Steatohepatitic hepatocellular carcinoma
Abstract
Subtypes of hepatocellular carcinoma are important for 2 primary reasons: they help improve diagnostic accuracy, as different subtypes have their own diagnostic pitfalls; they are an important building block to the personalization of patient care, as subtypes are enriched for shared genetic changes and biological associations. The most common subtype of hepatocellular carcinoma is steatohepatitic hepatocellular carcinoma (SH-HCC), a subtype that is strongly linked to tumorigenesis in the setting of the metabolic syndrome and metabolic-associated liver disease (MAFLD) and/or alcoholic hepatitis. SH-HCC shows macrovesicular steatosis, balloon cells, Mallory hyaline, intratumoral inflammation, and intratumoral fibrosis. This review examines the historical development of this subtype and explores in detail the histological features that are used to define SH-HCC. The strongest molecular correlates to-date include a low frequency of CTNNB1 mutations and possible activation of the IL6/JAK/STAT pathway. In addition, critical unresolved questions are discussed in detail to refine the histological definition of SH-HCC, including the minimal histological thresholds needed to make the diagnosis, as well as whether or not SH-HCC currently is a mixed category of tumors, containing some tumors where the distinctive morphology is driven by tumor-specific genetic changes, and other tumors where the findings are an epiphenomenon, a reflection of metabolic or alcohol-associated fatty liver disease, and not necessarily of genetic/epigenetic changes.
Keywords
INTRODUCTION
Hepatocellular carcinomas have a variety of morphological patterns, some of which form distinct subtypes of hepatocellular carcinoma[1,2]. Of these, the most common subtype is steatohepatitic hepatocellular carcinoma (SH-HCC)[1]. The observation that there can be fat in a hepatocellular carcinoma has been known for a very long time[3], but a 2010 paper by Salomao et al.[4] was the first to recognize steatohepatitic hepatocellular carcinoma (SH-HCC) as a distinct histological variant. They studied liver explants with cirrhosis from chronic hepatitis C, noting that a subset of these cases (36%) showed morphological findings reminiscent of steatohepatitis. Analysis of the clinical correlates showed cases with a SH-HCC morphology were highly enriched for features of the metabolic syndrome and the background livers were highly enriched for metabolic associated fatty liver disease (MAFLD). A 2012 follow-up study by the same group extended the findings to livers with other etiologies of underlying liver disease and to livers without cirrhosis, showing an overall frequency of 14% for SH-HCC; the study also affirmed the strong correlation with the metabolic syndrome, and reported that alcoholic hepatitis was another risk factor[5]. Subsequent studies have extended the core association of SH-HCC with the metabolic syndrome to a number of different countries including India[6], Japan[7], and Hong Kong[8].
HISTOLOGICAL DEFINITION
SH-HCC was originally defined as having the ordinary features of steatohepatitis, including at least 5% macrovesicular steatosis, at least mild intratumoral inflammation (neutrophilic or lymphocytic), balloon cells, Mallory-Denk bodies, and intratumoral pericellular fibrosis[4]. The percent of the tumor with these findings that was needed to qualify for SH-HCC was originally defined as ≥ 5%[4], but the cut-off was later moved to ≥ 50% by the same group of authors[5]. Nonetheless, subsequent studies have generally used the 5% or greater cut-off[6,7] [Table 1]. While there is little data to specifically decide on the best cut-off, a higher cut off, such as 50% seems more likely to capture a biologically/molecularly cohesive set of cases[9,10].
