Histopathology of hepatocellular carcinoma - when and what
Abstract
When do you need to take biopsies of the liver, and what information will you get is the topic of this review on hepatocellular carcinoma (HCC). If, clinically, the differential diagnosis of HCC after imaging is suggested, a biopsy has become obligatory as a diagnostic confirmation of HCC in the non-cirrhotic liver prior to definitive therapeutic interventions, as well as in a palliative therapy concept. In the case of hepatic lesions with an uncharacteristic contrast uptake, a biopsy should be performed immediately to confirm the diagnosis of HCC. After diagnosing HCC, a treatment strategy is evaluated. Further, the biopsy, or in case of surgical treatment, the resected tissue, shows us the different subtypes of HCC, with the steatohepatitic subtype being the most common and the lymphocyte-rich subtype being the least common. Further, the histological grade of HCC is determined according to the grading system of the WHO or the Edmonson and Steiner System. Through biopsies, HCC can be differentiated from intrahepatic cholangiocarcinoma or combined hepatocellular-cholangiocarcinoma or metastases of other malignant tumors, especially metastases of the gastrointestinal tract. In summary, biopsies are fundamental in the diagnosis of HCC.
Keywords
INTRODUCTION, DEFINITION, AND EPIDEMIOLOGY
Hepatocellular carcinoma (HCC) is a primary liver tumor with hepatocytic differentiation. HCC arises from the hepatocyte in various stages of differentiation. The incidence of HCC in Europe and the United States will continue to increase with the progression of hepatitis C, nonalcoholic steatohepatitis, and obesity with diabetes mellitus and metabolic syndrome[1,2]. In Germany, around 9000 new cases of HCC occur each year, with an incidence of 6.4/100,000 for men and 1.5/100,000 for women[3-5]. Clinically, the tumor marker for HCC is alpha-fetoprotein (AFP). However, it has significant limitations in terms of sensitivity (39% to 64%) and specificity (76% to 91%). AFP levels greater than 20 ng/mL, as well as lower but slowly increasing levels, are a serious indication for HCC. Levels greater than 200 ng/mL are highly suspicious for diagnosis of HCC if positive on imaging. However, AFP levels do not correlate very closely with the size of the HCC. Other specific and sensitive HCC markers have been searched for decades. Several alternative markers have been investigated, for example, des-gamma-carboxyprothrombin, glypican-3, AFP fractions, but they are not yet used in clinical practice[6-8]. In almost all cases, prior to therapy, a precise histopathological diagnosis is mandatory, especially in tumors without cirrhosis. The histological HCC subtyping should be done according to the latest World Health Organization (WHO) classification. Next to tumor typing (i.e., HCC determination, subtyping, and ruling out intrahepatic cholangiocarcinoma), the grade of tumor differentiation (“grading”) is necessary. There are three grading systems for the HCC. The HCC-specific grading of Edmondson and Steiner described by Edmondson and Steiner[9], the grading according to Nzeako et al.[10,11], and the International Union Against Cancer (UICC) grading[12]. The general UICC grading is not entity-specific, is less detailed, and is explicitly rarely applied in the context of HCC. Grading, according to Edmondson and Steiner, is recommended by UICC in HCC[12]. Nzeako et al.[10,11] grading is more commonly used in the European and American regions. This grading is based purely on nuclear features. Staging of HCC must be performed according to the current Malignant Tumours (TNM) classification[13]. Another point of histopathological diagnosis is the early detection of precancerous lesions in cirrhotic and non-cirrhotic liver.
WHEN-BIOPSY
Due to the increase of HCC to the 5th most common cancer in Europe and worldwide, there has been an update of the guidelines on treatment strategies, especially concerning when to take biopsies[14,15]. In 2013, it was recommended that a biopsy should only be obtained if it has a therapeutic consequence. In 2021, biopsy has been strengthened as an important diagnostic measure. The diagnostic algorithm has changed so that lesions greater than 1 cm in size should now receive an imaging examination rather than only lesions greater than 2 cm. If a lesion shows the characteristic contrast enhancement, a curative or palliative therapy concept can be initiated depending on the size and extension of the HCC. Interestingly, the detection rate of small lesions has increased in recent years, leading to new therapeutic strategies[16-18]. In particular, image-guided tumor ablation for early-stage HCC gained attention as a therapeutic strategy. This therapy is a non-surgical treatment that provides local tumor control, and the patients have more favorable survival benefits. Before ablation, a biopsy is taken for the histopathological examination to confirm the diagnose of HCC. The biopsy should be taken prior to ablation to reduce necrosis in the tissue to be examined[19-21]. All in all, biopsy has become obligatory as a diagnostic confirmation of HCC in the non-cirrhotic liver before definitive therapeutic interventions, as well as in a palliative therapy concept. In the case of hepatic lesions with an uncharacteristic contrast uptake, a biopsy should be performed immediately to confirm the diagnosis of HCC. Further, if a palliative therapy concept is considered, a biopsy should be performed to exclude other differential diagnoses and to confirm HCC[22]. In the circumstance of a curative concept, further imaging should be performed. If this second imaging continues to show uncharacteristic contrast enhancement, a biopsy is an obligatory procedure. The interpretation of the biopsy and the diagnosis of HCC can be made in many cases by conventional histology. Depending on the histopathological appearance, further examinations, in particular immunohistochemistry, can be used to confirm the diagnosis[23-25].
For an adequate histopathological interpretation of the biopsy, the specimen must be taken from the lesion and must be of sufficient size[26]. To obtain a relevant sample of a focal liver lesion, image guidance, with either real-time ultrasound, computer tomography, or magnetic resonance imaging, is necessary to place the needle accurately within the lesion[27]. Further studies claim that a longer sample size increases the chance of a possible adequate histopathological interpretation[28-30]. The guidelines on the use of liver biopsies in clinical practice from the British Society of Gastroenterology, the Royal College of Radiologists, and the Royal College of Pathology recommend a sample that should be at least 20 mm[26,31]. In general, only one pass is required if an adequate sample is obtained. Nevertheless, studies have demonstrated a more accurate diagnose with an increased number of needle passes. Three or more passes should not be performed as this significantly increases the risk of complications and morbidity[32-34]. The current state of knowledge doesn’t specify if a lesion biopsy should be taken through adjacent liver tissue to minimize the risk of so-called “punch channel tumor spreading”. How biopsies should be taken depends on the case and the localization of the lesion, and they should be taken with the lowest impact on the liver and patient. In consensus with our hepatologists, a biopsy should be taken as directly as possible. The complication rate of biopsy of HCC is low and includes the risk of hemorrhage and so-called “punch channel tumor spreading”. Minor bleeding requires no treatment and occurs in approximately 3%-4% of cases. Bleeding requiring transfusion is rare (0.5%)[35]. Meta-analyses have shown that “punch channel tumor spreading” is also rare (2.7%). They typically occur late, approximately after a mean of 17 months, and are usually well treatable and have no negative impact on survival as well as on the success of therapeutic measures[36-38]. Another indication for a biopsy can be a portal vein thrombosis. Portal vein thrombosis may be either portal vein tumor thrombosis or benign portal vein thrombosis. It is important to distinguish between them as they are relevant to patient outcome and therapeutic strategies[39-41]. In particular, when HCC occurs in a cirrhotic liver, the differentiation can be difficult, as benign portal vein thrombosis often occurs in cirrhotic livers. The first diagnostic procedure for differentiation is imaging[42-44]. However, if imaging does not fulfill the characteristic criteria of portal vein tumor thrombosis or benign portal vein thrombosis, an ultrasound-guided fine-needle aspiration biopsy can be performed for differentiation[44,45].
