Surgical management of cholangiocarcinoma
Abstract
Cholangiocarcinoma (CCA) is a rare but lethal tumor that arises from the intrahepatic, perihilar, or extrahepatic bile ducts. Complete surgical resection remains the only chance at long-term survival. Unfortunately, most cases of CCA are clinically silent until late in the disease process, and, combined with the lack of effective screening tests, many CCAs present as unresectable tumors. CCA workup typically includes a multiphasic chest, abdominal, and pelvic imaging, liver function tests, and tumor markers (CEA, CA 19-9). Tissue diagnosis is encouraged but not always necessary. In certain situations, esophagogastroduodenoscopy, colonoscopy, and mammography are recommended. If resectable, intrahepatic CCAs and perihilar CCAs require a hepatectomy ranging from a wedge resection to an extended hepatectomy with reconstruction depending on the location and tumor size. In certain specialized centers, portal vein and hepatic artery reconstruction can be performed with good outcomes and acceptable morbidity. For resectable extrahepatic CCAs, a pancreaticoduodenectomy is recommended. Traditionally, few effective adjuvant options have existed for patients after surgery. However, recent randomized controlled trials support the use of either adjuvant chemotherapy or chemoradiation therapy after surgical resection. In select patients, intra-arterial therapy options such as transarterial chemoembolization, hepatic artery infusion therapy, or yttrium-90 radioembolization, as well as liver transplant, are effective treatment modalities. Improved surgical techniques, regionalization of care to high-volume centers, and appropriate application of preoperative optimization techniques have safely expanded the candidates of potentially resectable patients and improved patient outcomes.
Keywords
INTRODUCTION
Cholangiocarcinomas (CCAs) include a heterogeneous group of tumors that arise from the epithelial cells of the bile ducts and, although rare, are highly lethal. In the United States, the annual incidence of CCA is approximately 1.26 cases per 100,000 people, a rate that has significantly increased over the last two decades[1,2]. An estimated 9000 cases occur annually in the United States, and due to aggressive tumor biology, low incidence of early detection, and poor efficacy of traditional therapies, less than 10% of all patients diagnosed with CCA survive 5 years[1,2]. While much work remains, recent improvements in multimodality care have led to decreasing CCA mortality rates since 2013[2].
In patients with CCA, surgical resection provides the only possibility for long-term survival. Unfortunately, most cases of CCA are clinically silent, especially early in the disease process, commonly presenting with locally advanced disease not amenable to resection[3]. Advances in the surgical management of patients with CCA (e.g., portal vein embolization, vascular reconstruction, locoregional and systemic therapies) have expanded the pool of surgical candidates. For patients with resectable disease, median overall survival (OS) is 51 months with a median relapse-free survival (RFS) of 24 months, significantly improved compared to patients with the unresectable disease (median OS 11 months and median RFS 8 months)[4]. However, recurrence rates after surgical resection remain high (> 60%)[5]. In this article, we review the evaluation and treatment of CCA with a focus on the surgical management of CCA.
Classification
CCA classification includes intrahepatic, perihilar, and extrahepatic or distal subtypes. Approximately 50% arise from perihilar ducts, 40% from distal ducts, and less than 10% from intrahepatic ducts[6]. Anatomically, intrahepatic cholangiocarcinoma (ICCA) arises from the segmental bile ducts or smaller branches of the intrahepatic biliary tree [Figure 1][7]. Per the 2019 WHO classification of tumors of the digestive system, ICCA has two main subtypes: large duct type, which resembles extrahepatic cholangiocarcinoma, and small duct type, which shares many characteristics with hepatocellular carcinoma[8]. Perihilar cholangiocarcinoma (PCCA) arises from the cystic duct-common duct junction to the second-order bile ducts[9]. Distal cholangiocarcinoma (DCCA) develops between the ampulla of Vater to the cystic duct[9]. However, some debate exists about the exact transitions between the CCA subtypes. Multiple classification systems exist for CCA, each with its own limitations. A well-known staging system by The American Joint Committee on Cancer (AJCC) stratifies disease prognosis by stage based on tumor size (T), lymph node disease (N), and metastasis (M) for the three subtypes of CCA[10]. The Bismuth-Corlette classification further subdivides perihilar tumors by the extent of ductal infiltration[11]. Type I PCCAs include tumors distal to the confluence of the left and right ducts; type II tumors involve the confluence; type III tumors extend into the right hepatic duct (IIIa) or left hepatic duct (IIIb) in addition to involving the confluence; type IV tumors extend into both the right and left hepatic bile ducts. An alternative classification system for PCCAs, the Blumgart staging system, includes clinically relevant factors such as the presence or absence of portal venous invasion and hepatic atrophy in addition to tumor location and extent of bile duct involvement[12,13]. This system classifies tumors into three stages (T1-T3) and predicts resectability, the potential for metastatic disease, and survival[12,13]. As understanding of the complex pathophysiology of CCA improves, new classification systems will continue to emerge; however, as of now, no universally accepted system exists[14-17].
Figure 1. Cholangiocarcinoma classification includes intrahepatic, hilar, and extrahepatic or distal subgroups. The majority of cholangiocarcinomas arise from perihilar or distal ducts, while less than 10% are from intrahepatic ducts. Additionally, through improved genetic analysis, there is a better understanding of the shared and distinct somatic genomic landscapes of cholangiocarcinoma and possible actionable mutation for which therapies may exist.
Risk factors
Large population-based studies have revealed several risk factors for CCA. While metabolic conditions (i.e., obesity, diabetes, and nonalcoholic fatty liver disease) and toxic exposures (i.e., alcohol, tobacco, 1,2-dichloropropane, dichloromethane, and thorotrast) are known risk factors for CCA[18-23], chronic inflammation of the bile ducts is associated with most cases of CCA. In the Western world, the etiology of long-standing bile duct inflammation includes primary sclerosing cholangitis (PSC) and choledochal cystic disease. Nearly 30% of CCAs are diagnosed in patients with PSC. Furthermore, patients with PSC tend to develop CCAs in the 5th decade of life, a much younger age compared with the general CCA cohort (age > 65 years); patients with PSC have a lifetime risk of developing CCA of approximately 10%[24-26]. Patients with untreated choledochal cysts also tend to develop CCA at an earlier age with a risk of malignancy approaching 20%[27,28]. Patients with a long common pancreaticobiliary channel, as a result of a congenital malformation in which the ducts join outside of the duodenal wall, are at higher risk of developing biliary tract cancers[29]. Liver fluke infection, more common in Asia, is associated with ICCA[30]. Multiple cohort studies demonstrate an association between viral hepatitis and cholangiocarcinoma; however, this risk is much lower than for hepatocellular carcinoma (HCC)[18,31-33]. To date, at least four genetic disorders are associated with an increased risk of developing cholangiocarcinoma: Lynch syndrome, BAP1 tumor predisposition syndrome, cystic fibrosis, and multiple biliary papillomatosis, the latter of which leads to malignant transformation in up to 80% of patients[34-37]. In most cases of CCA, an etiological factor cannot be identified[38].
Pathophysiology
Histologically, over 90% of CCAs are adenocarcinoma with squamous cell carcinoma comprising most of the remaining histological types[9,39]. Morphologic subtypes include sclerosing, nodular, or papillary. All three subtypes have a high rate of local invasion, slow growth, produce mucin, and tend to invade perineural sheath and spread along nerves[39]. Most CCAs start with molecular mutations as a result of chronic inflammation[40]. Prolonged exposure of cholangiocytes to inflammatory mediators such as tumor necrosis factor-α, interleukin-6, and cyclo-oxygenase-2, cause progressive mutations in oncologic regulatory genes. Furthermore, inflammation-induced impaired bile flow leads to cholestasis and bile acid accumulation, lowering pH. An acidic microenvironment activates numerous cellular pathways (e.g., ERK1/2, Akt, NF-κB, etc.) responsible for tumor growth, infiltration, and spread[41,42]. Other mediators upregulated in CCA include transforming growth factor-β, vascular endothelial growth factor, and hepatocyte growth factor facilitate cellular proliferation, angiogenesis, and cell migration[43-46]. Ongoing research continues to reveal underlying molecular alterations responsible for the pathogenesis of CCA providing potential marks for targeted therapies.
DIAGNOSTIC WORKUP
CCA commonly presents with non-specific symptoms such as abdominal pain, weight loss, fatigue, and night sweats[9]. While ICCAs usually remain asymptomatic until the mass is significantly large to cause mass effect, PCCAs and DCCAs can cause biliary obstruction producing earlier symptoms such as jaundice, pruritis, dark-colored urine, and clay-colored stools. Once suspected, workup of CCA includes a multiphasic abdominal/pelvic computed tomography (CT) or magnetic resonance imaging (MRI) with IV contrast, chest CT, liver function tests, tumor markers CEA, CA 19-9, ± Alpha-fetoprotein and EUS/biopsy[47]. Since most pathologic hepatic lesions are metastatic in origin, additional workup to include an esophagogastroduodenoscopy, colonoscopy, and mammography are strongly encouraged before a definitive diagnosis of ICCA is made[47,48]. Fluorodeoxyglucose-positron emission tomography can be helpful to rule out distant metastatic disease[49]. MRI/magnetic resonance cholangiopancreatogra (MRCP) is emerging as the preferred imaging modality, especially for the evaluation of ICCA and PCCA[28]. MRI/MRCP offers a comparable evaluation of the biliary system to endoscopic retrograde cholangiopancreatography (ERCP) or percutaneous transhepatic cholangiography in addition to enabling the surgeons to examine vascular involvement and local tumor extension, knowledge of which is critical in determining resectability and surgical approach[50]. All patients with CCAs should be reviewed by a multidisciplinary team. In cases where the diagnosis is in doubt (e.g., biliary stricture with negative brushings), surgical resection should be considered on an individual patient basis as certain clinical scenarios may be consistent with an oncologic etiology despite the lack of a tissue diagnosis.