Definition of steatohepatitic hepatocellular carcinoma
| Ref. | Features | Cut-off | Details on other criteria |
| Salomao et al.[4] | 1. Macrovesicular steatosis 2. Balloon cells 3. Mallory Hyaline 4. Intratumoral inflammation (lymphocytic or neutrophilic) 5. Intratumoral fibrosis; pericellular or scirrhous-like fibrosis with thick bands of collagen | 5% of tumor | All cases showed steatosis and ballooning, the frequency of the remaining features varied; 27% had minimal or no fibrosis |
| Salomao et al.[5] | Same | 50% of tumor | At least 3 of the features were required, usually all 5 features were present; 90% had fibrosis |
| Jain et al.[6] | Same | 5% of tumor | All cases showed steatosis, ballooning, and inflammation. 95% showed pericellular fibrosis |
| Shibahara et al.[7] | Same | 5% of tumor | Data analyzed separately for cases with (1) 4 of 5 features even when focal and (2) 5 of 5 features where steatohepatitis dominated morphology |
| Chan et al.[8] | Same | 5% of tumor | Data analyzed separately for cases with (1) steatosis only (2) cases with steatohepatitis, requiring additional features of ballooning and inflammation |
| Qin et al.[11] | Same | 5% of tumor 50% of tumor; subset analyzed with a 30% cutoff | At least 3 of 5 features. Data analyzed separately for cases both cut-offs |
CLINICAL AND OTHER CORRELATES
The association of SH-HCC with the metabolic syndrome (obesity, hypertension, diabetes, and hyperlipidemia) has been identified in essentially all studies to date. On the other hand, alcoholic liver disease was identified as a likely risk factor early on[5], but subsequent data were inconsistent, with some studies showing an association[11], and others showing the opposite; in fact, one study found alcoholic liver disease had a protective effect against developing the SH-HCC morphology[7].
Studies have fairly consistently found no difference in the overall survival and/or disease free survival for SH-HCC following resection, compared to conventional HCC[5,7,8]. One study, however, found a potential increased risk for late relapse of disease after resection[8]. Data are mixed on differences between SH-HCC and conventional HCC in terms of tumor size, tumor grade, and the presence or absence of vascular invasion, but several larger studies found that SH-HCC were more likely to be smaller than conventional HCC[7,8], although other studies have found no association with size[5,6], or even an association with larger size[9]. One large study found a lower frequency of small vessel and large vessel invasion[8] but this has not been widely confirmed. Other tentative associations found in larger studies include earlier tumor stage[8], lower serum AFP levels[8], and a higher frequency of bile duct invasion[7].
HISTOLOGICAL FINDINGS
Intratumoral steatosis, balloon cells, inflammation
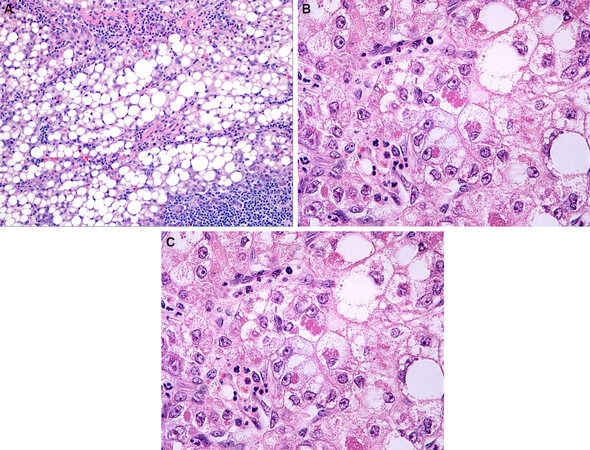
By definition, SH-HCC must have macrovesicular steatosis [Figure 1A]. While most studies have required 5% or greater of the tumor show macrovesicular steatosis, there is a wide range in the percentages of fat reported by various studies [Table 2]. Explanation for the varying degrees of fat within SH-HCC remains elusive, but studies have not found a direct association between the amount of fat in the tumor and the amount of fat in the background liver[8].
Figure 1. Steatohepatitic hepatocellular carcinoma (SH-HCC). The tumor shows fat, inflammation and fibrosis (Panel A). SH-HCC Numerous ballooned tumor cells and Mallory-Denk bodies are seen (Panel B). This SH-HCC showed scirrhous like intratumoral fibrosis (Panel C).
Percent steatosis in steatohepatitic hepatocellular carcinoma
In the non-neoplastic liver, fatty liver disease can be divided into patterns of steatosis and steatohepatitis based on the presence of balloon cells and lobular inflammation. A similar distinction can be made in HCC, as some cases show steatosis alone and some show the full features of SH-HCC [Figure 1]. Of the group of HCC that have at least mild steatosis within them, one study found that 58% had additional findings of ballooning and inflammation to qualify as SH-HCC[12]. Those HCC with fat but without steatohepatitis are called steatotic HCC[8]. While most studies have focused on SH-HCC, and excluded steatotic HCC, at least one study specifically compared the two, and found both were clinically and histologically similar, other than a lower frequency of diabetes in steatotic HCC versus SH-HCC[8].