In any case, a malignant liver tumor cannot be diagnosed without a histological examination since the classification of malignant liver tumors is based on histological criteria. Overall, histopathology is the basis of HCC diagnosis and its differential diagnoses. If any concerns occur concerning a clinical diagnose of HCC, a biopsy should be considered. The criteria for the current diagnosis of malignant liver tumors are summarized in the 5th edition of the WHO Classification of Tumors of the Digestive Tract[46-48]. If the patient’s history does not include information regarding the underlying liver disease, an additional biopsy of liver tissue could be obtained to evaluate whether fibrosis or cirrhosis is presented. This information is essential for further therapeutical decision-making and patient outcome. In this case, the Royal College of Pathologists updated guidelines suggesting a minimum adequacy requirement for samples being at least
WHAT YOU GET AFTER BIOPSY OR RESECTION
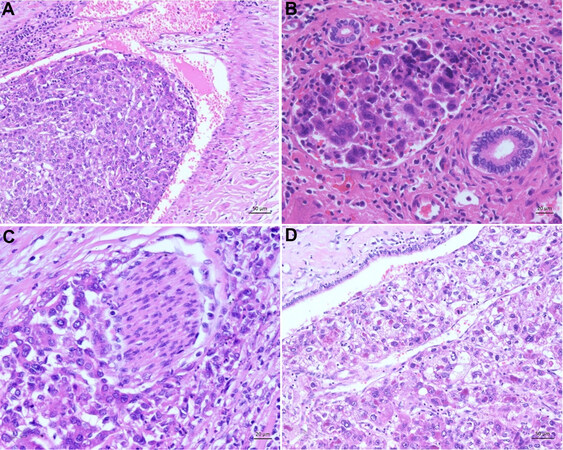
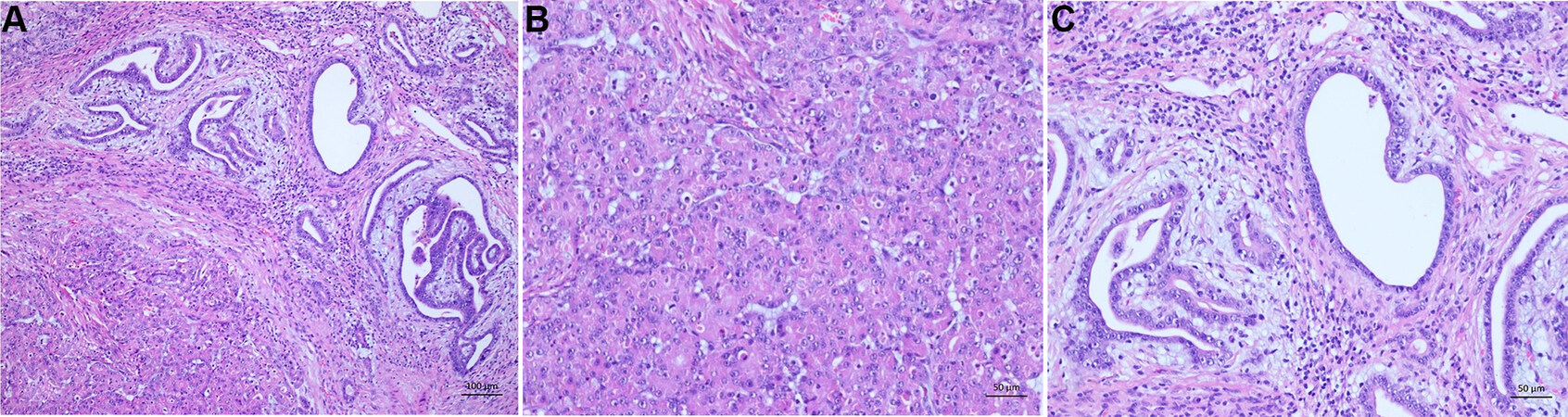
Not only the histological subtype, the primary resectability or the degree of liver cirrhosis are important factors to evaluate the prognosis and outcome for the patient. It is further important to look at the macroscopic appearance of the tumor. HCCs occur as solid, green to yellow nodes of various sizes in the liver. The green to yellow color is caused by the bilious imbibition and the fat content of the tumor. Compared to the non-neoplastic liver tissue and especially in cirrhotic liver, HCCs appear as a soft tumor. In cirrhotic livers, they have additionally a pseudocapsule. Little has been reported on the role of macroscopic classification of HCC and the prognostic role. However, the macroscopic classification of HCC might have a strong correlation with long-term prognosis after hepatectomy[50]. Historically, four types of HCC were described by Eggel in 1901. He divided the neoplasms into the expansive (nodule), infiltrative, mixed (expansive and infiltrative), and diffuse types. The prevalence among cases are: 20% nodule, 33% infiltrative, 42% mixed, and 5% diffuse. The data published by Eggel are based on HCCs arising in cirrhosis[51]. Nowadays, the exclusive morphological classification by Eggel has been mostly left behind since it does not allow conclusions to be made concerning the etiology, clinical course, or prognosis[52,53]. Besides this, the macroscopic description of the HCC still has an important clinical impact when it comes to describing the size of the primary tumor, the size of additional tumor nodes with correlation to the primary tumor, and tumor vessel invasion. For example, for tumor size, there was a significant difference between tumors smaller than 5 cm and those larger than this. In tumors measuring less than 5 cm, intrahepatic metastasis, portal vein involvement, and lymphogenous and hematogenous metastasis were not common. However, these frequencies became significantly higher if the tumor exceeded 5 cm. Comparing the percentages of tumors less than 5 cm and greater than 5 cm, intrahepatic metastases were observed in 60.6% and 95.7%, portal vein involvement in 40% and 74.5%, lymph node metastases in 0% and 40.4%, and hematogenous metastases in 6.7% and 51.1%, respectively[53]. Further, the macroscopic description of whether there is vessel invasion is relevant to TNM. HCCs often appear hypervascular and have close contact with the vessels [Figure 1A-D][54,55]. If a second tumor occurs in the liver, the distance to the first and biggest one must be described. Additionally, it must be ensured that they were separated by non-neoplastic liver tissue. Whether this second tumor is an intrahepatic metastasis or a second synchronic or heterochinic tumor is still discussed[56-59]. Tumors arising within a radius of less than 2 cm to the primary tumor, and if they are smaller than the primary tumor, are called satellite nodules. Another special anatomic appearance of HCC is the pedunculated HCC. It is a rare subtype, which can be further categorized by its presence or absence of a pedicle attaching it to the liver[60-62]. Especially the macroscopic described tumor size is relevant for the WHO grading system. This grading system for HCC proposed by the WHO takes not only the tumor size but also the architecture and the extent of cell and nuclear pleomorphism (including sarcomatoid or anaplastic morphology) into account[12,48].
HISTOLOGICAL SUBTYPES
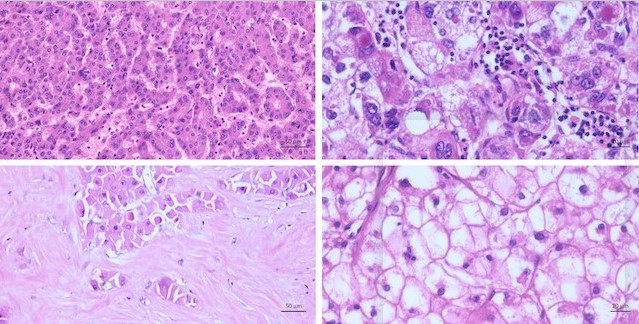
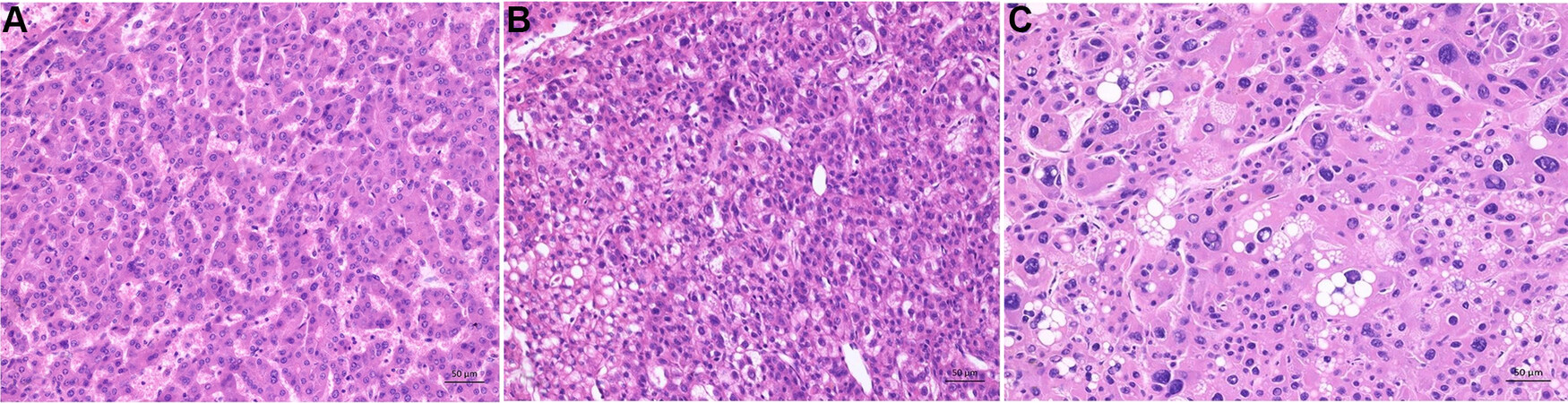
There are various numbers of different subtypes of HCC. They differ by morphology and/or immunohistochemistry. All different types of HCC show cytological atypia varying from minimal to marked, and the tumor cells show an increased proliferation. Characteristic cellular changes of HCC include bile production, lipofuscin deposits, glycogen accumulation leading to clear cell changes, and fatty changes
Figure 2. Characteristic cellular changes in hepatocellular carcinoma. (A) Tumor cells with bile production. (B) Fatty changes with intracytoplasmic accumulation of lipids. (C) Glycogenated nuclei. (D) Hyaline bodies (Mallory Denk bodies): eosinophilic cytoplasmic inclusions.