Once the diagnosis is established, resectability drives the prognosis for patients with CCA. Guidelines vary, but traditional criteria for resectability include the absence of retropancreatic or paraceliac lymph node involvement, absence of extrahepatic adjacent organ invasion, absence of disseminated disease, and absence of main portal vein or main hepatic artery invasion; however, certain specialized, high volume centers have acceptable outcomes with en bloc resection of the portal vein or hepatic artery followed by vascular reconstruction if microscopically negative margins (R0 resection) are achieved[51-55]. Additional factors for resection are specific to the tumor location and include bilateral duct involvement up to secondary radicles, atrophy of one lobe with contralateral secondary biliary radicle involvement, or involvement of bilateral hepatic arteries[56]. Staging laparoscopy provides limited benefit in the evaluation of locoregional involvement due to the improvement in cross-sectional imaging, but it can be used selectively to rule out small peritoneal disease in high-risk patients such as those with elevated CA 19-9 levels in the absence of biliary obstruction[57].
Preoperative optimization
Preoperative biliary decompression in CCA remains controversial[58-63]. Data from recent meta-analyses have come to different conclusions on the impact of preoperative stenting[62,63]. Proponents of preoperative biliary decompression cite the physiologic impact of hyperbilirubinemia on liver regeneration and immune function, as well as the potential increase in postoperative complications[64]. Preoperative biliary drainage is indicated in patients with severe symptomatic jaundice, cholangitis, or in patients with hyperbilirubinemia and planned chemotherapy[65]. Opponents argue that the procedure itself (i.e., endoscopic or percutaneous approach) can have complications such as bleeding or perforation as well as future complications from stent or drain blockage. Furthermore, unnecessary manipulation of the biliary tract may lead to cholangitis and sepsis[64]. When preoperative drainage is pursued, the interval of drainage (i.e., time from drainage procedure to surgery) should be short to decrease the risk of complications. Furthermore, debate continues surrounding the optimal approach for preoperative drainage, endoscopic versus percutaneous[66-68]. A recent multicenter randomized controlled trial examining these two approaches was stopped early due to higher all-cause mortality in the percutaneous transhepatic biliary drainage group; however, the results were hard to interpret due to low study enrollment[69]. Well-powered randomized controlled trials are needed to elucidate best practice, but the low incidence of CCA and the heterogeneous pathophysiology makes these studies difficult to perform.
An adequate future liver remnant (FLR) requires at least two continuous segments with adequate perfusion, venous outflow, and biliary drainage. The degree of underlying liver disease (e.g., steatohepatitis, cirrhosis, chemotherapy-associated liver injury, etc.) influences the amount of FLR needed to prevent postoperative hepatic complications. For patients undergoing major hepatectomy, surgeons should consider CT or MRI volumetry and liver function assessment with indocyanine green clearance testing to determine the maximum extent of major hepatic resection that will not lead to postoperative liver complications[70]. In situations when complete surgical resection will create an inadequate FLR, alternative approaches are needed. Historically, surgeons performed a 2-stage hepatectomy to induce liver regeneration after the first resection[71]. However, liver regeneration could be slow, and many patients never received the second stage of the procedure[72]. In turn, a novel 2-stage liver resection called associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) procedure was proposed. This procedure combines portal vein ligation with transection of the liver along the FLR. After adequate liver hypertrophy approximately 1-2 weeks later, the surgeon completes the hepatectomy by transecting the right hepatic artery, the biliary duct, and the hepatic vein(s) during the second stage of the procedure[73]. ALPPS induced greater liver hypertrophy, and patients had a higher rate of stage 2 hepatectomy completion compared with historical staged hepatectomy; however, high morbidity and mortality have prevented broader adoption of ALPPS[74]. Portal vein embolization (PVE) is another option to increase the FLR. The initial PVE approach through an ileocolic venous branch is a surgical procedure requiring general anesthesia and has fallen out of favor of minimally invasive approaches performed by interventional radiology[75]. In appropriately selected patients, PVE induces liver hypertrophy leading to high rates of planned hepatectomy and low rates of postoperative liver failure[76].
NEOADJUVANT THERAPY
Neoadjuvant therapy has gained favor in the treatment of CCA. Theoretical benefits include downstaging unresectable disease, early treatment of potential micro-metastatic disease, and allowing time to evaluate the aggressiveness of the tumor biology as disease progression on neoadjuvant therapy is a poor prognostic indicator[77]. Limited studies have revealed no improvement in OS among patients who underwent surgical resection after neoadjuvant chemotherapy compared with patients who underwent upfront surgery[78,79]. With multiple neoadjuvant chemotherapy trails currently recruiting, the role of neoadjuvant chemotherapy for patients with CCA will be better defined in the future[80].
Patients with unresectable CCA may benefit from liver-directed therapies such as transarterial radioembolization (TARE), transarterial chemoembolization (TACE), transarterial bland embolization (TAE), yttrium-90 (Y-90) radioembolization, as well as hepatic artery infusion (HAI) therapy. In HAI therapy, a surgically placed pump into the gastroduodenal artery delivers high-dose chemotherapy directly to the liver with few systemic side effects[81]. A retrospective review and follow-up phase 2 clinical trial demonstrated improved OS for CCA patients with unresectable disease that received combined HAI therapy and systemic chemotherapy even when patients had positive lymph nodes[81,82]. These therapies are discussed in greater detail in the Locoregional Options section of this review.
APPROACH TO RESECTION
Current best evidence to guide the surgical management of CCA comes from observational studies. Complete surgical resection with negative margins remains the only chance for cure from CCA. Improved surgical techniques, regionalization of care to high-volume centers, and appropriate application of preoperative optimization techniques (i.e., portal vein embolization, locoregional and systemic therapies) have safely expanded the candidates of potentially resectable patients and improved outcomes[53,83-88].
Management of intrahepatic cholangiocarcinoma
Hepatectomy with negative margins and a regional lymphadenectomy of the porta hepatis while maintaining an adequate liver remnant offers the best chance at long-term survival for patients with ICCA. Once the abdomen is entered, a thorough evaluation of the abdominal cavity for metastatic disease and resectability should be performed. If exploration reveals distant metastatic disease or lymph node disease beyond the porta hepatis, the procedure should be aborted as these are contraindications to resection. The liver should be fully evaluated with intraoperative ultrasound, specifically looking for multifocal hepatic disease and proximity of the tumor to intrahepatic structures that may prohibit resection. Intraoperative ultrasound utilization can change surgical management in up to one-third of cases[89]. Traditionally, multifocal ICCA represented metastatic disease and a contraindication to surgical resection. In a large database study, Yin et al.[90] examined 580 patients with multifocal ICCA and demonstrated significantly improved median survival in patients that underwent resection compared with patients who were managed non-operatively.
Patients with microscopically negative margins (R0 resection) have significantly better outcomes compared with patients who had a resection with microscopically positive margins (R1 resection)[91]. However, the optimal negative margin width is unclear as studies provide conflicting data[91,92]. While major hepatectomy is often required to completely excise ICCA, wedge resection or segmental resection is acceptable as long as an R0 resection is achieved. Conversely, aggressive surgical approaches may be an option for highly selected patients with otherwise unresectable tumors and good liver function treated at high-volume centers by experienced surgeons and multidisciplinary care teams. Initially described by Dr. Raab et al.[93], ex vivo liver resection techniques include in-situ, ante-situm, and ex-situ approaches, which require veno-venous bypass, liver perfusion, and major vascular reconstruction. The technically demanding procedure has a high complication and mortality rate and, when performed for CCA, can have a high tumor recurrence rate[94]. Overall, over 76% to 92% of patients with ICCA receive an R0 resection when taken to the operating room with curative intent based on preoperative workup[91,92,95-97].
Management of perihilar cholangiocarcinoma
Surgical resection of PCCA begins in a similar fashion to ICCA resection with abdominal exploration for metastatic disease and tumor evaluation with intraoperative ultrasound to confirm resectability. Particular attention should be paid to potential tumor involvement of the contralateral bile ducts and vascular structures.