The frequency of balloon cell change [Figure 1B] in SH-HCC is generally high, typically close to 100%[4,7,13], while the frequency of Mallory hyaline is lower but still found in 80% or more of cases[4,13]. The inflammation in SH-HCC [Table 3] is primarily lymphocytic [Figure 1A] but also shows neutrophils, often in the setting of satellitosis around ballooned hepatocytes, and occasional plasma cells[4-6].
Intratumoral fibrosis
Intratumoral fibrosis has not been a requirement for the diagnosis of SH-HCC in most studies, but most SH-HCC have at least mild pericellular fibrosis, with an overall prevalence of fibrosis that ranges from 75% to 100%[4,13]. The 1st paper to describe SH-HCC[4] astutely noted 2 distinct patterns of intratumoral fibrosis in SH-HCC: the first pattern is pericellular fibrosis, equivalent to the classic fibrosis pattern seen with steatohepatitis in the non-neoplastic liver, while the second pattern shows thick bundles of fibrosis more typical of the scirrhous pattern of HCC [Figure 1C]. These 2 distinct patterns were further confirmed in subsequent papers[5,7], one of which reported that about 9% of SH-HCC had scirrhous-like fibrosis[7]. Hatano et al.[14], when studying scirrhous HCC, noted that a subset of cases also had steatosis/steatohepatitis, and it appears likely that this group of scirrhous HCC with fatty changes is the same as SH-HCC that have scirrhous-like fibrosis. Whether these cases are best classified as scirrhous HCC or as SH-HCC remains unclear, but findings to-date suggest they share key similarities to SH-HCC[14]. On the other hand, the distinctly different fibrosis patterns suggest the tumors are likely to have some biological/molecular differences when compared to SH-HCC with conventional patterns of pericellular fibrosis.
Multifocal tumors
When a liver has multiple HCC, they can all show SH-HCC morphology or can show a mixture of SH-HCC and conventional HCC (about 50%-75% of cases)[7,8].
Background liver
The strong association between SH-HCC and the metabolic syndrome and/or alcohol use is evident at several levels. First, about 40%-70% of HCC in patients with the metabolic syndrome and/or alcoholic liver disease will have the SH-HCC morphology[5,11,15]. Second, when tumors show the SH-HCC morphology, studies have consistently found an association with steatosis/steatohepatitis in the background liver, with a frequency of 65%-95%, which is about 2X that seen in the background livers of conventional HCC[4,5,7]. In most cases of SH-HCC, the background liver shows mild macrovesicular steatosis and/or mild steatohepatitis[7]. Interestingly, one study found that SH-HCC tended to have a higher percentage of fat than that seen in the background liver[7], although other studies have not reported this association[5].
On the other hand, the strong association with the metabolic syndrome and/or alcohol use is not universal, as seen by several lines of evidence. First, about 1/3 of HCC that arise in patients with histories of the metabolic syndrome or significant alcohol use do not show the SH-HCC morphology. Secondly, a small group of SH-HCC has no fat in the background liver and lacks risk factors for the metabolic syndrome or alcohol use[10].
In other cases, patients may have known risk factors for fatty liver disease, such as alcoholic liver disease, but the background liver will no longer show steatosis or steatohepatitis, despite the tumor having a SH-HCC morphology[5]. This may reflect the broader observation that changes of steatohepatitis can be lost as livers become cirrhotic. Nonetheless, the frequency of cirrhosis is similar between conventional HCC and SH-HCC[7,16].
Confirmatory immunostain tests
No confirmatory immunostains have been developed to identify SH-HCC. Since the morphological findings are so distinctive, it’s not clear if they are needed, at least in most cases. On the other hand, as noted below in the section on ongoing challenges, there remains significant work to do to improve the H&E definition. Trichrome stains can identify intratumoral fibrosis, but are not necessary for the diagnosis. Likewise, immunostains such as CK8/18 or ubiquitin will highlight Mallory-Denk bodies[4,5], but are not necessary for the diagnosis.