There are four principal growth patterns of HCCs: trabecular, solid (syn.: compact), pseudoglandular (syn.: pseudocarina), and macrotrabecular [Figure 3A-C]. Half of all resected HCCs have mixed patterns. It is mostly a combination of trabecular plus one or two others. The pathologist may recognize the growth pattern, but it must not be described since it does not have any clinical relevance. However, in a recently published study suggests there might be an association between the macrotrabecular pattern and a worse prognosis. A special pattern is nodule-in-nodule growth. In this case, a nodule of poor differentiation arises within an existing HCC[63].
Figure 3. Histological growth pattern. (A) Trabecular pattern: thin tumor trabeculaes with not more than ten cells in thickness. (B) Pseudoglandular pattern: glandular-like or acinus-like structures with minimal atypia. (C) Macrotrabecular Pattern: trabecular structures with more than 10 cells in thickness, increased nuclear:cytoplasma ratio.
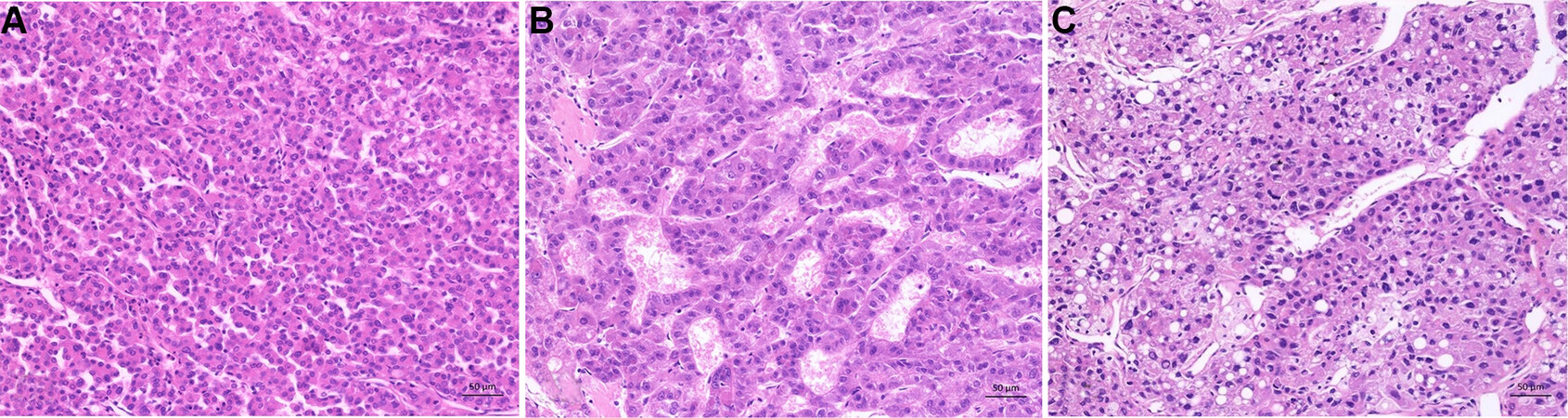
The histological grading system of the WHO is based on H&E staining and compares the differentiation of the tumor cell with the benign hepatocytes. The WHO proclaims a three-stage system. There are well-differentiated (G1), moderately differentiated (G2), and poorly differentiated (G3) HCCs [Table 1, Figure 4A-C]. Undifferentiated tumors are excluded from this system because they have no compelling evidence for being either hepatocellular or biliary and are not a grade of HCC [Table 1][12]. Some HCC may have intratumoral heterogeneity concerning the grade. In this case, the predominant grade or a combination of the worst can be reported, due to the fact that the worst grade tends to drive the prognosis. A uniform worldwide used grading system remains to be developed. One example of a currently often used grading system is the Edmonson and Steiner System, which favors a three-tiered grading system [Table 2]. It was developed by Edmonson and Steiner in 1954 and is based on nuclear-cytoplasmic ratio, the grade of acidophilic, chromatin content, the bile production, and the histological architecture[9].
Figure 4. Histological grading of hepatocellular carcinoma according to World Health Organization. (A) Well differentiated hepatocellular carcinoma: thin trabecular structures and minimal atypia of tumor cells resembling mature hepatocytes. (B) Moderately differentiated hepatocellular carcinoma: trabecular pattern with at least 3 cells in thickness, moderate nuclear atypia with increased nuclear:cytoplasma ratio, microvesicular fatty changes (lower left). (C) Poorly differentiated hepatocellular carcinoma: marked pleomorphic tumor cells with fatty changes, prominent nucleoli und anaplastic giant cells.
World Health Organization[9]
| Grades | Architecture | Cytology | Other features |
| Well differentiated | Thin trabecular, frequent acinar structures | Minimal atypia | Fatty change is frequent |
| Moderately differentiated | Trabecular (3 or more cells in thickness) and acinar | Abundant eosinophilic cytoplasm, round nuclei with distinct nucleoli | Bile or proteinaceous fluid within acini |
| Poor differentiated | Solid | Moderate to marked pleomorphism | Absence of sinusoid-like blood spaces |
| Undifferentiated | Solid | Little cytoplasm, spindle, or round-shaped cells | - |
Edmondson and Steiner[9]
| Grades | Architecture | Cytology | Other features |
| I | - | - | Areas of carcinoma where distinction from hyperplastic liver is difficult |
| II | Trabecular, frequent acini (lumen varying from tiny canaliculi to large thyroid-like spaces) | Resemblance to normal hepatic cells; larger nuclei; abundant acidophilic cytoplasm | Cell borders sharp and clear cut; acini containing bile or protein precipitate |
| III | Distortion of trabecular structure, acini less frequent than grade II | Larger, more hyperchromatic nuclei, granular but less acidophilic cytoplasm | Acini are less frequent; tumor giant cells may be numerous |
| IV | Medullary, less trabeculae, rare acini | Highly hyperchromatic nuclei, scanty cytoplasm, with fewer granules | Loss of cell cohesiveness; giant, spindle or short-plump cells can be found |
Of the HCCs, 35% can be further classified into eight distinct subtypes, which will be described below. All subtypes except the fibrolamellar, which only occurs in non-cirrhotic livers, have been described in cirrhotic and non-cirrhotic liver. The most common type is the steatohepatitic HCC[64,65].