Microscopically negative margins are critical to long-term outcomes for patients with PCCA as 5-year OS drops form between 27%-45% for patients that received an R0 resection compared to 0%-23% for patients with an R1 or R2 resection[12,54,98-100]. Aggressive surgical management is warranted in patients with adequate functional FLR. The intraoperative frozen section of the proximal ductal margin can be used to guide intraoperative decision-making. Patients that achieved R0 resection after re-resection of a positive frozen section margin had comparable survival outcomes as patients initially with R0 resection while patients with R1 resection had significantly decreased survival[101]. Complete resection of PCCA includes removal of the involved biliary tree, associated hemi-liver, and porta hepatis lymphadenectomy. The central location of PCCAs generally requires a caudate lobe resection. The contralateral bile duct is usually reconstructed with a Roux-en-Y hepaticojejunostomy. There may be tumors that require an extended liver resection and/or vascular resection to achieve negative margins. Extended hepatectomy (e.g., trisectionectomy) and vascular resection have acceptable outcomes when performed at specialized centers by high-volume surgeons and are encouraged to achieve R0 resection[87,102]. In an earlier study examining 80 patients with PCCA, extended hepatic resection, including right trisectionectomy and portal vein resections, resulted in higher rates of R0 resection and subsequent longer survival[102]. In a recent retrospective study, 216 patients with Bismuth type IV PCCA underwent resection, of which 112 patients underwent a left hepatic trisectionectomy, and 131 patients had a combined vascular resection[87]. Over 40% of patients experienced a Clavien-Dindo grade III or higher complication and 1.9% surgical mortality within 90 days. An R0 resection was achieved among 72% of patients. The high R0 resection rate in this challenging patient population corresponded to a significantly improved 5-year survival rate compared with patients who had unresected tumors (32.8% vs. 1.5%; P < 0.001). As recently as the AJCC 7th edition, type IV PCCAs were classified as stage IV disease and deemed unresectable due to the poor OS[103]. The feasibility of excellent oncologic outcomes with low mortality despite higher morbidity in a surgically complex patient population has been demonstrated. For example, portal vein resection can be routinely performed when there is suspicion of tumor invasion. However, arterial resection and reconstruction should be limited to highly selected patients as arterial resection can result in higher morbidity and mortality compared with portal vein resection[88]. For patients with Bismuth type I or II, limited bile duct resection has been attempted; however, en bloc liver resection typically has provided significantly better 5-year survival (50%) compared with extrahepatic bile duct resection alone (30%) and is the preferred management approach[104].
Lymphadenectomy
The presence of nodal metastases adversely impacts OS in patients with CCA. In addition, when nodal disease is present, prognostic factors including vascular invasion and multiple tumors no longer impact survival, suggesting that nodal spread is one of the most important prognostic indicators in CCA[105]. Lymphadenectomy provides prognostic and staging information but offers little therapeutic benefit[10]. Approximately half of surgeons perform a lymphadenectomy with the utilization of lymph node evaluation rising proportionally to tumor size[106]. When performed routinely for ICCA and PCCA, lymph node metastases appear in 40%-50% of patients, with the incidence increasing proportionally with T stage[107,108]. Standard dissection for patients with ICCA or PCCA involves a regional lymphadenectomy of the porta hepatitis[47]. A recent recommendation proposed that lymphadenectomy for ICCA and PCCA should include both station 12 (hepatoduodenal ligament) and 8 (common hepatic artery) lymph nodes. Lymphadenectomy of these stations covered 82% of metastatic cases regardless of tumor location[109]. While there is no universal consensus on the minimum number of lymph nodes needed for accurate staging, the 8th edition of the AJCC staging system recommends recovery of at least 6 lymph nodes[110]. Gross lymph node metastases to the porta hepatis portend a poor prognosis, and surgical resection should only be pursued in highly selected patients.
Management of distal cholangiocarcinoma
DCCA can also present later in the disease process, and only a minority of patients are resectable at the time of diagnosis[111]. Prior to resection, a thorough evaluation of the anticipated proximal biliary margin should be performed to confirm the absence of disease. The recommended surgical approach for DCCA is pancreaticoduodenectomy with lymphadenectomy and typical pancreaticojejunostomy, hepaticojejunostomy, and gastrojejunostomy or duojejunostomy reconstruction. Patients with R0 resection have a median survival of 25 months and approximately 30% 5-year survival[112,113]. The morbidity profile compares favorably to more proximal CCAs with lower mortality rates[114]. In situations with diffuse involvement of the biliary tract where the CCA extends to include the distal and perihilar segments, a hepatectomy can be added to a pancreaticoduodenectomy to achieve negative margins with acceptable morbidity and mortality[115,116]. These combined cases should be performed at high-volume centers with experienced multidisciplinary care teams for highly selected patients to achieve the best outcomes.
Periampullary carcinomas arise within 2 cm of the major duodenal papilla and include 4 possible sites of origin: biliary tract (periampullary CCA), pancreas, ampulla of Vater, and duodenum. Survival rates for ampullary and duodenal cancers are highest, followed by periampullary CCAs and pancreatic cancer. The majority of these cancers (80%) are resectable at the time of diagnosis. Like DCCA, the preferred surgical approach for periampullary CCA is pancreaticoduodenectomy. Transduodenal ampullectomy has been performed in highly selected patients with T1 disease. However, due to early lymph node metastases, over 10% in some series, even for these early-stage tumors, such local approaches carry higher risks of local recurrence and worst survival[117,118].
ADJUVANT THERAPY
Surgical resection for CCA offers the best chance for long-term survival, yet recurrence rates remain high, suggesting the need for more effective systemic therapy[119]. The low prevalence of biliary tract malignancies makes it rather difficult to study and find effective therapies. Clinical trials of adjuvant chemotherapy and radiation are limited. Additionally, in order to achieve sufficient statistical power, these studies often include a heterogenous cohort with ICCA, extrahepatic cholangiocarcinoma (ECCA), and gallbladder cancers[120]. In addition to studies having mixed pathologies, historically, studies of adjuvant therapy for biliary tract cancers have been small, nonrandomized, and retrospective[121].
While population-based cohort studies support the use of adjuvant chemotherapy, the results of recent randomized controlled trials demonstrate conflicting results[122]. Horgan et al.[123] performed a meta-analysis on studies published between 1960 and November 2010 on adjuvant chemotherapy, radiotherapy, or both compared with curative-intent surgery alone for resected biliary tract cancer. These authors reported a nonsignificant improvement in OS with any adjuvant therapy compared with surgery alone (P = 0.06). Patients who received chemotherapy or chemoradiotherapy had a greater benefit than individuals who received radiation alone. The greatest benefit with adjuvant therapy was noted in patients with lymph node metastasis or R1 resection[123]. Ghidini et al.[124] performed a meta-analysis of 50 studies including 22,499 patients, 3967 of which underwent surgical resection, to compare any adjuvant therapy, including chemotherapy and chemoradiation, and found any use of adjuvant therapy increased survival by 4.3 months compared to surgery alone.
The main deterrent to widespread acceptance of adjuvant therapy for biliary tract cancers has been the lack of sufficient data from randomized clinical trials. Clinical trials such as ABC-02, PRODIGE-12/ACCORD-18, and BILCAP have attempted to answer the need for effective adjuvant therapy [Table 1]. As there was no established adjuvant chemotherapy for patients with resected pancreaticobiliary malignancies, in 2002, Takada et al.[125] investigated the use of mitomycin C at the time of surgery and 5-FU in 2 courses for 5 consecutive days during postoperative weeks 1 and 3, followed by 5-FU daily from postoperative week 5 until disease recurrence. In total, 436 patients were randomized: 158 patients with pancreatic adenocarcinoma, 118 with bile duct cancer, 112 with gallbladder cancer, and 48 with cancer of the ampulla of Vater. The 5-year OS was significantly better in patients with gallbladder cancer who received adjuvant mitomycin C and 5-FU compared with control, but there was no difference in OS or DFS in patients with pancreatic, bile duct, or ampullary cancer[125].
Randomized clinical trials of chemotherapy for biliary tract cancer
| Takada et al.[125] | PRODIGE12/ACCORD18 | BILCAP | BCAT | |
| Study arms | 5FU + mitomycin vs. observation | GEMOX vs. observation | Capecitabine vs. observation | Gemcitabine vs. observation |
| Recruitment period | April 1986-June 1992 | July 2009-February 2014 | March 2006-December 2017 | September 2007-January 2011 |
| Total sample size | 436 | 196 | 447 | 225 |
| Disease distribution | CCA 118 (27%) PDAC 158 (36%) GBC 112 (26%) Ampulla 48 (11%) | ICCA 86 (44%) PCCA 15 (8%) DCCA 55 (28%) GBC 38 (20%) | ICCA 84 (19%) PCCA 128 (28%) DCCA 156 (35%) GBC 79 (18%) | PCCA 101 DCCA 124 |
| Primary endpoints | OS | RFS and time to definitive deterioration of HRQOL | OS | OS |
| Secondary endpoints | DFS, ECOG PS, improvement in body weight, adverse events | OS, toxicity, and exploratory translational endpoint | Per-protocol analysis of OS/RFS, RFS, toxicity, health economics, and quality of life | RFS and toxicity |
| Completion of therapy | 80% completion | Median of 10 cycles of 10 for gemcitabine and oxaliplatin | 55% completed chemotherapy, 10 patients (4%) had 0 cycles, 32% discontinued therapy due to toxicity | 52.1% completed chemotherapy 18 patients stopped Gem due to needing tor dose reduction |
| Results | 5-year OS improved in patients with GBC who received adjuvant therapy (26.0% vs. 14.4%, P = 0.0367) and 5 year DFS (20.3% vs. 11.6%, P = 0.0210) No difference in OS or DFS in patients with PDAC, CCA, or ampullary cancer | No difference in OS, RFS, or deterioration of HRQOL | No significant difference in OS in intention to treat population Significant improvement with capecitabine in OS and RFS in prespecified per-protocol analysis | Gemcitabine provided no difference in OS or RFS |
| Relapse rate | 79.9% adjuvant therapy 88.4% observation | 62.1% adjuvant therapy 67.7% observation | 60% adjuvant therapy 65% observation | 53.8% adjuvant therapy 56.5% observation |
ABC-02
This phase III randomized clinical trial by Valle et al.[126] was performed comparing cisplatin plus gemcitabine vs. gemcitabine alone in patients with locally advanced or metastatic cholangiocarcinoma, gallbladder cancer, or ampullary cancer. Four hundred and ten patients were randomized to receive cisplatin plus gemcitabine for eight cycles or gemcitabine alone for six cycles for up to 24 weeks. The primary endpoint was OS with secondary endpoints of progression-free survival, tumor response, and adverse events. Of the 410 patients randomized, 149 patients had gallbladder cancer, 241 had cholangiocarcinoma, and 20 had ampullary cancer; 204 received cisplatin plus gemcitabine, and 206 received gemcitabine alone.
