Non-invasive detection of liver cirrhosis - the “Essen algorithm”
The global prevalence of chronic liver disease (CLD) is relatively high and reported to occur in up to 20% of people[1]. Regarding pathogenesis, chronic inflammation leads to hepatocyte destruction and progressive fibrosis of the liver parenchyma. Cirrhosis is the (mostly irreversible) late-stage of scarring of the liver caused by various forms of CLD. Arising complications due to portal hypertension and/or hepatocellular carcinoma are responsible for the dramatically increasing morbidity and mortality among this patient collective[2,3]. Identification of (advanced) fibrosis is relevant in the course of CLD since it delivers prognostic information and assists in establishing treatment priorities in various clinical conditions. However, liver regeneration is highly efficient and fibrosis potentially reversible whilst a certain “point of no return” is not surpassed. Diagnosis and assessment of the severity of fibrosis is therefore important not only to allow risk stratification but also to deliver adequate therapy to avoid progression of CLD[4-6].
Today, histological analysis of the liver parenchyma obtained by either percutaneous or laparoscopic biopsy is considered as standard procedure for evaluating the severity of fibrosis. However, due to its invasiveness, this method is impracticable as a widespread tool for screening and monitoring CLD in routine clinical visits[7,8]. Thus, there is a certain requirement for non-invasive techniques to assess the severity of CLD which is reflected by different stages of fibrosis. Therein, different diagnostic instruments have been developed and, in part, implemented in clinical practice and international guidelines. However, proposed serum biomarkers such as aspartate aminotransferase (AST) to alanine aminotransferase (ALT) ratio, AST-to-platelet ratio index, and fibrosis-4 score do not seem to be reliable enough since these enzymes are not exclusively expressed in hepatocytes and influenced by multiple confounders such as acute inflammation, cholestasis, or half-life of the enzymes. In addition, laboratory parameters are compensated and within normal ranges in many cases even though (decompensated) cirrhosis is present and liver function is yet significantly impaired[9-11]. In the early years of this millennium, measurement of liver stiffness by transient elastography (TE, FibroScan) has been introduced and extensively examined. Herein, liver stiffness (or hardness) is evaluated by measuring the velocity of a vibration wave (shear wave). This non-invasive technique has been well-established in the assessment of liver fibrosis with convincing diagnostic accuracy for different stages of fibrosis and cirrhosis of varying etiology. However, TE cannot be used in individuals with ascites, and is associated with higher failure rates or unreliable results in obese patients. Furthermore, diagnostic accuracy of TE is impaired by the state of acute hepatitis and accompanying inflammatory activity[12-15]. More recently, liver maximum capacity (LiMAx) breath test has been developed as a novel non-invasive technique to determine actual enzymatic liver function. It is based on the metabolic turnover of intravenously injected 13C-labelled methacetin solution and produces a quantitative measurement of maximal liver function capacity. The test reveals metabolic (dys)function at cellular level in real-time, which correlates with disease severity in various clinical conditions as previously investigated[16-18].
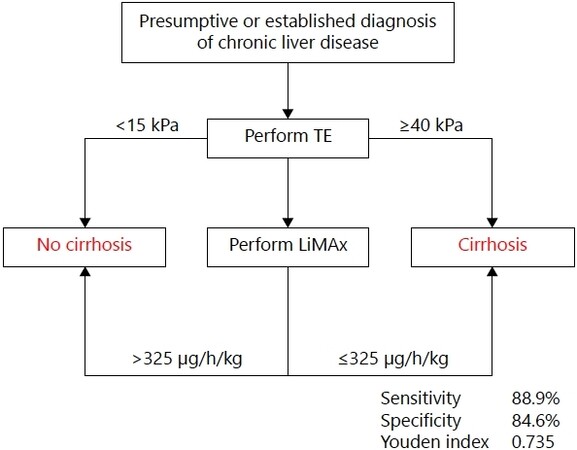
In a currently published work of our group, we evaluated the diagnostic accuracy of the parameters mentioned above in comparison with the “gold standard” determined by histology in a cohort of over 100 individuals suffering from CLD with different etiologies. Histological analysis of liver fibrosis was therefore graduated according to the widely accepted “Desmet scoring system”[19]. Herein, TE and LiMAx showed good diagnostic accuracy in detecting cirrhosis [≥ F4; TE: cut-off 15.0 kilo Pascals, sensitivity 88.9%, specificity 76.9%, positive predictive value (PPV) 47.1%, negative predictive value (NPV) 96.8%, and Youden index 0.66; LiMAx: cut-off 249 μg/h/kg, sensitivity 75.0%, specificity 89.0%, PPV 62.5%, NPV 93.6%, and Youden index 0.64]. However, combination of these two methods significantly increased the diagnostic accuracy to rule out cirrhosis, which we summarized by the novel “Essen algorithm” (sensitivity 88.9%, specificity 84.6%, PPV 0.57, NPV 0.97, and Youden index 0.735; Figure 1)[20]. We firmly believe that this novel algorithm will improve clinical management of patients suffering from CLD.