Immunohistochemically, HCCs show an antibody reaction against carbamoylphosphat-synthetase-1 (HEP PAR1). The poorer the degree of differentiation of HCC, the lower the expression of HEP PAR1[66]. Further, an expression of CD10, cytokeratin-8, and cytokeratin-18 can be obtained[67]. There is no expression of cytokeratin-20 as well as epithelial membrane antigen (EMA). Cytokeratin-7 and -19 usually are not expressed but may be observed in high-grade HCC. AFP expression usually indicates malignancy and can be seen in 50% of cases. Further, glypican 3 can be used as a diagnostic marker for differentiating between HCC from non-malignant liver lesions. A high glypican 3 positive staining significantly correlates with later tumor stage, higher tumor grade, presence of vascular invasion, shortened overall survival, and shortened disease-free survival. Shafizadeh et al.[68] reported an expression of glypican 3 in 9/16 (56%) well, 15/18 (83%) moderately, and 22/24 (89%) poorly differentiated HCC. A diffuse expression can be seen of the 70 kilodalton heat shock proteins (HSP 70) and glutamine synthetase[68-71]. In routine clinical work, immunohistochemistry is of limited value in the differential diagnosis. In most cases, the diagnosis of HCC can be based on the H&E staining.
Steatohepatitic hepatocellular carcinoma
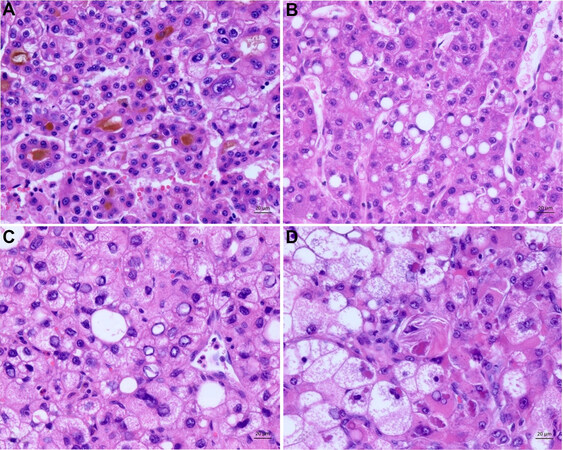
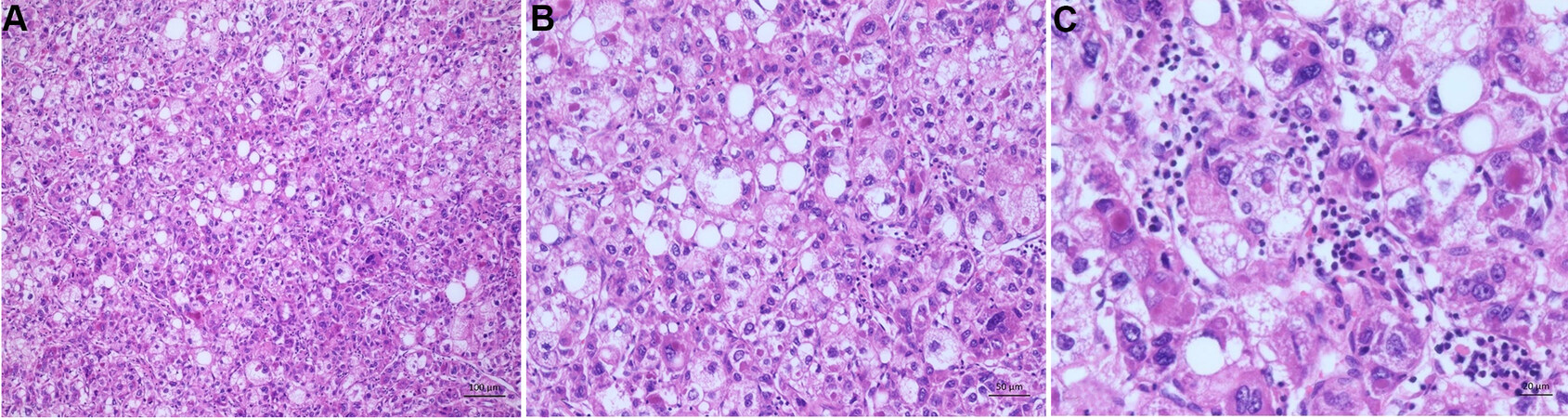
The steatohepatitic subtype is the most common, with 5%-20% prevalence among HCCs. The tumor cells show histological features of steatohepatitis, such as containing fatty vacuoles, an intratumoral stroma reaction, and inflammation. Further, it is often associated with metabolic syndrome and steatohepatitis or even cirrhosis of the liver [Figure 5A-C][72-75].
Figure 5. Steatohepatitic subtype. (A) Overview of the steatohepatitic tumor. The tumor cells show histological features of steatohepatitis by containing fatty vacuoles. Further inflammation and an intratumoral stroma reaction is seen. (B) Higher magnification of the tumor. Tumor shows features of steatohepatitis tumor cells with fatty vacuoles and tumor cell ballooning. (C) The tumors characteristic intratumoral inflammation and hyaline bodies (Mallory Denk bodies) are seen here.
Clear cell hepatocellular carcinoma
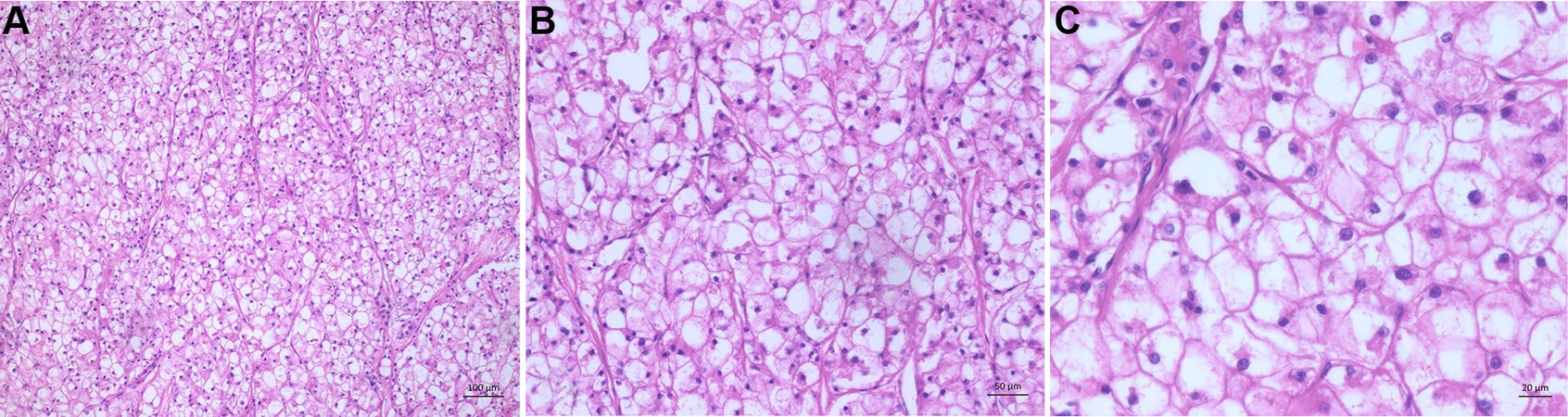
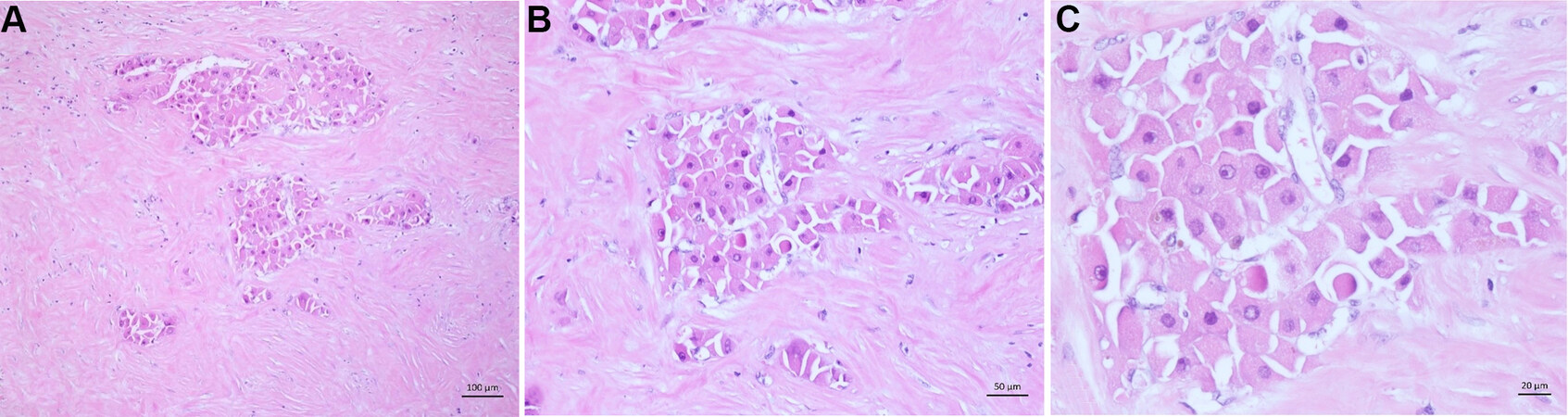
With 3%-7% prevalence, it is the second most common subtype. Of the tumor cells, 80% show a clear cell morphology with a light cytoplasm and minor nuclear atypia. The “brightness” of the cells occurs because the tumor cells accumulate glycogen [Figure 6A-C][76-78].