At a median follow-up of 8.2 months, the median OS was significantly higher in patients who received cisplatin-gemcitabine than the gemcitabine alone (11.7 months vs. 8.1 months; hazard ratio = 0.64; 95%CI: 0.52-0.80; P < 0.001). Additionally, patients who received cisplatin-gemcitabine had improved PFS (8.0 months vs. 5.0 months, P < 0.001) and tumor control rate (81.4% vs. 71.8%, P = 0.049). On prespecified subgroup analysis, there was no difference in the hazard ratio for death according to the primary tumor site. These data provided evidence that cisplatin plus gemcitabine is an effective treatment for locally advanced or metastatic biliary tract cancer[126]. These results were utilized as the basis of using gemcitabine with cisplatin in the adjuvant setting and set the stage for following clinical trials[120].
PRODIGE-12/ACCORD-18
In a phase II study, Gemcitabine Oxaliplatin (GEMOX) was demonstrated to be tolerable and active in patients with advanced biliary tract cancers[127]. The PRODIGE-12/ACCORD-18 was a phase III multi-institutional study performed to determine if adjuvant GEMOX could improve outcomes compared to surgery alone in patients who received R0 or R1 resection of localized biliary tract cancer[128]. The primary endpoints were relapse-free survival and time to definitive deterioration of health-related quality of life (HRQOL). Secondary endpoints included OS, toxicity, and exploratory translational endpoints. Of the 196 patients included, 38 patients had gallbladder cancer, while 86 patients had ICCA, 15 PCCA, and 55 DCCA. At a median follow-up of 46.5 months, there was no significant difference in relapse-free survival between patients who received GEMOX and those who had surgery alone (30.4 months vs. 18.5 months; hazard ratio = 0.88; 95%CI: 0.62-1.25; P = 0.48). In addition, there was no difference in time to deterioration of HRQOL. OS was not statistically significant between groups (75.8 months vs. 50.8 months; hazard ratio = 1.08; 95%CI: 0.70-1.66; P = 0.74). Furthermore, on pre-planned subgroup analysis, disease site, lymph node status, or margin status did not identify a subgroup that would benefit from GEMOX. This study has been criticized as being underpowered to detect an effect size hazard ratio of 0.6, as well as including a low proportion of patients who are considered to be high-risk (13% had R1 resections and 37% had lymph node-positive disease) who would benefit the most from adjuvant therapy[120].
BILCAP
The BILCAP study was a phase III multi-institutional study that compared adjuvant capecitabine to observation in patients with biliary tract cancer who underwent macroscopically complete resection with curative intent[129]. The primary endpoint was OS, and secondary endpoints included a per-protocol analysis of outcomes, RFS, toxicity, health economics, and quality of life. Of the 447 patients randomized, 84 patients had ICCA, 128 PCCA, 156 DCCA, and 79 gallbladder cancer. At a median follow-up of 60 months, in the intention-to-treat analysis, the median OS was 51.1 months in the capecitabine group compared to 36.4 months in the observation group (adjusted hazard ratio = 0.81, 95%CI: 0.63-1.04; P = 0.097) and RFS of 24.4 months in the capecitabine group and 17.5 months in the observation group (P = 0.033). In a prespecified per-protocol analysis, median OS was 53 months in the capecitabine group and 36 months in the observation group (adjusted hazard ratio = 0.75, 95%CI: 0.58-0.97; P = 0.028), as well as a median RFS of 25.9 months in the capecitabine group and 17.4 months in the observation group (P = 0.0093)[129]. However, there was no evidence of a difference in RFS beyond 24 months, indicating capecitabine may delay recurrence[120,129].
Based on the results of the BILCAP trial, patients with resected biliary tract cancers should receive 6 months of adjuvant capecitabine[47]. However, the BILCAP trial has been criticized as only having a significant improvement in OS in the adjusted and per-protocol analyses. Additionally, the R1 resection margin and lymph node metastasis rate were relatively high, which has been proposed to account for the different results between the BILCAP trail and PRODIGE-12/ACCORD-18 and Bile Duct Cancer Adjuvant Trial (BCAT) trials[119].
BCAT
The BCAT trial was a randomized controlled multi-institutional phase III Japanese trial that investigated adjuvant gemcitabine compared to observation in patients with resected bile duct cancer[130]. The primary endpoint was OS. Secondary endpoints included relapse-free survival, subgroup analysis, and toxicity. Two hundred and twenty-five patients were included: 45% had PCCA while 55% had DCCA. Gemcitabine provided no difference in OS or RFS.
TOSBIC01 (Tokyo Study Group for Biliary Cancer)
The TOSBIC01 was a phase II study that examined S-1, an oral fluoropyrimidine derivative, in 46 patients with resected biliary malignancies. The regimen consisted of S-1 given within 10 weeks post-surgery and continued up to 1-year post-surgery. Of the 46 patients that met inclusion criteria, 19 had ECCA, 10 had gallbladder carcinoma, 9 had ampullary carcinoma, and 8 had ICCA. There was a 54.3% completion rate, while the completion rate without recurrence during the 1-year administration was 62.5%. OS and DFS rates at 1 year were 91.2% and 80.0%, and 84.3% and 77.2% at 2 years, respectively[131].
Chemoradiotherapy - SWOG0809
The SWOG0809 trial is the only clinical trial utilizing chemoradiotherapy for ECCA or gallbladder cancer. In this study, 54 of 79 patients had ECCA, while the remainder had gallbladder cancer. Results were similar for gallbladder and ECCA. The two-year OS was 68% (95%CI: 54%-79%) for ECCA and 56% (95%CI: 35%-73%) for gallbladder cancer patients (P = 0.87). The two-year DFS was 54% (95%CI: 39%-66%) for ECCA and 48% (95%CI: 28%-66%) for gallbladder cancer (P = 0.71)[132].
LOCOREGIONAL OPTIONS
Unfortunately, most patients with cholangiocarcinoma present with advanced disease and are not candidates for surgical resection. Locoregional therapies may improve outcomes in patients with advanced disease. Intra-arterial therapy (IAT) options such as TACE, TAE, drug-eluting beads, or Y-90 radioembolization were found to be safe and effective in patients with ICCA. In a retrospective review of five major hepatobiliary institutions, IAT produced a partial or complete response in 25.5% of patients and stable disease in 61.5%, while 13.0% had progressive disease[133]. In addition to treating patients with advanced disease, locoregional therapies have been used in an attempt to downstage patients to render the tumor resectable[119]. Patients with locally advanced cholangiocarcinoma who underwent neoadjuvant therapy followed by resection after tumor downstaging were found to have similar short and long-term results compared to patients with initially resectable cholangiocarcinoma[79]. This highlights the importance of surgical resection in the treatment of cholangiocarcinoma.
The concept of liver-directed arterial infusion therapy was initially developed in the 1950s for the treatment of primary and secondary liver cancers. The concept of liver regional therapy was conceived by the landmark study from Sullivan et al.[134], which described their experience with placement of a catheter in the common hepatic artery and directly infusing chemotherapy for one to two months to treat primary and secondary liver cancer. Hepatic artery infusion therapy has gained a foothold in the treatment of patients with colorectal liver metastasis[135]. However, the application of HAI therapy to other primary or secondary liver tumors, such as ICCA, is under investigation.
A phase II clinical trial included 34 patients with unresectable primary liver cancer (26 ICCA and 8 HCC) treated with hepatic artery infusion with floxuridine (HAI-FUDR). Sixteen patients had a partial response, 14 patients had stable disease, and three patients had progressive disease. Patients who responded to therapy had a median disease-specific survival of 29.5 months. The patients with ICCA had a greater response rate than HCC (53.8% vs. 25%, respectively)[136]. A retrospective review analyzed 104 patients with unresectable ICCA confined to the liver who were treated with combined HAI and systemic therapy (gemcitabine-based) or systemic therapy alone[81]. The HAI combination therapy demonstrated a greater response rate than systemic therapy alone[81].
Systemic gemcitabine and oxaliplatin was then investigated in combination with FUDR via HAI. The study included patients with unresectable ICCA and also allowed for resectable regional lymphadenopathy. Eighty-four percent of patients achieved disease control at 6 months, with 58% of patients showing a partial response. The 6-month PFS was 67% for pretreated patients and 89% for chemotherapy-naïve patients. The median OS was 25 months. Similar to prior studies, nodal disease did not alter OS or PFS. Four patients experienced a significant enough response to undergo resection of their tumor[82]. Although there is a limitation in the number of studies, the use of HAI pump therapy, particularly in ICCA, is gaining interest, and a randomized controlled trial is needed to establish the role of this therapy for ICCA.