Molecular correlations
Polymorphisms in the gene PNPLA3 have been linked to an increased risk for fatty liver disease in the non-neoplastic liver, but are not associated with SH-HCC[8]. In comparison to conventional hepatocellular carcinoma, SH-HCC are less likely to show beta-catenin activation; they have a lower frequency of beta-catenin nuclear accumulation (6% vs. 25%), a lower frequency of glutamine synthetase over expression (4% vs. 26%), and a lower frequency of CTNNB1 mutations[17]. A role for the sonic hedgehog pathway has been postulated[17], but not confirmed by subsequent studies. SH-HCC also have a lower frequency of TP53 and TERT promoter mutations, compared to conventional HCC, and more frequent activation of the IL6/JAK/STAT pathway[18]. SH-HCC are more likely than conventional HCC to overexpress serum amyloid A (50% vs. 13%) and C-reactive protein (42% vs. 16%)[9], further supporting a potential role for the IL6/JAK/STAT pathway, although such observation may be attributed to activation by other factors (i.e., underlying HBV or alcoholic hepatitis and the inflammation from therein). In fact, an association between HBV-associated HCC and CRP has been suggested[19].
Transcriptome studies have suggested a link between SH-HCC and what is called the S1 molecular subtype of HCC (which is characterized by TGF-beta pathway signaling)[11,16]. These molecular observations, however, are not yet strong enough to help refine the definition of SH-HCC, nor to guide therapy.
ONGOING CHALLENGES
Subtypes of hepatocellular carcinoma are important for 2 primary reasons[2]. First, different histological subtypes of hepatocellular carcinoma have their own distinct diagnostic pitfalls. For example, when SH-HCC is well differentiated, it can closely mimic ordinary steatohepatitis or benign lesions showing steatohepatitic features, i.e., steatosis, ballooning, and inflammation, such as some cases of focal nodular hyperplasia and some hepatic adenomas[20,21]. The second important reason is, in many ways, more aspirational, at least for many subtypes including SH-HCC: to recognize subtypes of HCC in order to provide more personalized patient care. To better accomplish the second goal, morphological subtypes of HCC should meet these four criteria[22], understanding that it takes many years for a morphologically based subtype to be fully defined:
1. Distinct histological findings that can be reasonably identified on H&E. The histological findings should have high sensitivity; specificity is enhanced by additional tests (see 2, 4)
2. Additional tissue based tests (immunohistochemistry, FISH, or other molecular studies) to confirm the specific subtype of HCC. The H&E findings should be compatible. These confirmatory testing should not be used in isolation from the H&E.
3. Clinical correlates. These can include demographics, risk factors, survival, and therapeutic responses.
4. Unique molecular findings. When identified, the unique molecular findings can be incorporated into Step 2 for confirmatory testing and even potential targeted therapy.
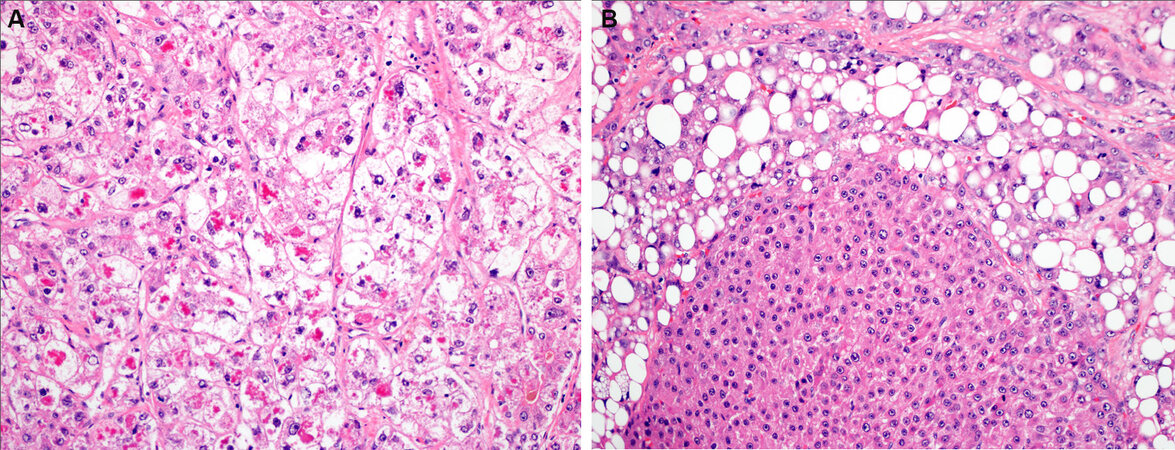
In terms of the SH-HCC variant, there are unresolved definitional challenges (criterion 1). How much of the tumor needs to show the SH morphology (best minimum cut-off)? And does it matter when some cases are mostly ballooning with little fat [Figure 2A], and some are mostly fat with little ballooning? Secondly, it remains unclear if the 2 distinct patterns of fibrosis that have been described-pericellular versus scirrhous-like - have additional clinical or biological significance. Thirdly, should steatotic HCC be combined with SH-HCC, and considered as a continuum/spectrum?