Macrotrabecular (massive) hepatocellular carcinoma
To classify this subtype, there must be at least 50% of a macrotrabecular growth pattern[79,80]. The tumor occurs in 5% of the cases and is associated with a worse prognosis. Clinically, a high serum
Scirrhous hepatocellular carcinoma
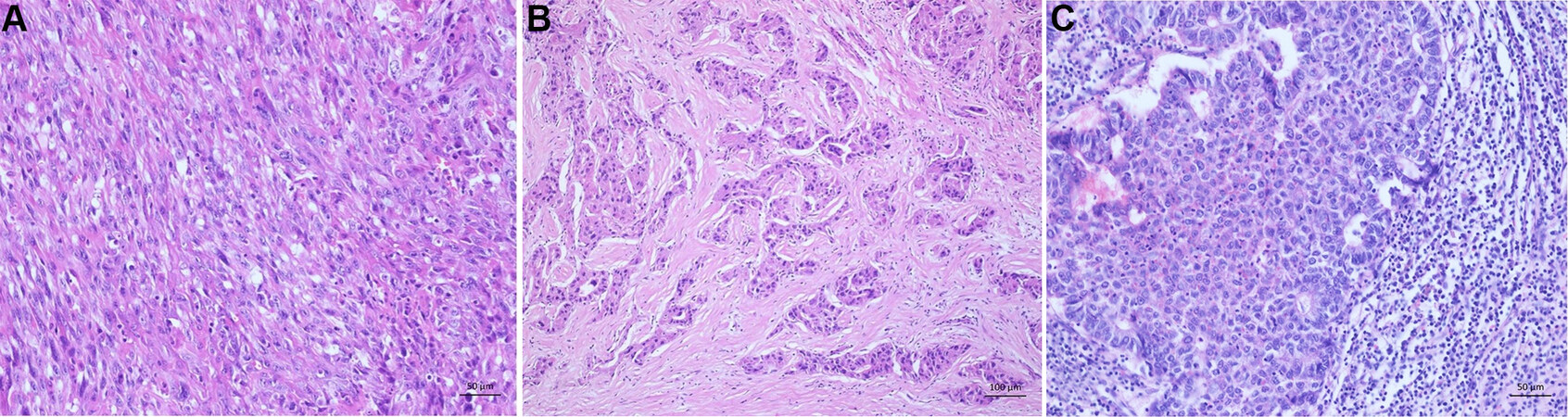
Four percent of all HCCs show a scirrhous subtype. This tumor subtype shows dense intratumoral fibrosis of greater than 50%, which arouses especially in association with the sinusoidal capillarization leading to an atrophy of the tumor trabecular[60,82-84]. After chemotherapy or radiation of HCC, the tumor may mimic this subtype. Clinically, this subtype mimics a cholangiocarcinoma on imaging [Figure 7B][60,82,85].
Figure 7. Rare histological subtypes. (A) Sarcomatoid subtype: poorly differentiated HCC with spindle cell morphology resembling sarcoma. (B) Scirrhous subtype: dense intratumoral fibrosis. (C) Neutrophil-rich subtype: poorly differentiated hepatocellular carinoma with numerous intratumoral neutrophils.
Chromophobe hepatocellular carcinoma
Three percent of the subtypes show a light cytoplasm with outlined cell borders. The tumor nuclei are mainly bland. However, focal areas have striking nuclear atypia, such as two nuclei within one tumor cell. Further, giant cells can be found.
Fibrolamellar hepatocellular carcinoma
Of all subtypes, 0.5%-1% are fibrolamellar HCCs. Compared to the other subtypes, they often occur in young patients with a median age of 25 years and only occur in non-cirrhotic livers[86-88]. So far, no association with chronic liver disease or specific risk factors has been described[89-91]. Due to their macroscopic appearance with a central scar and the fact that they are more common in women, they can be misdiagnosed as focal nodular hyperplasia[88,92,93]. The tumor cells are large and eosinophilic with prominent nuclei. Another characteristic for diagnosing this subtype is dense intratumoral fibrosis consisting of acellular collagen and pale bodies. The pale bodies are characteristic but not specific, and occur in the classic HCC as well[94] [Figure 8]. In some cases, glandular tumor cell configurations occur, which may lead to the misdiagnosis of combined hepatocellular cholangiocarcinoma [Figure 9][80].
Figure 8. Fibrolamellar subtype. (A) Overview of a fibrolamellar subtype. Scattered tumor cells with eosinophilic cytoplasm in a dense intratumoral fibrosis. (B) The dense intratumoral fibrosis consists of acellular collagen. Some tumor cells contain pale bodies. (C) The tumor cells are large and eosinophilic with prominent nuclei and some tumor cells contain pale bodies.
Figure 9. Combined hepatocellular-cholangiocarcinoma. (A) Typical form with moderately differentiated hepatocellular carcinoma (lower left) and well differentiated cholangiocarcinoma (upper right). Between both tumor components a fibrotic border is seen. (B) Hepatocellular carcinoma - component with trabecular pattern and moderate atypia. (C) Cholangiocarcinoma -component with tubular structures and mild atypia.
Neutrophil-rich hepatocellular carcinoma
This tumor has an incidence of less than 1% and mainly occurs in older individuals. It shows a histomorphologically significant intratumoral neutrophil-rich inflammation and can have sarcomatoid areas (for an illustration of sarcomatoid morphology refer to Figure 7A). The tumor’s key molecular finding is the production of granulocyte colony-stimulating factor, leading to dense areas of infiltrates with neutrophils. Further, some clinical findings, such as elevated leukocyte counts, C-reactive protein, and IL-6, support the diagnosis of a neutrophil-rich HCC [Figure 7C][64,95].
Lymphocyte-rich hepatocellular carcinoma
To diagnose this rare tumor, there must be more lymphocytes than tumor cells in most fields of the H&E staining. Characteristically, the cords of tumor cells lie in a dense lymphoid stroma. An association with Epstein-Barr virus has been frequently described, but new evidence shows no Epstein-Barr virus relationship, and most cases are negative for Epstein-Barr virus[95].