TRANSPLANT
Liver transplantation (LT) is a widely accepted treatment modality for HCC. HCC typically occurs in patients with underlying liver disease, which may hinder a patient’s ability to undergo resection. In these patients, LT has the advantage of not only addressing the HCC but also the underlying liver disease. Similarly, patients with CCA may experience limitations for resection secondary to chronic liver disease and inadequate future liver remnant[137]. The use of LT for CCA is becoming more widely accepted, specifically for early-stage non-resectable PCCA, while the role of LT in patients with ICCA is currently debated.
Initial series investigating LT for patients with ICCA resulted in poor outcomes with an 18%-25% OS and RFS at 5 years and is considered by most centers a contraindication for LT[138-141]. There is limited, largely retrospective data to support LT for ICCA. A 2016 retrospective study suggested LT may be a viable treatment option for patients with small (< 2 cm), solitary ICCA in patients who did not receive neoadjuvant chemotherapy[142]. Lunsford et al.[140] investigated the utility of LT in patients with locally advanced, unresectable ICCA without vascular involvement or extrahepatic disease who received neoadjuvant chemotherapy in a prospective case series. Patients received gemcitabine-based chemotherapy and were required to have a minimum of six months of stable disease prior to listing for transplantation. Twenty-one patients were evaluated, and twelve patients were listed for transplant. Nine patients were not listed for transplant because seven had extrahepatic disease or disease progression, and two were downstaged to resectable disease. Six of the twelve patients listed for LT underwent transplantation, three of the six not transplanted were still on the transplant list, two did not receive a transplant because of severe adhesions, and the remaining patient was found to have resectable disease on exploration. In patients who underwent LT, OS at 1 year was 100% with a 3 and 5 year OS of 83.3%. Three patients developed recurrent disease at a median of 7.6 months with a 50% recurrence-free survival at 1, 3, and 5 years[140]. Liver transplant for ICCA remains controversial and, as of this writing, has not made its way into the National Comprehensive Cancer Network (NCCN) guidelines for the treatment of ICCA[47].
Unlike patients with ICCA, LT is more widely accepted for the treatment of PCCA. In patients with PCCA, LT highly focuses on patient selection. The NCCN guidelines recommend LT in highly selected patients with tumors < 3 cm in radial diameter, no intrahepatic or extrahepatic metastasis, and no nodal disease[47]. Protocols for LT of PCCA often test underlying tumor biology with neoadjuvant therapy followed by repeat staging[143-145]. The Mayo Clinic Protocol utilizes external beam radiation (45 Gy in 30 fractions) with continuous infusion of 5 FU over 3 weeks followed by brachytherapy administered 2 weeks following completion of external beam radiation therapy and then capecitabine until the time of LT[145]. Diagnostic laparoscopy is essential in these patients to look for metastatic disease, or lymph node involvement as up to 20% of patients may harbor occult disease[137]. It is recommended that at least one lymph node along the proper hepatic artery and common bile duct are excised and pathologically evaluated even if it appears normal as nodal disease would prohibit LT[145]. Additionally, percutaneous or endoscopic ultrasound directed transperitoneal biopsy has been observed to cause peritoneal seeding, these interventions preclude transplant[145]. Indication and contraindications for LT for PCCA are outlined in Table 2[145]. After neoadjuvant therapy and in highly selected patients, 5-year OS was found to be 75%[145] and 5 year RFS of 65%[146].
Inclusion and exclusion criteria for liver transplantation for hilar-cholangiocarcinoma
| Liver transplantation for hilar-cholangiocarcinoma | |
| Inclusion criteria | Exclusion criteria |
| Diagnosis of cholangiocarcinoma | Uncontrolled infection |
| Transcatheter biopsy or brush cytology | Prior radiation or chemotherapy |
| CA19-9 > 100 mg/mL with mass or malignant appearing stricture | Prior or attempted biliary resection |
| Biliary ploidy by FISH with mass or malignant appearing stricture | Intrahepatic metastasis |
| Unresectable tumor above cystic duct | Extrahepatic disease |
| Radial tumor diameter < 3 cm | History of other malignancy within 5 years |
| Absence of intra and extrahepatic metastasis | Transperitoneal biopsy |
| Medically fit for transplantation | |
MINIMALLY INVASIVE SURGERY
Minimally invasive surgery has been used in the treatment of multiple hepato-pancreatico-biliary malignancies, including CCA. Over the past decade, multiple consensus statements have been written stating that minimally invasive liver surgery is safe in the hands of experienced surgeons[147-149]. In a study performed to evaluate laparoscopic versus open liver resection in patients with ICCA, the authors found that the Pringle Maneuver was used less frequently, and blood loss was less in the laparoscopic group. Additionally, there was no difference in complication rates between open and laparoscopic surgery. Importantly, there was no difference in oncologic outcomes[150]. Conversely, a recent retrospective study of 149 patients with PCCA who underwent laparoscopic or open resection reported that while most short-term surgical outcomes were similar, patients who underwent open surgical resection compared with laparoscopic resection had better OS and DFS[151]. In another study, a review of the National Cancer Database stratified patients by laparoscopic liver resection vs. open liver resection. In total, 2309 patients with ICCA underwent liver resection between 2010 and 2015. During that time, laparoscopic liver resection increased from 12% to 16% and was more common for wedge and segmental resections. However, nodal evaluation was only performed in 58% of all patients with ICCA. The use of laparoscopic surgery was found to exacerbate the lack of lymph node dissection, where patients who underwent laparoscopic surgery had significantly worse nodal evaluation than patients who underwent open surgery[152]. Recent meta-analyses demonstrated laparoscopic surgery for ICCA is safe and may provide improved short-term outcomes with no difference in long-term oncologic results[153,154]. Additionally, although very technically demanding even in the open setting with high morbidity[155], surgeons have performed robotic resections and associated reconstruction of PCCA[156]. Overall, data comparing minimally invasive vs. open hepatic resection among patients with CCA has been limited to retrospective reviews. Randomized controlled trials are needed to clarify the role of minimally invasive surgery in this patient population. Although minimally invasive surgery can be performed, the first priority is to perform safe surgery followed by achieving good oncologic outcomes.
CONCLUSION
CCAs are a heterogeneous group of tumors with poor long-term survival. Due to the complexity of care required to maximize patient outcomes, diagnostic workup and management decisions should be performed under the guidance of an experienced multidisciplinary team. Although many CCAs present as unresectable tumors, aggressive surgical management performed at specialized centers utilizing appropriate preoperative optimizing modalities have expanded the candidates suitable for resection and led to improved outcomes with decreasing mortality over the last decade. Furthermore, emerging efficacious adjuvant therapies will contribute to extending long-term survival for patients with CCA.
DECLARATIONS
Authors’ contributionsStudy concept and design: Hewitt DB, Brown ZJ, Pawlik TM
Literature research: Hewitt DB, Brown ZJ
Drafting of the manuscript and critical revision of the manuscript: Hewitt DB, Brown ZJ, Pawlik TM
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflict of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2021.
REFERENCES
1. Patel N, Benipal B. Incidence of cholangiocarcinoma in the USA from 2001 to 2015: a US cancer statistics analysis of 50 states. Cureus 2019;11:e3962.
2. Gad MM, Saad AM, Faisaluddin M, et al. Epidemiology of cholangiocarcinoma; United States incidence and mortality trends. Clin Res Hepatol Gastroenterol 2020;44:885-93.
3. Forner A, Vidili G, Rengo M, Bujanda L, Ponz-Sarvisé M, Lamarca A. Clinical presentation, diagnosis and staging of cholangiocarcinoma. Liver Int 2019;39 Suppl 1:98-107.
4. Banales JM, Marin JJG, Lamarca A, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol 2020;17:557-88.
5. Hyder O, Hatzaras I, Sotiropoulos GC, et al. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery 2013;153:811-8.
6. DeOliveira ML, Cunningham SC, Cameron JL, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg 2007;245:755-62.
8. Nagtegaal ID, Odze RD, Klimstra D, et al. WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020;76:182-8.
9. Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol 2011;8:512-22.
10. Chun YS, Pawlik TM, Vauthey JN. 8th edition of the AJCC cancer staging manual: pancreas and hepatobiliary cancers. Ann Surg Oncol 2018;25:845-7.
11. Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg 1992;215:31-8.
12. Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg 2001;234:507-17; discussion 517-9.
13. Matsuo K, Rocha FG, Ito K, et al. The Blumgart preoperative staging system for hilar cholangiocarcinoma: analysis of resectability and outcomes in 380 patients. J Am Coll Surg 2012;215:343-55.
14. Chaiteerakij R, Harmsen WS, Marrero CR, et al. A new clinically based staging system for perihilar cholangiocarcinoma. Am J Gastroenterol 2014;109:1881-90.
15. Nguyen Canh H, Takahashi K, Yamamura M, et al. Diversity in cell differentiation, histology, phenotype and vasculature of mass-forming intrahepatic cholangiocarcinomas. Histopathology 2021;79:731-50.
16. Deoliveira ML, Schulick RD, Nimura Y, et al. New staging system and a registry for perihilar cholangiocarcinoma. Hepatology 2011;53:1363-71.
17. Yamashita S, Morine Y, Imura S, et al. A new pathological classification of intrahepatic cholangiocarcinoma according to protein expression of SSTR2 and Bcl2. World J Surg Oncol 2021;19:142.
18. Welzel TM, Graubard BI, El-Serag HB, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study. Clin Gastroenterol Hepatol 2007;5:1221-8.