Figure 2. Steatohepatitic hepatocellular carcinoma (SH-HCC), challenging areas. This case showed 5% patchy macrovesicular steatosis, but most of the case was composed of ballooned tumor cells with Mallory-Denk bodies (Panel A). This SH-HCC shows an area of clonal progression (Panel B).
Another significant challenge revolves around the question of whether or not the SH-morphology is, in some cases, largely a reflection of systemic conditions, wherein the entire liver shows fatty changes, and so does the tumor; in this setting the steatohepatitis morphology might not imply shared tumor genetics, as would be the case for a true subtype, and be an epiphenomenon. If this is true, it would be helpful to separate SH-HCC into those cases where the morphology is driven by tumor specific changes from those tumors where it is largely an epiphenomenon.
There are data that the SH-HCC morphology can be tumor specific, at least in those cases where the tumor shows steatohepatitis but the background liver does not, and patients have no histories of alcohol use or of the metabolic syndrome[10]. Another observation also provides potential support for SH-HCC having tumor specific changes: when the background liver shows steatosis/steatohepatitis, not all HCC have the SH-HCC morphology. It is possible that those HCC without the SH-HCC morphology are poorly differentiated, while those with the SH-HCC morphology are well to moderately differentiated, in which case the changes may still be epiphenomenal. While this possibility has yet to be formally assessed, at least in many anecdotal cases, well to moderately differentiated HCC without fatty changes can be seen in livers that have underlying fatty liver disease. Additional support potentially comes from tumors that are multifocal; it has been well documented that in this setting some HCC show steatohepatitic morphology and some do not[7,8].
Another piece of the SH-HCC puzzle comes from studies that examined early HCC in the setting of cirrhosis. These studies found that dysplastic nodules and early HCC often had more steatosis than the background liver[23,24]; the fatty changes were not associated with alcohol use, metabolic syndrome, or fat in the background liver, but did correlate with the HCC grade. In these cases, the frequency of steatosis decreased significantly between well differentiated and moderately differentiated tumors[24]. Taken together, these observations from disparate study designs strongly suggest that at least a subgroup of early, well-differentiated SH-HCC have tumor-specific changes that lead to the distinctive morphology.
Yet, several fundamental questions remain unanswered. For example, if it is true that fatty change is an early finding in the formation of dysplastic nodules/early HCC, a finding that is independent of the presence of clinical risk factors for fatty liver disease and of fat in the liver, then doesn’t this suggest that fatty change in early malignancy is different from that found in SH-HCC associated with MALFD or alcoholic liver disease? HCC are known to undergo clonal progression[25] - another unanswered question is what happens to SH-HCC morphology when this happens [Figure 2B]. If, as a hypothetical example, a SH-HCC loses much of its SH-HCC morphology through clonal progression, wherein a poorly differentiated clone has overgrown most of the original HCC, should it still be classified as a SH-HCC if there is a small rim of residual SH-HCC? Answers to these types of questions will help refine the classification of SH-HCC and improve its relevance to patient findings.
CURRENT CLINICAL PRACTICES
Currently, subtype determination of SH-HCC is not mandatory in the pathology report for clinical care, since it does not alter clinical management. Nonetheless, in our experience, clinical and radiology colleagues are often still interested in the HCC subtype, so we provide this information when requested. To accomplish this goal, designations of SH-HCC or steatotic-HCC, depending on the histological findings, are both appropriate. The information can also be reasonably provided descriptively, in the microscopic description portion of the pathology report. Key findings include the percent of fat (most important) as well as amount of balloon cells, intratumoral inflammation, and intratumoral fibrosis.
CONCLUSION
SH-HCC is the most common subtype of hepatocellular carcinoma, one that is routinely encountered in clinical practice. SH-HCC is strongly associated with the metabolic syndrome and likely with alcoholic hepatitis. It has a distinctive morphology characterized by varying degrees of fat, balloon cells, Mallory hyaline, intratumoral inflammation, and intratumoral fibrosis. Several molecular correlates have been identified, including a lower frequency of beta catenin mutations. Key unanswered questions revolve around the minimal histological thresholds needed to make the diagnosis, as well as whether or not SH-HCC currently is a mixed category, containing tumors where the distinctive morphology is driven by tumor specific genetic changes and other tumors where the findings may be an epiphenomenon of MAFLD or of alcohol-associated fatty liver disease.