HISTOLOGICAL DIFFERENTIAL DIAGNOSIS
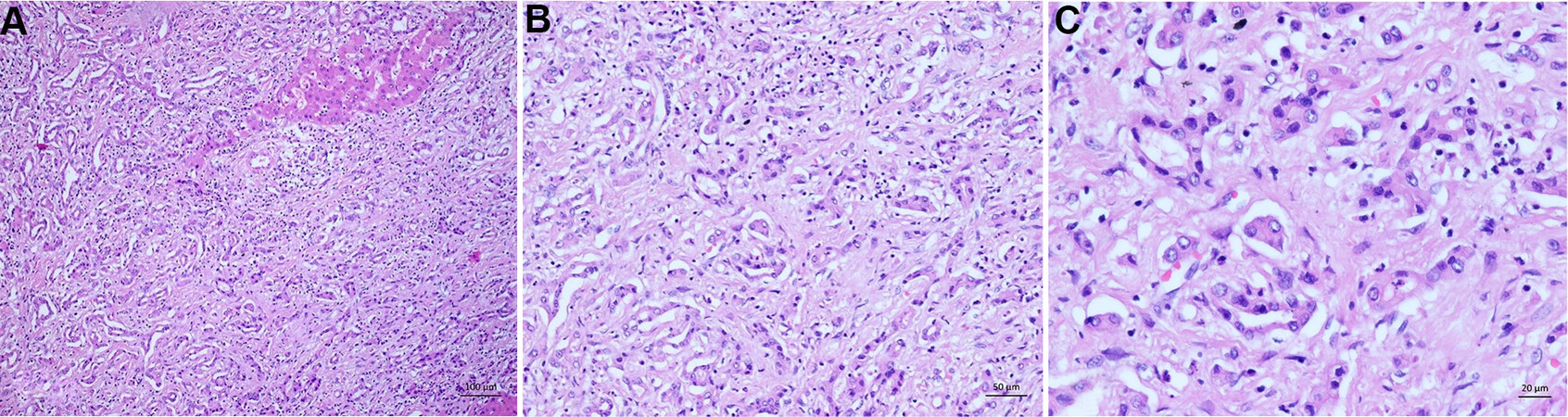
Histologically, HCC must be differentiated from combined hepatocellular-cholangiocarcinoma
Figure 10. Intrahepatic cholangiocarcinoma. (A) Overview of a moderately differentiated adenocarcinoma with tubular pattern infiltrating a dense fibrous stroma. (B) Infiltrative irregular glands with a prominent fibrodesmoplastic stromal reaction. (C) Higher magnification of the tumor showing the moderately pleomorphic tumor cells with eosinophilic cytoplasm.
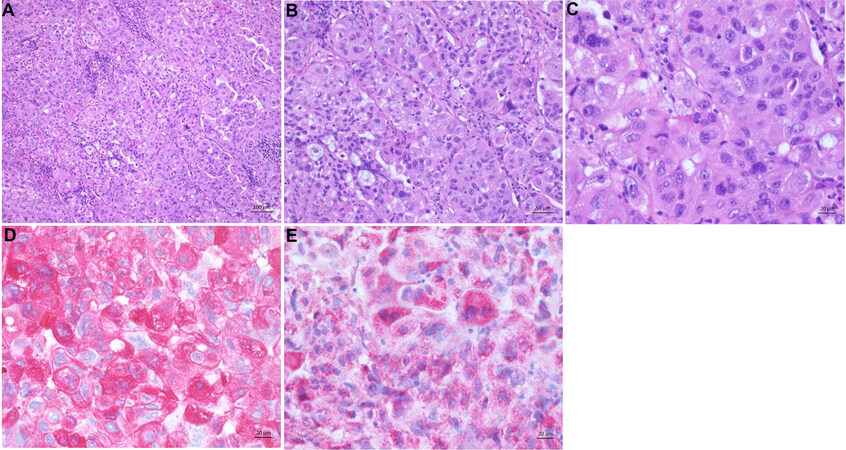
Figure 11. Hepatoid adenocarcinoma. (A) The tumor shows hepatocyte-like tumor cells and lymphoplasmacytic infiltrates localized to stroma and tumor parenchyma. (B) Moderate differentiated tumor cells forming thick trabeculae in the tumor. (C) Hepatocyte-like tumor cells with round nuclei and eosinophilic cytoplasm. (D) Tumor cells are strongly positive for CK7. (E) HepPar1 is also expressed.
CONCLUSION FOR THE CLINIC
Due to the increasing incidence and the histological variety of HCC, the histopathological diagnosis is essential for diagnosis and therapy of the tumor. Biopsy indications are well established in the diagnostic algorithm of HCC, even in cirrhotic liver. A biopsy should always be used in the palliative situation and when non-invasive methods are unequivocal. Especially if there is any doubt about the diagnosis of HCC, a biopsy should be considered in the first place.
DECLARATIONS
Authors’ contributionsMade substantial contributions to conception and design of the script: Gisder DM
Made substantial contributions to conception and design of the script: Tannapfel A
Made substantial contributions to conception and design of the script and selected the case images:
Not applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. GCO Global Cancer Observatory. The Global Cancer Observatory (GCO) is an interactive web-based platform presenting global cancer statistics to inform cancer control and research. Available from: https://gco.iarc.fr [Last accessed on 31 Dec 2021].
2. European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236.
3. ZfKD Zentrum für Krebsregisterdaten. Das Zentrum für Krebsregisterdaten (ZfKD) am Robert Koch-Institut in Berlin führt Daten der Landeskrebsregister auf Bundesebene zusammen. Available from: http://krebsdaten.de/ [Last accessed on 31 Dec 2021].
4. De Toni EN, Schlesinger-Raab A, Fuchs M, et al. Age independent survival benefit for patients with hepatocellular carcinoma (HCC) without metastases at diagnosis: a population-based study. Gut 2020;69:168-76.
5. Erhardt A, Theobald I, Petry W, et al. [Hepatocellular carcinoma: rising incidence of hepatitis C virus-associated cases at a university clinic in Germany]. Dtsch Med Wochenschr 2002;127:2665-8.
6. Zhou L, Liu J, Luo F. Serum tumor markers for detection of hepatocellular carcinoma. World J Gastroenterol 2006;12:1175-81.
7. Spangenberg HC, Thimme R, Blum HE. Serum markers of hepatocellular carcinoma. Semin Liver Dis 2006;26:385-90.
8. Nassar A, Cohen C, Siddiqui MT. Utility of glypican-3 and survivin in differentiating hepatocellular carcinoma from benign and preneoplastic hepatic lesions and metastatic carcinomas in liver fine-needle aspiration biopsies. Diagn Cytopathol 2009;37:629-35.
9. Edmondson HA, Steiner PE. Primary carcinoma of the liver. A study of 100 cases among 48,900 necropsies. Cancer 1954;7:462-503.
10. Nzeako UC, Goodman ZD, Ishak KG. Hepatocellular carcinoma in cirrhotic and noncirrhotic livers. A clinico-histopathologic study of 804 North American patients. Am J Clin Pathol 1996;105:65-75.
11. Nzeako UC, Goodman ZD, Ishak KG. Comparison of tumor pathology with duration of survival of north american patients with hepatocellular carcinoma. Cancer 1995;76:579-88.
12. Paradis VFM. PYSP, tumors of the liver and intrahepatic bile ducts. In: WHO Classification of Tumours Editorial Board WHO-Classification of Tumours. 5th ed. Digestive system tumours. International Agency for Research on Cancer; 2019. p. 215-64.
13. Wittekind C. TNM Klassifikation maligner Tumoren: Korrigierter Nachdruck 2020 mit allen Ergänzungen der UICC aus den Jahren 2017 b is 2019. 8th ed. Weinheim: Wiley-VCH; 2020.
14. Bray F, Ferlay J, Laversanne M, et al. Cancer incidence in five continents: inclusion criteria, highlights from volume X and the global status of cancer registration. Int J Cancer 2015;137:2060-71.
15. Altekruse SF, Henley SJ, Cucinelli JE, McGlynn KA. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol 2014;109:542-53.
16. Horigome H, Nomura T, Saso K, Itoh M, Joh T, Ohara H. Limitations of imaging diagnosis for small hepatocellular carcinoma: comparison with histological findings. J Gastroenterol Hepatol 1999;14:559-65.
17. Ikai I, Arii S, Kojiro M, et al. Reevaluation of prognostic factors for survival after liver resection in patients with hepatocellular carcinoma in a Japanese nationwide survey. Cancer 2004;101:796-802.
18. Kojiro M. Focus on dysplastic nodules and early hepatocellular carcinoma: an Eastern point of view. Liver Transpl 2004;10:S3-8.
19. Krücker J, Xu S, Glossop N, et al. Electromagnetic tracking for thermal ablation and biopsy guidance: clinical evaluation of spatial accuracy. J Vasc Interv Radiol 2007;18:1141-50.
20. Hakime A, Deschamps F, De Carvalho EG, Barah A, Auperin A, De Baere T. Electromagnetic-tracked biopsy under ultrasound guidance: preliminary results. Cardiovasc Intervent Radiol 2012;35:898-905.