19. Jing W, Jin G, Zhou X, et al. Diabetes mellitus and increased risk of cholangiocarcinoma: a meta-analysis. Eur J Cancer Prev 2012;21:24-31.
20. Sahani D, Prasad SR, Tannabe KK, Hahn PF, Mueller PR, Saini S. Thorotrast-induced cholangiocarcinoma: case report. Abdom Imaging 2003;28:72-4.
21. McGee EE, Jackson SS, Petrick JL, et al. Smoking, alcohol, and biliary tract cancer risk: a pooling project of 26 prospective studies. J Natl Cancer Inst 2019;111:1263-78.
22. Welzel TM, Graubard BI, Zeuzem S, El-Serag HB, Davila JA, McGlynn KA. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology 2011;54:463-71.
23. Kubo S, Kinoshita M, Takemura S, et al. Characteristics of printing company workers newly diagnosed with occupational cholangiocarcinoma. J Hepatobiliary Pancreat Sci 2014;21:809-17.
24. Bergquist A, Ekbom A, Olsson R, et al. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol 2002;36:321-7.
26. Claessen MM, Vleggaar FP, Tytgat KM, Siersema PD, van Buuren HR. High lifetime risk of cancer in primary sclerosing cholangitis. J Hepatol 2009;50:158-64.
27. Lipsett PA, Pitt HA, Colombani PM, Boitnott JK, Cameron JL. Choledochal cyst disease. A changing pattern of presentation. Ann Surg 1994;220:644-52.
28. Khan SA, Thomas HC, Davidson BR, Taylor-robinson SD. Cholangiocarcinoma. Lancet 2005;366:1303-14.
29. Roukounakis N, Manolakopoulos S, Tzourmakliotis D, Bethanis S, McCarty TM, Cuhn J. Biliary tract malignancy and abnormal pancreaticobiliary junction in a Western population. J Gastroenterol Hepatol 2007;22:1949-52.
31. Shaib YH, El-Serag HB, Davila JA, Morgan R, McGlynn KA. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology 2005;128:620-6.
32. Kobayashi M, Ikeda K, Saitoh S, et al. Incidence of primary cholangiocellular carcinoma of the liver in Japanese patients with hepatitis C virus-related cirrhosis. Cancer 2000;88:2471-7.
33. Hsing AW, Zhang M, Rashid A, et al. Hepatitis B and C virus infection and the risk of biliary tract cancer: a population-based study in China. Int J Cancer 2008;122:1849-53.
34. Mecklin JP, Järvinen HJ, Virolainen M. The association between cholangiocarcinoma and hereditary nonpolyposis colorectal carcinoma. Cancer 1992;69:1112-4.
35. Lee SS, Kim MH, Lee SK, et al. Clinicopathologic review of 58 patients with biliary papillomatosis. Cancer 2004;100:783-93.
36. Yamada A, Komaki Y, Komaki F, Micic D, Zullow S, Sakuraba A. Risk of gastrointestinal cancers in patients with cystic fibrosis: a systematic review and meta-analysis. Lancet Oncol 2018;19:758-67.
37. Chen XX, Yin Y, Cheng JW, et al. BAP1 acts as a tumor suppressor in intrahepatic cholangiocarcinoma by modulating the ERK1/2 and JNK/c-Jun pathways. Cell Death Dis 2018;9:1036.
39. Akiba J, Nakashima O, Hattori S, et al. Clinicopathologic analysis of combined hepatocellular-cholangiocarcinoma according to the latest WHO classification. Am J Surg Pathol 2013;37:496-505.
40. Labib PL, Goodchild G, Pereira SP. Molecular pathogenesis of cholangiocarcinoma. BMC Cancer 2019;19:185.
41. Jones H, Alpini G, Francis H. Bile acid signaling and biliary functions. Acta Pharm Sin B 2015;5:123-8.
42. Chen B, Liu J, Ho TT, Ding X, Mo YY. ERK-mediated NF-κB activation through ASIC1 in response to acidosis. Oncogenesis 2016;5:e279.
43. Araki K, Shimura T, Suzuki H, et al. E/N-cadherin switch mediates cancer progression via TGF-β-induced epithelial-to-mesenchymal transition in extrahepatic cholangiocarcinoma. Br J Cancer 2011;105:1885-93.
44. Ogasawara S. Expression of angiogenic factors, basic fibroblast growth factor and vascular endothelial growth factor, in human biliary tract carcinoma cell lines. Hepatol Res 2001;20:97-113.
45. Leelawat K, Leelawat S, Tepaksorn P, et al. Involvement of c-Met/hepatocyte growth factor pathway in cholangiocarcinoma cell invasion and its therapeutic inhibition with small interfering RNA specific for c-Met. J Surg Res 2006;136:78-84.
46. Sirica AE. Role of ErbB family receptor tyrosine kinases in intrahepatic cholangiocarcinoma. World J Gastroenterol 2008;14:7033-58.
47. Network NCC. Hepatobiliary cancers v2.2021. https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf [Last accessed on 15 Nov 2021].
48. Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 2014;60:1268-89.
49. Lamarca A, Barriuso J, Chander A, et al. 18F-fluorodeoxyglucose positron emission tomography (18FDG-PET) for patients with biliary tract cancer: systematic review and meta-analysis. J Hepatol 2019;71:115-29.
50. Masselli G, Gualdi G. Hilar cholangiocarcinoma: MRI/MRCP in staging and treatment planning. Abdom Imaging 2008;33:444-51.
51. Tsao JI, Nimura Y, Kamiya J, et al. Management of hilar cholangiocarcinoma: comparison of an American and a Japanese experience. Ann Surg 2000;232:166-74.
52. Rajagopalan V, Daines WP, Grossbard ML, Kozuch P. Gallbladder and biliary tract carcinoma: a comprehensive update, part 1. Oncology (Williston Park) 2004;18:889-96.
53. Ebata T, Nagino M, Kamiya J, Uesaka K, Nagasaka T, Nimura Y. Hepatectomy with portal vein resection for hilar cholangiocarcinoma: audit of 52 consecutive cases. Ann Surg 2003;238:720-7.
54. Hemming AW, Reed AI, Fujita S, Foley DP, Howard RJ. Surgical management of hilar cholangiocarcinoma. Ann Surg 2005;241:693-9; discussion 699-702.
55. Mizuno T, Ebata T, Yokoyama Y, et al. Combined vascular resection for locally advanced perihilar cholangiocarcinoma. Ann Surg 2020; doi: 10.1097/SLA.0000000000004322.
56. Sano T, Shimizu Y, Senda Y, Kinoshita T, Nimura Y. Assessing resectability in cholangiocarcinoma. Hepat Oncol 2014;1:39-51.
57. Mansour JC, Aloia TA, Crane CH, Heimbach JK, Nagino M, Vauthey JN. Hilar cholangiocarcinoma: expert consensus statement. HPB (Oxford) 2015;17:691-9.
58. Liu F, Li Y, Wei Y, Li B. Preoperative biliary drainage before resection for hilar cholangiocarcinoma: whether or not? Dig Dis Sci 2011;56:663-72.
59. Mehrabi A, Khajeh E, Ghamarnejad O, et al. Meta-analysis of the efficacy of preoperative biliary drainage in patients undergoing liver resection for perihilar cholangiocarcinoma. Eur J Radiol 2020;125:108897.
60. Laurent A, Tayar C, Cherqui D. Cholangiocarcinoma: preoperative biliary drainage (Con). HPB (Oxford) 2008;10:126-9.
61. Nimura Y. Preoperative biliary drainage before resection for cholangiocarcinoma (Pro). HPB (Oxford) 2008;10:130-3.
62. Moole H, Bechtold M, Puli SR. Efficacy of preoperative biliary drainage in malignant obstructive jaundice: a meta-analysis and systematic review. World J Surg Oncol 2016;14:182.
63. Scheufele F, Schorn S, Demir IE, et al. Preoperative biliary stenting versus operation first in jaundiced patients due to malignant lesions in the pancreatic head: a meta-analysis of current literature. Surgery 2017;161:939-50.
64. Iacono C, Ruzzenente A, Campagnaro T, Bortolasi L, Valdegamberi A, Guglielmi A. Role of preoperative biliary drainage in jaundiced patients who are candidates for pancreatoduodenectomy or hepatic resection: highlights and drawbacks. Ann Surg 2013;257:191-204.
65. Dumonceau JM, Tringali A, Papanikolaou IS, et al. Endoscopic biliary stenting: indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - updated October 2017. Endoscopy 2018;50:910-30.
66. Paik WH, Loganathan N, Hwang JH. Preoperative biliary drainage in hilar cholangiocarcinoma: when and how? World J Gastrointest Endosc 2014;6:68-73.
67. Hameed A, Pang T, Chiou J, et al. Percutaneous vs. endoscopic pre-operative biliary drainage in hilar cholangiocarcinoma - a systematic review and meta-analysis. HPB (Oxford) 2016;18:400-10.
68. Al Mahjoub A, Menahem B, Fohlen A, et al. Preoperative biliary drainage in patients with resectable perihilar cholangiocarcinoma: is percutaneous transhepatic biliary drainage safer and more effective than endoscopic biliary drainage? J Vasc Interv Radiol 2017;28:576-82.