Figure 1. The “Essen algorithm”: diagnostic algorithm to rule out cirrhosis in patients with chronic liver disease. TE: Transient elastography; LiMAx: liver maximum capacity.
DECLARATIONS
Authors’ contributionsWrote the article: Buechter M
Conzeptualized and corrected the article: Gerken G
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestBoth authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2021.
REFERENCES
1. Bellentani S, Tiribelli C, Saccoccio G, et al. Prevalence of chronic liver disease in the general population of northern Italy: the Dionysos Study. Hepatology 1994;20:1442-9.
2. Engelmann G, Quader J, Teufel U, et al. Limitations and opportunities of non-invasive liver stiffness measurement in children. World J Hepatol 2017;9:409-17.
3. Wong GL-H. Non-invasive assessments for liver fibrosis: the crystal ball we long for. J Gastroenterol Hepatol 2018;33:1009-15.
4. Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370-98.
5. Stasi C, Milani S. Evolving strategies for liver fibrosis staging: non-invasive assessment. World J Gastroenterol 2017;23:191-6.
6. Ellis EL, Mann DA. Clinical evidence for the regression of liver fibrosis. J Hepatol 2012;56:1171-80.
7. Atwell TD, Smith RL, Hesley GK, et al. Incidence of bleeding after 15,181 percutaneous biopsies and the role of aspirin. AJR Am J Roentgenol 2010;194:784-9.
9. Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388-402.
10. Association for Study of Liver, Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol 2015;63:237-64.
11. Peleg N, Issachar A, Sneh-Arbib O, et al. AST to Platelet Ratio Index and fibrosis 4 calculator scores for non-invasive assessment of hepatic fibrosis in patients with non-alcoholic fatty liver disease. Dig Liver Dis 2017;49:1133-8.
12. Wu HM, Sheng L, Wang Q, et al. Performance of transient elastography in assessing liver fibrosis in patients with autoimmune hepatitis-primary biliary cholangitis overlap syndrome. World J Gastroenterol 2018;24:737-43.
13. Karlas T, Petroff D, Sasso M, et al. Impact of controlled attenuation parameter on detecting fibrosis using liver stiffness measurement. Aliment Pharmacol Ther 2018;47:989-1000.
14. Guo L, Zheng L, Hu L, et al. Transient Elastography (FibroScan) Performs Better Than Non-Invasive Markers in Assessing Liver Fibrosis and Cirrhosis in Autoimmune Hepatitis Patients. Med Sci Monit 2017;23:5106-12.
15. Wilder J, Patel K. The clinical utility of FibroScan(®) as a noninvasive diagnostic test for liver disease. Med Devices Auckl NZ 2014;7:107-14.
16. Buechter M, Kersting S, Gerken G, et al. Enzymatic liver function measured by LiMAx - a reliable diagnostic and prognostic tool in chronic liver disease. Sci Rep 2019;9:13577.
17. Jara M, Bednarsch J, Valle E, et al. Reliable assessment of liver function using LiMAx. J Surg Res 2015;193:184-9.
18. Malinowski M, Jara M, Lüttgert K, et al. Enzymatic liver function capacity correlates with disease severity of patients with liver cirrhosis: a study with the LiMAx test. Dig Dis Sci 2014;59:2983-91.
19. Desmet VJ, Gerber M, Hoofnagle JH, et al. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology 1994;19:1513-20.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Buechter M, Gerken G. Non-invasive detection of liver cirrhosis - the “Essen algorithm”. Hepatoma Res 2021;7:57. http://dx.doi.org/10.20517/2394-5079.2021.99
AMA Style
Buechter M, Gerken G. Non-invasive detection of liver cirrhosis - the “Essen algorithm”. Hepatoma Research. 2021; 7: 57. http://dx.doi.org/10.20517/2394-5079.2021.99
Chicago/Turabian Style
Buechter, Matthias, Guido Gerken. 2021. "Non-invasive detection of liver cirrhosis - the “Essen algorithm”" Hepatoma Research. 7: 57. http://dx.doi.org/10.20517/2394-5079.2021.99
ACS Style
Buechter, M.; Gerken G. Non-invasive detection of liver cirrhosis - the “Essen algorithm”. Hepatoma. Res. 2021, 7, 57. http://dx.doi.org/10.20517/2394-5079.2021.99
About This Article
Copyright
Data & Comments
Data

 Cite This Article 6 clicks
Cite This Article 6 clicks










Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.