21. Kang TW, Rhim H. Recent advances in tumor ablation for hepatocellular carcinoma. Liver Cancer 2015;4:176-87.
22. S3-leitlin. Diagnostik und Ther. des Hepatozellulären Karzinoms und biliärer Karzinome. Available from: https://www.leitlinienprogramm-onkologie.de/leitlinien/hcc-und-biliaere-karzinome/ [Last accessed on 19 October 2021].
23. Di Tommaso L, Destro A, Seok JY, et al. The application of markers (HSP70 GPC3 and GS) in liver biopsies is useful for detection of hepatocellular carcinoma. J Hepatol 2009;50:746-54.
24. Di Tommaso L, Franchi G, Park YN, et al. Diagnostic value of HSP70, glypican 3, and glutamine synthetase in hepatocellular nodules in cirrhosis. Hepatology 2007;45:725-34.
25. Koehne de Gonzalez AK, Salomao MA, Lagana SM. Current concepts in the immunohistochemical evaluation of liver tumors. World J Hepatol 2015;7:1403-11.
26. Neuberger J, Patel J, Caldwell H, et al. Guidelines on the use of liver biopsy in clinical practice from the British Society of Gastroenterology, the Royal College of Radiologists and the Royal College of Pathology. Gut 2020;69:1382-403.
27. Ichim VA, Chira RI, Mircea PA, Nagy GA, Crisan D, Socaciu MA. Accuracy of endoscopic ultrasound-guided biopsy of focal liver lesions. Med Ultrason 2020;22:20-5.
28. Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol 2003;39:239-44.
29. Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 2003;38:1449-57.
30. Schiano T, Azeem S, Bodian C, et al. Importance of specimen size in accurate needle liver biopsy evaluation of patients with chronic hepatitis C. Clin Gastroenterol Hepatol 2005;3:930-5.
31. Wyatt J, Hubscher S, Bellamy C. Tissue pathways for liver biopsies for the investigation of medical disease and for focal leasions. The Royal College of Pathologists 2014; doi: 10.1002/lt.21781.
32. Chi H, Hansen BE, Tang WY, et al. Multiple biopsy passes and the risk of complications of percutaneous liver biopsy. Eur J Gastroenterol Hepatol 2017;29:36-41.
33. Maharaj B, Bhoora IG. Complications associated with percutaneous needle biopsy of the liver when one, two or three specimens are taken. Postgrad Med J 1992;68:964-7.
34. Perrault J, Mcgill DB, Ott BJ, Taylor WF. Liver biopsy: complications in 1000 inpatients and outpatients. Gastroenterology 1978;74:103-6.
35. Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. American Association for the Study of Liver Diseases. Liver biopsy. Hepatology 2009;49:1017-44.
36. Silva MA, Hegab B, Hyde C, Guo B, Buckels JA, Mirza DF. Needle track seeding following biopsy of liver lesions in the diagnosis of hepatocellular cancer: a systematic review and meta-analysis. Gut 2008;57:1592-6.
37. Müllhaupt B, Durand F, Roskams T, Dutkowski P, Heim M. Is tumor biopsy necessary? Liver Transpl 2011;17 Suppl 2:S14-25.
38. Fuks D, Cauchy F, Fusco G, Paradis V, Durand F, Belghiti J. Preoperative tumour biopsy does not affect the oncologic course of patients with transplantable HCC. J Hepatol 2014;61:589-93.
39. Kokudo T, Hasegawa K, Yamamoto S, et al. Surgical treatment of hepatocellular carcinoma associated with hepatic vein tumor thrombosis. J Hepatol 2014;61:583-8.
40. Lee YH, Hsu CY, Huang YH, et al. Vascular invasion in hepatocellular carcinoma: prevalence, determinants and prognostic impact. J Clin Gastroenterol 2014;48:734-41.
41. Amitrano L, Guardascione MA, Brancaccio V, et al. Risk factors and clinical presentation of portal vein thrombosis in patients with liver cirrhosis. J Hepatol 2004;40:736-41.
42. Psutka SP, Boorjian SA, Thompson RH, et al. Clinical and radiographic predictors of the need for inferior vena cava resection during nephrectomy for patients with renal cell carcinoma and caval tumour thrombus. BJU Int 2015;116:388-96.
43. Aslam Sohaib S, Teh J, Nargund VH, Lumley JS, Hendry WF, Reznek RH. Assessment of tumor invasion of the vena caval wall in renal cell carcinoma cases by magnetic resonance imaging. J Urol 2002;167:1271-5.
44. Quencer KB, Friedman T, Sheth R, Oklu R. Tumor thrombus: incidence, imaging, prognosis and treatment. Cardiovasc Diagn Ther 2017;7:S165-77.
45. Yang L, Lin LW, Lin XY, et al. Ultrasound-guided fine needle aspiration biopsy in differential diagnosis of portal vein tumor thrombosis. Hepatobiliary Pancreat Dis Int 2005;4:234-8.
46. Lukianchenko AB, Medvedeva BM, Karseladze AI, Romanova KA. Morphology classification of liver tumors (comparison of the last and previous WHO classifications 2010 and 2019). Medicinskaâ vizualizaciâ 2020;24:138-43.
47. Kim H, Jang M, Park YN. Histopathological variants of hepatocellular carcinomas: an update according to the 5th edition of the WHO classification of digestive system tumors. J Liver Cancer 2020;20:17-24.
48. Nagtegaal ID, Odze RD, Klimstra D, et al. WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020;76:182-8.
49. Kleiner DE, Bedossa P. Liver histology and clinical trials for nonalcoholic steatohepatitis-perspectives from 2 pathologists. Gastroenterology 2015;149:1305-8.
50. Shimada M, Rikimaru T, Hamatsu T, et al. The role of macroscopic classification in nodular-type hepatocellular carcinoma. Am J Surg 2001;182:177-82.
52. Zimmermann A. Hepatocellular carcinoma (Ordinary Hepatocellular Carcinoma). Tumors and tumor-like lesions of the hepatobiliary tract. Cham: Springer International Publishing; 2016. p. 1-38.
53. Yuki K, Hirohashi S, Sakamoto M, Kanai T, Shimosato Y. Growth and spread of hepatocellular carcinoma: a review of 240 consecutive autopsy cases. Cancer 1990;66:2174-9.
54. Schlageter M, Terracciano LM, D'Angelo S, Sorrentino P. Histopathology of hepatocellular carcinoma. World J Gastroenterol 2014;20:15955-64.
55. Paradis V. Histopathology of Hepatocellular Carcinoma. In: Vauthey J, Brouquet A, editors. Multidisciplinary treatment of hepatocellular carcinoma. Berlin: Springer Berlin Heidelberg; 2013. p. 21-32.
56. Feo F, Pascale RM. Multifocal hepatocellular carcinoma: intrahepatic metastasis or multicentric carcinogenesis? Ann Transl Med 2015;3:4.
57. Chianchiano P, Pezhouh MK, Kim A, et al. Distinction of intrahepatic metastasis from multicentric carcinogenesis in multifocal hepatocellular carcinoma using molecular alterations. Hum Pathol 2018;72:127-34.
58. Furuta M, Ueno M, Fujimoto A, et al. Whole genome sequencing discriminates hepatocellular carcinoma with intrahepatic metastasis from multi-centric tumors. J Hepatol 2017;66:363-73.
59. Shimada S, Mogushi K, Akiyama Y, et al. Comprehensive molecular and immunological characterization of hepatocellular carcinoma. EBioMedicine 2019;40:457-70.
60. Kurogi M, Nakashima O, Miyaaki H, Fujimoto M, Kojiro M. Clinicopathological study of scirrhous hepatocellular carcinoma. J Gastroenterol Hepatol 2006;21:1470-7.
61. Nakashima T, Okuda K, Kojiro M, et al. Pathology of hepatocellular carcinoma in Japan: 232 consecutive cases autopsied in ten years. Cancer 1983;51:863-77.
62. Yeh CN, Lee WC, Jeng LB, Chen MF. Pedunculated hepatocellular carcinoma: clinicopathologic study of 18 surgically resected cases. World J Surg 2002;26:1133-8.