69. Coelen RJS, Roos E, Wiggers JK, et al. Endoscopic versus percutaneous biliary drainage in patients with resectable perihilar cholangiocarcinoma: a multicentre, randomised controlled trial. Lancet Gastroenterol Hepatol 2018;3:681-90.
70. Hwang S, Ha TY, Song GW, et al. Quantified risk assessment for major hepatectomy via the indocyanine green clearance rate and liver volumetry combined with standard liver volume. J Gastrointest Surg 2015;19:1305-14.
71. Adam R, Laurent A, Azoulay D, Castaing D, Bismuth H. Two-stage hepatectomy: a planned strategy to treat irresectable liver tumors. Ann Surg 2000;232:777-85.
72. Lam VW, Laurence JM, Johnston E, Hollands MJ, Pleass HC, Richardson AJ. A systematic review of two-stage hepatectomy in patients with initially unresectable colorectal liver metastases. HPB (Oxford) 2013;15:483-91.
73. Popescu GA, Alexandrescu ST, Grigorie RT, Stoica L, Apavaloaie CA, Hrehoreţ D. GOOD TO KNOW: the ALPPS procedure - embracing a new technique. Chirurgia (Bucur) 2017;112:332-41.
74. Liu Y, Yang Y, Gu S, Tang K. A systematic review and meta-analysis of associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) versus traditional staged hepatectomy. Medicine (Baltimore) 2019;98:e15229.
75. May BJ, Madoff DC. Portal vein embolization: rationale, technique, and current application. Semin Intervent Radiol 2012;29:81-9.
76. Abulkhir A, Limongelli P, Healey AJ, et al. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg 2008;247:49-57.
77. Akateh C, Ejaz AM, Pawlik TM, Cloyd JM. Neoadjuvant treatment strategies for intrahepatic cholangiocarcinoma. World J Hepatol 2020;12:693-708.
78. Kato A, Shimizu H, Ohtsuka M, et al. Downsizing chemotherapy for initially unresectable locally advanced biliary tract cancer patients treated with gemcitabine plus cisplatin combination therapy followed by radical surgery. Ann Surg Oncol 2015;22 Suppl 3:S1093-9.
79. Le Roy B, Gelli M, Pittau G, et al. Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Br J Surg 2018;105:839-47.
80. Rizzo A, Brandi G. Neoadjuvant therapy for cholangiocarcinoma: a comprehensive literature review. Cancer Treat Res Commun 2021;27:100354.
81. Konstantinidis IT, Groot Koerkamp B, Do RK, et al. Unresectable intrahepatic cholangiocarcinoma: systemic plus hepatic arterial infusion chemotherapy is associated with longer survival in comparison with systemic chemotherapy alone. Cancer 2016;122:758-65.
82. Cercek A, Boerner T, Tan BR, et al. Assessment of hepatic arterial infusion of floxuridine in combination with systemic gemcitabine and oxaliplatin in patients with unresectable intrahepatic cholangiocarcinoma: a phase 2 clinical trial. JAMA Oncol 2020;6:60-7.
83. Imamura H, Seyama Y, Kokudo N, et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg 2003;138:1198-206; discussion 1206.
84. Dimick JB, Cowan JA Jr, Knol JA, Upchurch GR Jr. Hepatic resection in the United States: indications, outcomes, and hospital procedural volumes from a nationally representative database. Arch Surg 2003;138:185-91.
85. Colavita PD, Tsirline VB, Belyansky I, et al. Regionalization and outcomes of hepato-pancreato-biliary cancer surgery in USA. J Gastrointest Surg 2014;18:532-41.
86. Kinoshita H, Sakai K, Hirohashi K, Igawa S, Yamasaki O, Kubo S. Preoperative portal vein embolization for hepatocellular carcinoma. World J Surg 1986;10:803-8.
87. Ebata T, Mizuno T, Yokoyama Y, Igami T, Sugawara G, Nagino M. Surgical resection for Bismuth type IV perihilar cholangiocarcinoma. Br J Surg 2018;105:829-38.
88. Abbas S, Sandroussi C. Systematic review and meta-analysis of the role of vascular resection in the treatment of hilar cholangiocarcinoma. HPB (Oxford) 2013;15:492-503.
89. Leen E, Ceccotti P, Moug SJ, et al. Potential value of contrast-enhanced intraoperative ultrasonography during partial hepatectomy for metastases: an essential investigation before resection? Ann Surg 2006;243:236-40.
90. Yin L, Zhao S, Zhu H, Ji G, Zhang X. Primary tumor resection improves survival in patients with multifocal intrahepatic cholangiocarcinoma based on a population study. Sci Rep 2021;11:12166.
91. Ribero D, Pinna AD, Guglielmi A, et al. Italian Intrahepatic Cholangiocarcinoma Study Group. Surgical approach for long-term survival of patients with intrahepatic cholangiocarcinoma: a multi-institutional analysis of 434 patients. Arch Surg 2012;147:1107-13.
92. Spolverato G, Yakoob MY, Kim Y, et al. The Impact of surgical margin status on long-term outcome after resection for intrahepatic cholangiocarcinoma. Ann Surg Oncol 2015;22:4020-8.
93. Raab R, Schlitt HJ, Oldhafer KJ, Bornscheuer A, Lang H, Pichlmayr R. Ex-vivo resection techniques in tissue-preserving surgery for liver malignancies. Langenbecks Arch Surg 2000;385:179-84.
94. Zawistowski M, Nowaczyk J, Jakubczyk M, Domagała P. Outcomes of ex vivo liver resection and autotransplantation: a systematic review and meta-analysis. Surgery 2020;168:631-42.
95. Farges O, Fuks D, Boleslawski E, et al. Influence of surgical margins on outcome in patients with intrahepatic cholangiocarcinoma: a multicenter study by the AFC-IHCC-2009 study group. Ann Surg 2011;254:824-29; discussion 830.
96. Shimada K, Sano T, Sakamoto Y, Esaki M, Kosuge T, Ojima H. Clinical impact of the surgical margin status in hepatectomy for solitary mass-forming type intrahepatic cholangiocarcinoma without lymph node metastases. J Surg Oncol 2007;96:160-5.
97. Tamandl D, Herberger B, Gruenberger B, Puhalla H, Klinger M, Gruenberger T. Influence of hepatic resection margin on recurrence and survival in intrahepatic cholangiocarcinoma. Ann Surg Oncol 2008;15:2787-94.
98. Hong JC, Jones CM, Duffy JP, et al. Comparative analysis of resection and liver transplantation for intrahepatic and hilar cholangiocarcinoma: a 24-year experience in a single center. Arch Surg 2011;146:683-9.
99. Nuzzo G, Giuliante F, Ardito F, et al. Italian Chapter of the International Hepato-Pancreato-Biliary Association. Improvement in perioperative and long-term outcome after surgical treatment of hilar cholangiocarcinoma: results of an Italian multicenter analysis of 440 patients. Arch Surg 2012;147:26-34.
100. Hasegawa S, Ikai I, Fujii H, Hatano E, Shimahara Y. Surgical resection of hilar cholangiocarcinoma: analysis of survival and postoperative complications. World J Surg 2007;31:1256-63.
101. Zhang XF, Squires MH 3rd, Bagante F, et al. The impact of intraoperative re-resection of a positive bile duct margin on clinical outcomes for hilar cholangiocarcinoma. Ann Surg Oncol 2018;25:1140-9.
102. Neuhaus P, Jonas S, Bechstein WO, et al. Extended resections for hilar cholangiocarcinoma. Ann Surg 1999;230:808-18; discussion 819.
103. Edge SB, Compton CC. The American Joint Committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
104. Lim JH, Choi GH, Choi SH, Kim KS, Choi JS, Lee WJ. Liver resection for Bismuth type I and Type II hilar cholangiocarcinoma. World J Surg 2013;37:829-37.
105. de Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol 2011;29:3140-5.
106. Zhang XF, Chen Q, Kimbrough CW, et al. Lymphadenectomy for intrahepatic cholangiocarcinoma: has nodal evaluation been increasingly adopted by surgeons over time? J Gastrointest Surg 2018;22:668-75.
107. Ercolani G, Grazi GL, Ravaioli M, et al. The role of lymphadenectomy for liver tumors: further considerations on the appropriateness of treatment strategy. Ann Surg 2004;239:202-9.
108. Kitagawa Y, Nagino M, Kamiya J, et al. Lymph node metastasis from hilar cholangiocarcinoma: audit of 110 patients who underwent regional and paraaortic node dissection. Ann Surg 2001;233:385-92.
109. Kim SH, Han DH, Choi GH, Choi JS, Kim KS. Extent of lymph node dissection for accurate staging in intrahepatic cholangiocarcinoma. J Gastrointest Surg 2021; doi: 10.1007/s11605-021-05039-5.
110. Amin MB, Greene FL, Edge SB, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin 2017;67:93-9.
111. Marsh Rde W, Alonzo M, Bajaj S, et al. Comprehensive review of the diagnosis and treatment of biliary tract cancer 2012. Part I: diagnosis-clinical staging and pathology. J Surg Oncol 2012;106:332-8.
112. DeOliveira ML, Kambakamba P, Clavien PA. Advances in liver surgery for cholangiocarcinoma. Curr Opin Gastroenterol 2013;29:293-8.
113. Razumilava N, Gores GJ. Classification, diagnosis, and management of cholangiocarcinoma. Clin Gastroenterol Hepatol 2013;11:13-21.e1; quiz e3-4.
