenylbenzenes in basil-containing sauce of pesto. Toxicol Rep 2017;4:1-8.
36. Mm, Hinton De, Klaunig Je, Trump Bf. Biology of hepatocellular neoplasia in the mouse. I. histogenesis of safrole-induced hepatocellular carcinoma. J Natl Cancer Inst 1981;67:365-76.
37. Lipsky MM, Hinton DE, Klaunig JE, Goldblatt PJ, Trump BF. Gamma glutamyl transpeptidase in safrole-induced, presumptive premalignant mouse hepatocytes. Carcinogenesis 1980;1:151-6.
38. Kobets T, Duan JD, Brunnemann KD, Etter S, Smith B, Williams GM. Structure-activity relationships for DNA damage by alkenylbenzenes in turkey egg fetal liver. Toxicol Sci 2016;150:301-11.
39. Rw, Fennell Tr, Miller Ja, Miller Ec. Further characterization of the DNA adducts formed by electrophilic esters of the hepatocarcinogens 1’-hydroxysafrole and 1’-hydroxyestragole. in vitro ;45:3096-105.
40. Randerath K, Haglund RE, Phillips DH, Reddy MV. 32P-post-labelling analysis of DNA adducts formed in the livers of animals treated with safrole, estragole and other naturally-occurring alkenylbenzenes. I. Adult female CD-1 mice. Carcinogenesis 1984;5:1613-22.
41. Rw, Miller Ec, Miller Ja, Liem A. Structure-activity studies of the hepatocarcinogenicities of alkenylbenzene derivatives related to estragole and safrole on administration to preweanling male C57bl/6j X C3h/Hej F1 mice. Cancer Res 1987;47:2275-83.
42. Shafique K, Zafar M, Ahmed Z, Khan NA, Mughal MA, Imtiaz F. Areca nut chewing and metabolic syndrome: evidence of a harmful relationship. Nutr J 2013;12:67.
43. Yamada T, Hara K, Kadowaki T. Chewing betel quid and the risk of metabolic disease, cardiovascular disease, and all-cause mortality: a meta-analysis. PLoS One 2013;8:e70679.
44. Chou YT, Li CH, Sun ZJ, et al. A Positive relationship between betel nut chewing and significant liver fibrosis in NAFLD subjects, but not in non-NAFLD ones. Nutrients 2021;13:914.
45. Wu GH, Boucher BJ, Chiu YH, Liao CS, Chen TH. Impact of chewing betel-nut (Areca catechu) on liver cirrhosis and hepatocellular carcinoma: a population-based study from an area with a high prevalence of hepatitis B and C infections. Public Health Nutr 2009;12:129-35.
46. Wang L. You Sl, Lu Sn, et al. Risk of hepatocellular carcinoma and habits of alcohol drinking, betel quid chewing and cigarette smoking: a cohort of 2416 HBsAg-seropositive and 9421 HBsAg-seronegative male residents in Taiwan. Cancer Causes Control 2003;14:241-50.
47. Tsai JF, Chuang LY, Jeng JE, et al. Betel quid chewing as a risk factor for hepatocellular carcinoma: a case-control study. Br J Cancer 2001;84:709-13.
48. Tsai JF, Jeng JE, Chuang LY, et al. Habitual betel quid chewing and risk for hepatocellular carcinoma complicating cirrhosis. Medicine (Baltimore) 2004;83:176-87.
49. Jeng JE, Tsai MF, Tsai HR, et al. Impact of chronic hepatitis B and hepatitis C on adverse hepatic fibrosis in hepatocellular carcinoma related to betel quid chewing. Asian Pac J Cancer Prev 2014;15:637-42.
50. Choudhury MD, Chetia P, Choudhury KD, Talukdar AD, Datta-Choudhari M. Atherogenic effect of arecoline: a computational study. Bioinformation 2012;8:229-32.
51. Dasgupta R, Saha I, Pal S, et al. Immunosuppression, hepatotoxicity and depression of antioxidant status by arecoline in albino mice. Toxicology 2006;227:94-104.
52. Marques MM, Beland FA, Lachenmeier DW, et al. Carcinogenicity of acrolein, crotonaldehyde, and arecoline. The Lancet Oncology 2021;22:19-20.