63. Bosman FT, Carneiro F, Hruban RH, et al. WHO classification of tumors of digestive system. WHO press; 2010. p. 304.
64. Torbenson MS. Morphologic subtypes of hepatocellular carcinoma. Gastroenterol Clin North Am 2017;46:365-91.
65. Rastogi A, Maiwall R, Ramakrishna G, et al. Hepatocellular carcinoma: clinicopathologic associations amidst marked phenotypic heterogeneity. Pathol Res Pract 2021;217:153290.
66. Leong AS, Sormunen RT, Tsui WM, Liew CT. Hep Par 1 and selected antibodies in the immunohistological distinction of hepatocellular carcinoma from cholangiocarcinoma, combined tumours and metastatic carcinoma. Histopathology 1998;33:318-24.
67. Lin F, Abdallah H, Meschter S. Diagnostic utility of CD10 in differentiating hepatocellular carcinoma from metastatic carcinoma in fine-needle aspiration biopsy (FNAB) of the liver. Diagn Cytopathol 2004;30:92-7.
68. Shafizadeh N, Ferrell LD, Kakar S. Utility and limitations of glypican-3 expression for the diagnosis of hepatocellular carcinoma at both ends of the differentiation spectrum. Mod Pathol 2008;21:1011-8.
69. Wee A. Diagnostic utility of immunohistochemistry in hepatocellular carcinoma, its variants and their mimics. Appl Immunohistochem Mol Morphol 2006;14:266-72.
70. Minervini MI, Demetris AJ, Lee RG, et al. Utilization of hepatocyte-specific antibody in the immunocytochemical evaluation of liver tumors. Mod Pathol 1997;10:686-92.
71. Liu H, Yang C, Lu W, Zeng Y. Prognostic significance of glypican-3 expression in hepatocellular carcinoma: a meta-analysis. Medicine (Baltimore) 2018;97:e9702.
72. Salomao M, Siegel A, Lefkowitch JH, et al. Steatohepatitic hepatocellular carcinoma: a novel histologic variant associated with steatohepatitis and metabolic syndrome. Lab Invest 2011;91.
73. Salomao M, Remotti H, Vaughan R, Siegel AB, Lefkowitch JH, Moreira RK. The steatohepatitic variant of hepatocellular carcinoma and its association with underlying steatohepatitis. Hum Pathol 2012;43:737-46.
74. Salomao M, Yu WM, Brown RS Jr, Emond JC, Lefkowitch JH. Steatohepatitic hepatocellular carcinoma (SH-HCC): a distinctive histological variant of HCC in hepatitis C virus-related cirrhosis with associated NAFLD/NASH. Am J Surg Pathol 2010;34:1630-6.
75. Yeh MM, Liu Y, Torbenson M. Steatohepatitic variant of hepatocellular carcinoma in the absence of metabolic syndrome or background steatosis: a clinical, pathological, and genetic study. Hum Pathol 2015;46:1769-75.
76. Bannasch P, Ribback S, Su Q, Mayer D. Clear cell hepatocellular carcinoma: origin, metabolic traits and fate of glycogenotic clear and ground glass cells. Hepatobiliary Pancreat Dis Int 2017;16:570-94.
77. Liu Z, Ma W, Li H, Li Q. Clinicopathological and prognostic features of primary clear cell carcinoma of the liver. Hepatol Res 2008;38:291-9.
78. Ji SP, Li Q, Dong H. Therapy and prognostic features of primary clear cell carcinoma of the liver. World J Gastroenterol 2010;16:764-9.
79. Ziol M, Poté N, Amaddeo G, et al. Macrotrabecular-massive hepatocellular carcinoma: a distinctive histological subtype with clinical relevance. Hepatology 2018;68:103-12.
80. Calderaro J, Couchy G, Imbeaud S, et al. Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification. J Hepatol 2017;67:727-38.
81. Renne SL, Woo HY, Allegra S, et al. Vessels encapsulating tumor clusters (VETC) is a powerful predictor of aggressive hepatocellular carcinoma. Hepatology 2020;71:183-95.
82. Lee JH, Choi MS, Gwak GY, et al. Clinicopathologic characteristics and long-term prognosis of scirrhous hepatocellular carcinoma. Dig Dis Sci 2012;57:1698-707.
83. Matsuura S, Aishima S, Taguchi K, et al. 'Scirrhous' type hepatocellular carcinomas: a special reference to expression of cytokeratin 7 and hepatocyte paraffin 1. Histopathology 2005;47:382-90.
84. Kim YJ, Rhee H, Yoo JE, et al. Tumour epithelial and stromal characteristics of hepatocellular carcinomas with abundant fibrous stroma: fibrolamellar versus scirrhous hepatocellular carcinoma. Histopathology 2017;71:217-26.
85. Choi SY, Kim YK, Min JH, et al. Added value of ancillary imaging features for differentiating scirrhous hepatocellular carcinoma from intrahepatic cholangiocarcinoma on gadoxetic acid-enhanced MR imaging. Eur Radiol 2018;28:2549-60.
86. Craig JR, Peters RL, Edmondson HA, Omata M. Fibrolamellar carcinoma of the liver: a tumor of adolescents and young adults with distinctive clinico-pathologic features. Cancer 1980;46:372-9.
87. Eggert T, McGlynn KA, Duffy A, Manns MP, Greten TF, Altekruse SF. Fibrolamellar hepatocellular carcinoma in the USA, 2000-2010: a detailed report on frequency, treatment and outcome based on the Surveillance, Epidemiology, and End Results database. United European Gastroenterol J 2013;1:351-7.
88. Stipa F, Yoon SS, Liau KH, et al. Outcome of patients with fibrolamellar hepatocellular carcinoma. Cancer 2006;106:1331-8.
89. Kakar S, Burgart LJ, Batts KP, Garcia J, Jain D, Ferrell LD. Clinicopathologic features and survival in fibrolamellar carcinoma: comparison with conventional hepatocellular carcinoma with and without cirrhosis. Mod Pathol 2005;18:1417-23.
90. Berman MA, Burnham JA, Sheahan DG. Fibrolamellar carcinoma of the liver: an immunohistochemical study of nineteen cases and a review of the literature. Human Pathology 1988;19:784-94.
91. Ringe B, Wittekind C, Weimann A, et al. Results of hepatic resection and transplantation for fibrolamellar carcinoma. Surg Gynecol Obstet 1992;175:299-305.
92. Titelbaum DS, Burke DR, Meranze SG, Saul SH. Fibrolamellar hepatocellular carcinoma: pitfalls in nonoperative diagnosis. Radiology 1988;167:25-30.
93. McLarney JK, Rucker PT, Bender GN, Goodman ZD, Kashitani N, Ros PR. Fibrolamellar carcinoma of the liver: radiologic-pathologic correlation. Radiographics 1999;19:453-71.
94. Edmondson HA. Differential diagnosis of tumors and tumor-like lesions of liver in infancy and childhood. AMA J Dis Child 1956;91:168-86.
96. Lin F, Liu H. Immunohistochemistry in undifferentiated neoplasm/tumor of uncertain origin. Arch Pathol Lab Med 2014;138:1583-610.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Gisder DM, Tannapfel A, Tischoff I. Histopathology of hepatocellular carcinoma - when and what. Hepatoma Res 2022;8:4. http://dx.doi.org/10.20517/2394-5079.2021.106
AMA Style
Gisder DM, Tannapfel A, Tischoff I. Histopathology of hepatocellular carcinoma - when and what. Hepatoma Research. 2022; 8: 4. http://dx.doi.org/10.20517/2394-5079.2021.106
Chicago/Turabian Style
Gisder, Doreen Maria, Andrea Tannapfel, Iris Tischoff. 2022. "Histopathology of hepatocellular carcinoma - when and what" Hepatoma Research. 8: 4. http://dx.doi.org/10.20517/2394-5079.2021.106
ACS Style
Gisder, DM.; Tannapfel A.; Tischoff I. Histopathology of hepatocellular carcinoma - when and what. Hepatoma. Res. 2022, 8, 4. http://dx.doi.org/10.20517/2394-5079.2021.106
About This Article
Copyright
Data & Comments
Data
 Cite This Article 98 clicks
Cite This Article 98 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.