114. Loehrer AP, House MG, Nakeeb A, Kilbane EM, Pitt HA. Cholangiocarcinoma: are North American surgical outcomes optimal? J Am Coll Surg 2013;216:192-200.
115. Ebata T, Yokoyama Y, Igami T, et al. Hepatopancreatoduodenectomy for cholangiocarcinoma: a single-center review of 85 consecutive patients. Ann Surg 2012;256:297-305.
116. Hemming AW, Magliocca JF, Fujita S, et al. Combined resection of the liver and pancreas for malignancy. J Am Coll Surg 2010;210:808-14, 814-6.
117. Sarmiento JM, Nagorney DM, Sarr MG, Farnell MB. Periampullary cancers: are there differences? Surg Clin North Am 2001;81:543-55.
118. Askew J, Connor S. Review of the investigation and surgical management of resectable ampullary adenocarcinoma. HPB (Oxford) 2013;15:829-38.
119. Cloyd JM, Ejaz A, Pawlik TM. The landmark series: intrahepatic cholangiocarcinoma. Ann Surg Oncol 2020;27:2859-65.
120. Gamboa AC, Maithel SK. The landmark series: gallbladder cancer. Ann Surg Oncol 2020;27:2846-58.
121. Shroff RT, Kennedy EB, Bachini M, et al. Adjuvant therapy for resected biliary tract cancer: ASCO clinical practice guideline. J Clin Oncol 2019;37:1015-27.
122. Cloyd JM, Pawlik TM. Adjuvant therapy for biliary tract cancers: new evidence to resolve old questions. J Oncol Pract 2018;14:723-4.
123. Horgan AM, Amir E, Walter T, Knox JJ. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol 2012;30:1934-40.
124. Ghidini M, Tomasello G, Botticelli A, et al. Adjuvant chemotherapy for resected biliary tract cancers: a systematic review and meta-analysis. HPB (Oxford) 2017;19:741-8.
125. Takada T, Amano H, Yasuda H, et al. Study Group of Surgical Adjuvant Therapy for Carcinomas of the Pancreas and Biliary Tract. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? Cancer 2002;95:1685-95.
126. Valle J, Wasan H, Palmer DH, et al. ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81.
127. André T, Reyes-Vidal JM, Fartoux L, et al. Gemcitabine and oxaliplatin in advanced biliary tract carcinoma: a phase II study. Br J Cancer 2008;99:862-7.
128. Edeline J, Benabdelghani M, Bertaut A, et al. Gemcitabine and oxaliplatin chemotherapy or surveillance in resected biliary tract cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): a randomized phase III study. J Clin Oncol 2019;37:658-67.
129. Primrose JN, Fox RP, Palmer DH, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol 2019;20:663-73.
130. Ebata T, Hirano S, Konishi M, et al. Bile Duct Cancer Adjuvant Trial (BCAT) Study Group. Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. Br J Surg 2018;105:192-202.
131. Itano O, Takemura Y, Kishida N, et al. A prospective feasibility study of one-year administration of adjuvant S-1 therapy for resected biliary tract cancer in a multi-institutional trial (Tokyo Study Group for Biliary Cancer: TOSBIC01). BMC Cancer 2020;20:688.
132. Ben-Josef E, Guthrie KA, El-Khoueiry AB, et al. SWOG S0809: a phase II intergroup trial of adjuvant capecitabine and gemcitabine followed by radiotherapy and concurrent capecitabine in extrahepatic cholangiocarcinoma and gallbladder carcinoma. J Clin Oncol 2015;33:2617-22.
133. Hyder O, Marsh JW, Salem R, et al. Intra-arterial therapy for advanced intrahepatic cholangiocarcinoma: a multi-institutional analysis. Ann Surg Oncol 2013;20:3779-86.
134. Sullivan RD, Norcross JW, Watkins Jr E. Chemotherapy of metastatic liver cancer by prolonged hepatic-artery infusion. N Engl J Med 1964;270(7):321-327.
136. Jarnagin WR, Schwartz LH, Gultekin DH, et al. Regional chemotherapy for unresectable primary liver cancer: results of a phase II clinical trial and assessment of DCE-MRI as a biomarker of survival. Ann Oncol 2009;20:1589-95.
137. Zamora-Valdes D, Heimbach JK. Liver transplant for cholangiocarcinoma. Gastroenterol Clin North Am 2018;47:267-80.
138. Becker NS, Rodriguez JA, Barshes NR, O'Mahony CA, Goss JA, Aloia TA. Outcomes analysis for 280 patients with cholangiocarcinoma treated with liver transplantation over an 18-year period. J Gastrointest Surg 2008;12:117-22.
139. Goldstein RM, Stone M, Tillery GW, et al. Is liver transplantation indicated for cholangiocarcinoma? Am J Surg 1993;166:768-72.
140. Lunsford KE, Javle M, Heyne K, et al. Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: a prospective case-series. Lancet Gastroenterol Hepatol 2018;3:337-48.
141. Pichlmayr R, Weimann A, Oldhafer KJ, et al. Role of liver transplantation in the treatment of unresectable liver cancer. World J Surg 1995;19:807-13.
142. Sapisochin G, Facciuto M, Rubbia-Brandt L, et al. iCCA International Consortium. Liver transplantation for "very early" intrahepatic cholangiocarcinoma: international retrospective study supporting a prospective assessment. Hepatology 2016;64:1178-88.
143. Heimbach JK, Haddock MG, Alberts SR, et al. Transplantation for hilar cholangiocarcinoma. Liver Transpl 2004;10:S65-8.
144. Rea DJ, Heimbach JK, Rosen CB, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg 2005;242:451-8; discussion 458-61.
145. Rosen CB, Heimbach JK, Gores GJ. Liver transplantation for cholangiocarcinoma. Transpl Int 2010;23:692-7.
146. Darwish Murad S, Kim WR, Harnois DM, et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology 2012;143:88-98.e3; quiz e14.
147. Abu Hilal M, Aldrighetti L, Dagher I, et al. The southampton consensus guidelines for laparoscopic liver surgery: from indication to implementation. Ann Surg 2018;268:11-8.
148. Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29.
149. Buell JF, Cherqui D, Geller DA, et al. World Consensus Conference on Laparoscopic Surgery. The international position on laparoscopic liver surgery: the Louisville statement, 2008. Ann Surg 2009;250:825-30.
150. Lee W, Park JH, Kim JY, et al. Comparison of perioperative and oncologic outcomes between open and laparoscopic liver resection for intrahepatic cholangiocarcinoma. Surg Endosc 2016;30:4835-40.
151. Ma D, Wang W, Wang J, et al. Laparoscopic versus open surgery for hilar cholangiocarcinoma: a retrospective cohort study on short-term and long-term outcomes. Surg Endosc 2021; doi: 10.1007/s00464-021-08686-6.
152. Martin SP, Drake J, Wach MM, et al. Laparoscopic approach to intrahepatic cholangiocarcinoma is associated with an exacerbation of inadequate nodal staging. Ann Surg Oncol 2019;26:1851-7.
153. Machairas N, Kostakis ID, Schizas D, Kykalos S, Nikiteas N, Sotiropoulos GC. Meta-analysis of laparoscopic versus open liver resection for intrahepatic cholangiocarcinoma. Updates Surg 2021;73:59-68.
154. Ziogas IA, Esagian SM, Giannis D, et al. Laparoscopic versus open hepatectomy for intrahepatic cholangiocarcinoma: an individual patient data survival meta-analysis. Am J Surg 2021;222:731-8.
155. Wiggers JK, Groot Koerkamp B, Cieslak KP, et al. Postoperative mortality after liver resection for perihilar cholangiocarcinoma: development of a risk score and importance of biliary drainage of the future liver remnant. J Am Coll Surg 2016;223:321-31.e1.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Hewitt DB, Brown ZJ, Pawlik TM. Surgical management of cholangiocarcinoma. Hepatoma Res 2021;7:75. http://dx.doi.org/10.20517/2394-5079.2021.83
AMA Style
Hewitt DB, Brown ZJ, Pawlik TM. Surgical management of cholangiocarcinoma. Hepatoma Research. 2021; 7: 75. http://dx.doi.org/10.20517/2394-5079.2021.83
Chicago/Turabian Style
Hewitt, D. Brock, Zachary J. Brown, Timothy M. Pawlik. 2021. "Surgical management of cholangiocarcinoma" Hepatoma Research. 7: 75. http://dx.doi.org/10.20517/2394-5079.2021.83
ACS Style
Hewitt, DB.; Brown ZJ.; Pawlik TM. Surgical management of cholangiocarcinoma. Hepatoma. Res. 2021, 7, 75. http://dx.doi.org/10.20517/2394-5079.2021.83
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 41 clicks
Cite This Article 41 clicks


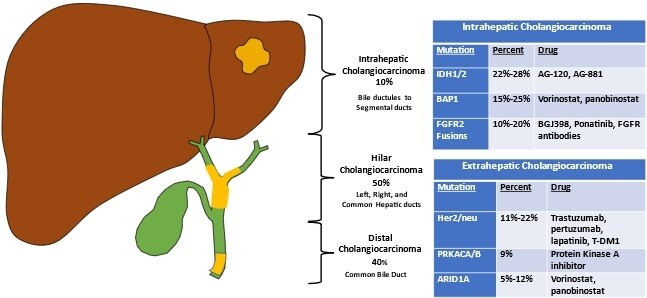








Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.