53. Chou WW, Guh JY, Tsai JF, et al. Arecoline-induced growth arrest and p21WAF1 expression are dependent on p53 in rat hepatocytes. Toxicology 2008;243:1-10.
54. Wang TS, Lin CP, Chen YP, Chao MR, Li CC, Liu KL. CYP450-mediated mitochondrial ROS production involved in arecoline N-oxide-induced oxidative damage in liver cell lines. Environ Toxicol 2018;33:1029-38.
55. Mesallamy HO, Metwally NS, Soliman MS, Ahmed KA, Abdel Moaty MM. The chemopreventive effect of Ginkgo biloba and Silybum marianum extracts on hepatocarcinogenesis in rats. Cancer Cell Int 2011;11:38.
56. Varghese L, Agarwal C, Tyagi A, Singh RP, Agarwal R. Silibinin efficacy against human hepatocellular carcinoma. Clin Cancer Res 2005;11:8441-8.
57. Brandon-Warner E, Sugg JA, Schrum LW, McKillop IH. Silibinin inhibits ethanol metabolism and ethanol-dependent cell proliferation in an in vitro model of hepatocellular carcinoma. Cancer Lett 2010;291:120-9.
58. Mao J, Yang H, Cui T, et al. Combined treatment with sorafenib and silibinin synergistically targets both HCC cells and cancer stem cells by enhanced inhibition of the phosphorylation of STAT3/ERK/AKT. Eur J Pharmacol 2018;832:39-49.
59. Yurtcu E, Darcansoy Iseri O, Iffet Sahin F. Effects of silymarin and silymarin-doxorubicin applications on telomerase activity of human hepatocellular carcinoma cell line Hepg2. J Buon 2015;20:555-61.
60. Miethe C, Nix H, Martin R, Hernandez AR, Price RS. Silibinin reduces the impact of obesity on invasive liver cancer. Nutr Cancer 2017;69:1272-80.
61. Zhang S, Yang Y, Liang Z, et al. Silybin-mediated inhibition of notch signaling exerts antitumor activity in human hepatocellular carcinoma cells. PLoS One 2013;8:e83699.
62. Chen Q, Guo H, Zong Y, Zhao X. Curcumin restrains hepatocellular carcinoma progression depending on the regulation of the circ_0078710/miR-378b/PRIM2 axis. J Recept Signal Transduct Res 2021:1-12.
63. Duan W, Chang Y, Li R, et al. Curcumin inhibits hypoxia inducible factor 1α induced epithelial mesenchymal transition in HepG2 hepatocellular carcinoma cells. Mol Med Rep 2014;10:2505-10.
64. Cao MT, Liu HF, Liu ZG, et al. Curcumin downregulates the expression of snail via suppressing Smad2 pathway to inhibit TGF-β1-induced epithelial-mesenchymal transitions in hepatoma cells. Oncotarget 2017;8:108498-508.
65. Wang WH, Chiang IT, Ding K, et al. Curcumin-induced apoptosis in human hepatocellular carcinoma j5 cells: critical role of Ca(+2)-dependent pathway. Evid Based Complement Alternat Med 2012;2012:512907.
66. Zhou C, Hu C, Wang B, Fan S, Jin W. Curcumin suppresses cell proliferation, migration, and invasion through modulating miR-21-5p/. SOX6 :axis in hepatocellular carcinoma.
67. Kim HJ, Park SY, Park OJ, Kim YM. Curcumin suppresses migration and proliferation of Hep3B hepatocarcinoma cells through inhibition of the Wnt signaling pathway. Mol Med Rep 2013;8:282-6.
68. Xu MX, Zhao L, Deng C, et al. Curcumin suppresses proliferation and induces apoptosis of human hepatocellular carcinoma cells via the wnt signaling pathway. Int J Oncol 2013;43:1951-9.
69. Shao J, Shi CJ, Li Y, et al. LincROR Mediates the suppressive effects of curcumin on hepatocellular carcinoma through inactivating Wnt/β-catenin signaling. Front Pharmacol 2020;11:847.
70. Hu P, Ke C, Guo X, et al. Both glypican-3/Wnt/β-catenin signaling pathway and autophagy contributed to the inhibitory effect of curcumin on hepatocellular carcinoma. Dig Liver Dis 2019;51:120-6.
71. Chiablaem K, Lirdprapamongkol K, Keeratichamroen S, Surarit R, Svasti J. Curcumin suppresses vasculogenic mimicry capacity of hepatocellular carcinoma cells through Stat3 And Pi3k/Akt inhibition. Anticancer Res 2014;34:1857-64.
72. Zhang K, Rui X, Yan X. Curcumin inhibits the proliferation and invasiveness of MHCC97-H cells via p38 signaling pathway. Drug Dev Res 2014;75:463-8.
73. Yj, Xiang H, Liu Js, Li D, Fang Zy, Zhang H. Study on the mechanism of Ampk signaling pathway and its effect on apoptosis of human hepatocellular carcinoma Smmc-7721 cells by curcumin. Eur Rev Med Pharmacol Sci 2017;21:1144-50.
74. Han L, Wang Y, Sun S. Curcumin inhibits proliferation of hepatocellular carcinoma cells through down regulation of DJ-1. Cancer Biomark 2020;29:1-8.
75. Liang WF, Gong YX, Li HF, et al. Curcumin activates ROS signaling to promote pyroptosis in hepatocellular carcinoma HepG2 cells. In Vivo 2021;35:249-57.
76. Cheng CY, Lin YH, Su CC. Curcumin inhibits the proliferation of human hepatocellular carcinoma J5 cells by inducing endoplasmic reticulum stress and mitochondrial dysfunction. Int J Mol Med 2010;26:673-8.
77. You Z, Li B, Xu J, Chen L, Ye H. Curcumin suppress the growth of hepatocellular carcinoma via down-regulating. SREBF1 :.
78. Wang WZ, Li L, Liu MY, et al. Curcumin induces FasL-related apoptosis through p38 activation in human hepatocellular carcinoma Huh7 cells. Life Sci 2013;92:352-8.
79. Kim J, Ha HL, Moon HB, et al. Chemopreventive effect of Curcuma longa Linn on liver pathology in HBx transgenic mice. Integr Cancer Ther 2011;10:168-77.
80. El-Shahat M, El-Abd S, Alkafafy M, El-Khatib G. Potential chemoprevention of diethylnitrosamine-induced hepatocarcinogenesis in rats: myrrh (Commiphora molmol) vs. turmeric (Curcuma longa). Acta Histochem 2012;114:421-8.
81. Cheng SB, Wu LC, Hsieh YC, et al. Supercritical carbon dioxide extraction of aromatic turmerone from curcuma longa linn. induces apoptosis through reactive oxygen species-triggered intrinsic and extrinsic pathways in human hepatocellular carcinoma HepG2 cells. J Agric Food Chem 2012;60:9620-30.
82. Abdel-Lateef E, Mahmoud F, Hammam O, et al. Bioactive chemical constituents of Curcuma longa L. rhizomes extract inhibit the growth of human hepatoma cell line (HepG2). Acta Pharm 2016;66:387-98.
83. Marquardt JU, Gomez-Quiroz L, Arreguin Camacho LO, et al. Curcumin effectively inhibits oncogenic NF-κB signaling and restrains stemness features in liver cancer. J Hepatol 2015;63:661-9.
84. Yoysungnoen P, Wirachwong P, Bhattarakosol P, Niimi H, Patumraj S. Antiangiogenic activity of curcumin in hepatocellular carcinoma cells implanted nude mice. Clin Hemorheol Microcirc 2005;33:127-35.
85. Yoysungnoen P, Wirachwong P, Bhattarakosol P, Niimi H, Patumraj S. Effects of curcumin on tumor angiogenesis and biomarkers, Cox-2 and Vegf, in hepatocellular carcinoma cell-implanted nude mice. Clin Hemorheol Microcirc 2006;34:109-15.
86. Pan Z, Zhuang J, Ji C, Cai Z, Liao W, Huang Z. Curcumin inhibits hepatocellular carcinoma growth by targeting VEGF expression. Oncol Lett 2018;15:4821-6.
87. Mohammed ES, El-Beih NM, El-Hussieny EA, El-Ahwany E, Hassan M, Zoheiry M. Effects of free and nanoparticulate curcumin on chemically induced liver carcinoma in an animal model. Arch Med Sci 2021;17:218-27.
88. Elmansi AM, El-Karef AA, Shishtawy MMEl, Eissa LA. Hepatoprotective effect of curcumin on hepatocellular carcinoma through autophagic and apoptic pathways. Ann Hepatol 2017;16:607-18.
89. Li J, Wei H, Liu Y, et al. Curcumin inhibits hepatocellular carcinoma via regulating miR-21/TIMP3 axis. Evid Based Complement Alternat Med 2020;2020:2892917.
90. Tork OM, Khaleel EF, Abdelmaqsoud OM. Altered cell to cell communication, autophagy and mitochondrial dysfunction in a model of hepatocellular carcinoma: potential protective effects of curcumin and stem cell therapy. Asian Pac J Cancer Prev 2015;16:8271-9.
91. Bortel N, Armeanu-Ebinger S, Schmid E, et al. Effects of curcumin in pediatric epithelial liver tumors: inhibition of tumor growth and alpha-fetoprotein in vitro and in vivo involving the NFkappaB- and the beta-catenin pathways. Oncotarget 2015;6:40680-91.
92. Liu J, Shen H, Ong C. Salvia miltiorrhiza inhibits cell growth and induces apoptosis in human hepatoma HepG2 cells. Cancer Letters 2000;153:85-93.
93. Liu J, Shen HM, Ong CN. Role of intracellular thiol depletion, mitochondrial dysfunction and reactive oxygen species in Salvia Miltiorrhiza-induced apoptosis in human hepatoma HepG2 cells. Life Sciences 2001;69:1833-50.
94. Jiang Y, Zhang L, Rupasinghe HP. Antiproliferative effects of extracts from Salvia officinalis L. and Saliva miltiorrhiza Bunge on hepatocellular carcinoma cells. Biomed Pharmacother 2017;85:57-67.
95. Hu S, Chen SM, Li XK, Qin R, Mei ZN. Antitumor effects of chi-shen extract from Salvia miltiorrhiza and Paeoniae radix on human hepatocellular carcinoma cells. Acta Pharmacol Sin 2007;28:1215-23.
96. Huang XY, Wang L, Huang ZL, Zheng Q, Li QS, Tang ZY. Herbal extract “Songyou Yin” inhibits tumor growth and prolongs survival in nude mice bearing human hepatocellular carcinoma xenograft with high metastatic potential. J Cancer Res Clin Oncol 2009;135:1245-55.
97. Liu X, Yang Y, Zhang X, et al. Compound Astragalus and Salvia miltiorrhiza extract inhibits cell invasion by modulating transforming growth factor-beta/Smad in HepG2 cell. J Gastroenterol Hepatol 2010;25:420-6.
98. Rui W, Xie L, Liu X, et al. Compound Astragalus and Salvia miltiorrhiza extract suppresses hepatocellular carcinoma progression by inhibiting fibrosis and PAI-1 mRNA transcription. J Ethnopharmacol 2014;151:198-209.
99. Hu X, Rui W, Wu C, et al. Compound Astragalus and Salvia miltiorrhiza extracts suppress hepatocarcinogenesis by modulating transforming growth factor-β/Smad signaling. J Gastroenterol Hepatol 2014;29:1284-91.
100. Boye A, Wu C, Jiang Y, et al. Compound Astragalus and Salvia miltiorrhiza extracts modulate MAPK-regulated TGF-β/Smad signaling in hepatocellular carcinoma by multi-target mechanism. J Ethnopharmacol 2015;169:219-28.
101. Wu C, Kan H, Hu M, et al. Compound Astragalus and Salvia miltiorrhiza extract inhibits hepatocarcinogenesis via modulating TGF-β/TβR and Imp7/8. Exp Ther Med 2018;16:1052-60.
102. Wu C, Chen W, Fang M, et al. Compound Astragalus and Salvia miltiorrhiza extract inhibits hepatocellular carcinoma progression via miR-145/miR-21 mediated Smad3 phosphorylation. J Ethnopharmacol 2019;231:98-112.
103. Liu L, Jia J, Zeng G, et al. Studies on immunoregulatory and anti-tumor activities of a polysaccharide from Salvia miltiorrhiza Bunge. Carbohydr Polym 2013;92:479-83.
104. Lee WY, Cheung CC, Liu KW, et al. Cytotoxic effects of tanshinones from Salvia miltiorrhiza on doxorubicin-resistant human liver cancer cells. J Nat Prod 2010;73:854-9.
105. Lee WY, Liu KW, Yeung JH. Reactive oxygen species-mediated kinase activation by dihydrotanshinone in tanshinones-induced apoptosis in HepG2 cells. Cancer Lett 2009;285:46-57.
106. Zheng L, Zhang Y, Liu G, et al. Tanshinone I regulates autophagic signaling via the activation of AMP-activated protein kinase in cancer cells. Anticancer Drugs 2020;31:601-8.
107. Chang JH, Lin CH, Shibu MA, et al. Cryptotanshinone (Dsh-003) from Salvia miltiorrhiza Bunge inhibits prostaglandin E2-induced survival and invasion effects in HA22T hepatocellular carcinoma cells. Environ Toxicol 2018;33:1254-60.
108. Zhu P, Liu Z, Zhou J, Chen Y. Tanshinol inhibits the growth, migration and invasion of hepatocellular carcinoma cells via regulating the PI3K-AKT signaling pathway. Onco Targets Ther 2019;12:87-99.
109. Lin CY, Chang TW, Hsieh WH, et al. Simultaneous induction of apoptosis and necroptosis by Tanshinone IIA in human hepatocellular carcinoma HepG2 cells. Cell Death Discov 2016;2:16065.
110. Yuxian X, Feng T, Ren L, Zhengcai L. Tanshinone Ii-A Inhibits invasion and metastasis of human hepatocellular carcinoma cells in vitro and in vivo. Tumori 2009;95:789-95.
111. Dai ZK, Qin JK, Huang JE, Luo Y, Xu Q, Zhao HL. Tanshinone IIA activates calcium-dependent apoptosis signaling pathway in human hepatoma cells. J Nat Med 2012;66:192-201.
112. Hong JY, Park SH, Park HJ, Lee SK. Anti-proliferative effect of 15,16-dihydrotanshinone i through cell cycle arrest and the regulation of AMP-activated protein kinase/Akt/mTOR and mitogen-activated protein kinase signaling pathway in human hepatocellular carcinoma cells. J Cancer Prev 2018;23:63-9.
113. Han Z, Liu S, Lin H, et al. Inhibition of murine hepatoma tumor growth by cryptotanshinone involves TLR7-dependent activation of macrophages and induction of adaptive antitumor immune defenses. Cancer Immunol Immunother 2019;68:1073-85.
114. Zhang X, Zhang P, An L, et al. Miltirone induces cell death in hepatocellular carcinoma cell through GSDME-dependent pyroptosis. Acta Pharm Sin B 2020;10:1397-413.
115. Lu HL, Su YC, Lin MC, Sun MF, Huang ST. Integrating Chinese and Western medicines reduced the incidence of hepatocellular carcinoma in patients with diabetes mellitus: a Taiwanese population-based cohort study. Complement Ther Med 2020;49:102332.
116. Padma V, Arul Diana Christie S, Ramkuma KM. Induction of apoptosis by ginger in HEp-2 cell line is mediated by reactive oxygen species. Basic Clin Pharmacol Toxicol 2007;100:302-7.
117. Elkady AI, Abu-Zinadah OA, Hussein RAEH. Crude flavonoid extract of medicinal herb. Zingibar officinale ;25:897-912.
118. Wang Y, Wang S, Song R, et al. Ginger polysaccharides induced cell cycle arrest and apoptosis in human hepatocellular carcinoma HepG2 cells. Int J Biol Macromol 2019;123:81-90.
119. Weng CJ, Wu CF, Huang HW, Ho CT, Yen GC. Anti-invasion effects of 6-shogaol and 6-gingerol, two active components in ginger, on human hepatocarcinoma cells. Mol Nutr Food Res 2010;54:1618-27.
120. Weng CJ, Chou CP, Ho CT, Yen GC. Molecular mechanism inhibiting human hepatocarcinoma cell invasion by 6-shogaol and 6-gingerol. Mol Nutr Food Res 2012;56:1304-14.
121. Nazim UM, Park SY. Attenuation of autophagy flux by 6-shogaol sensitizes human liver cancer cells to TRAIL-induced apoptosis via p53 and ROS. Int J Mol Med 2019;43:701-8.
122. Wu JJ, Omar HA, Lee YR, et al. 6-Shogaol induces cell cycle arrest and apoptosis in human hepatoma cells through pleiotropic mechanisms. Eur J Pharmacol 2015;762:449-58.
123. Hu R, Zhou P, Peng YB, et al. 6-Shogaol induces apoptosis in human hepatocellular carcinoma cells and exhibits anti-tumor activity. in vivo ;7:e39664.
124. Chen CY, Tai CJ, Cheng JT, et al. 6-dehydrogingerdione sensitizes human hepatoblastoma Hep G2 cells to TRAIL-induced apoptosis via reactive oxygen species-mediated increase of DR5. J Agric Food Chem 2010;58:5604-11.
125. Yang G, Zhong L, Jiang L, et al. Genotoxic effect of 6-gingerol on human hepatoma G2 cells. Chem Biol Interact 2010;185:12-7.
126. Yang G, Wang S, Zhong L, et al. 6-Gingerol induces apoptosis through lysosomal-mitochondrial axis in human hepatoma G2 cells. Phytother Res 2012;26:1667-73.
127. Hamza AA, Heeba GH, Hamza S, Abdalla A, Amin A. Standardized extract of ginger ameliorates liver cancer by reducing proliferation and inducing apoptosis through inhibition oxidative stress/ inflammation pathway. Biomed Pharmacother 2021;134:111102.
128. Habib SH, Makpol S, Abdul Hamid NA, Das S, Ngah WZ, Yusof YA. Ginger extract (Zingiber officinale) has anti-cancer and anti-inflammatory effects on ethionine-induced hepatoma rats. Clinics (Sao Paulo) 2008;63:807-13.
129. Mansour MA, Bekheet SA, Al-Rejaie SS, et al. Ginger ingredients inhibit the development of diethylnitrosoamine induced premalignant phenotype in rat chemical hepatocarcinogenesis model. Biofactors 2010;36:483-90.
130. Yusof YA, Ahmad N, Das S, Sulaiman S, Murad NA. Chemopreventive efficacy of ginger (Zingiber officinale) in ethionine induced rat hepatocarcinogenesis. Afr J Tradit Complement Altern Med 2008;6:87-93.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Khalil M, Calasso M, Bonfrate L, Di Ciaula A, De Angelis M, Wang DQH, Portincasa P. The pros and cons of biological effects of herbs and herb-derived compounds on liver tumorigenesis . Hepatoma Res 2022;8:23. http://dx.doi.org/10.20517/2394-5079.2022.04
AMA Style
Khalil M, Calasso M, Bonfrate L, Di Ciaula A, De Angelis M, Wang DQH, Portincasa P. The pros and cons of biological effects of herbs and herb-derived compounds on liver tumorigenesis . Hepatoma Research. 2022; 8: 23. http://dx.doi.org/10.20517/2394-5079.2022.04
Chicago/Turabian Style
Khalil, Mohamad, Maria Calasso, Leonilde Bonfrate, Agostino Di Ciaula, Maria De Angelis, David Q. H. Wang, Piero Portincasa. 2022. "The pros and cons of biological effects of herbs and herb-derived compounds on liver tumorigenesis " Hepatoma Research. 8: 23. http://dx.doi.org/10.20517/2394-5079.2022.04
ACS Style
Khalil, M.; Calasso M.; Bonfrate L.; Di Ciaula A.; De Angelis M.; Wang DQH.; Portincasa P. The pros and cons of biological effects of herbs and herb-derived compounds on liver tumorigenesis . Hepatoma. Res. 2022, 8, 23. http://dx.doi.org/10.20517/2394-5079.2022.04
About This Article
Copyright
Data & Comments
Data
 Cite This Article 9 clicks
Cite This Article 9 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.