DECLARATIONS
Authors’ contributionsMade substantial contributions to the conception, design, writing, and final editing: Torbenson MS, Yeh MM
Availability of data and materialsNot applicable.
Financial support and sponsorshipP50 CA210964 (MT).
Conflicts of interestBoth authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2021.
REFERENCES
1. Torbenson MS. Morphologic subtypes of hepatocellular carcinoma. Gastroenterol Clin North Am 2017;46:365-91.
2. Torbenson MS, Zen Y, Yeh MM, American Registry of Pathology. Tumors of the liver. : Washington, DC: American Registry of Pathology; 2018. p. 449.
3. Edmondson HA. Tumors of the liver and intrahepatic bile ducts. Washington DC: Armed Forces Institute of Pathology; 1958.
4. Salomao M, Yu WM, Brown RS Jr, Emond JC, Lefkowitch JH. Steatohepatitic hepatocellular carcinoma (SH-HCC): a distinctive histological variant of HCC in hepatitis C virus-related cirrhosis with associated NAFLD/NASH. Am J Surg Pathol 2010;34:1630-6.
5. Salomao M, Remotti H, Vaughan R, Siegel AB, Lefkowitch JH, Moreira RK. The steatohepatitic variant of hepatocellular carcinoma and its association with underlying steatohepatitis. Hum Pathol 2012;43:737-46.
6. Jain D, Nayak NC, Kumaran V, Saigal S. Steatohepatitic hepatocellular carcinoma, a morphologic indicator of associated metabolic risk factors: a study from India. Arch Pathol Lab Med 2013;137:961-6.
7. Shibahara J, Ando S, Sakamoto Y, Kokudo N, Fukayama M. Hepatocellular carcinoma with steatohepatitic features: a clinicopathological study of Japanese patients. Histopathology 2014;64:951-62.
8. Chan AW, Yu S, Yu YH, et al. Steatotic hepatocellular carcinoma: a variant associated with metabolic factors and late tumour relapse. Histopathology 2016;69:971-84.
9. Taniai M, Hashimoto E, Tobari M, et al. Clinicopathological investigation of steatohepatitic hepatocellular carcinoma: A multicenter study using immunohistochemical analysis of adenoma-related markers. Hepatol Res 2018;48:947-55.
10. Yeh MM, Liu Y, Torbenson M. Steatohepatitic variant of hepatocellular carcinoma in the absence of metabolic syndrome or background steatosis: a clinical, pathological, and genetic study. Hum Pathol 2015;46:1769-75.
11. Qin J, Higashi T, Nakagawa S, et al. Steatohepatitic variant of hepatocellular carcinoma is associated with both alcoholic steatohepatitis and nonalcoholic steatohepatitis: A study of 2 cohorts with molecular insights. Am J Surg Pathol 2020;44:1406-12.
12. Aigelsreiter A, Neumann J, Pichler M, et al. Hepatocellular carcinomas with intracellular hyaline bodies have a poor prognosis. Liver Int 2017;37:600-10.
13. Inui S, Kondo H, Tanahashi Y, et al. Steatohepatitic hepatocellular carcinoma: imaging findings with clinicopathological correlation. Clin Radiol 2021;76:160.e15- e25.
14. Hatano M, Ojima H, Masugi Y, et al. Steatotic and nonsteatotic scirrhous hepatocellular carcinomas reveal distinct clinicopathological features. Hum Pathol 2019;86:222-32.
15. de Campos PB, Oliveira CP, Stefano JT, et al. Hepatocellular carcinoma in non-alcoholic fatty liver disease (NAFLD) - pathological evidence for a predominance of steatohepatitic inflammatory non-proliferative subtype. Histol Histopathol 2020;35:729-40.
16. Tan PS, Nakagawa S, Goossens N, et al. Clinicopathological indices to predict hepatocellular carcinoma molecular classification. Liver Int 2016;36:108-18.
17. Ando S, Shibahara J, Hayashi A, Fukayama M. Beta-catenin alteration is rare in hepatocellular carcinoma with steatohepatitic features: immunohistochemical and mutational study. Virchows Arch 2015;467:535-42.
18. Calderaro J, Couchy G, Imbeaud S, et al. Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification. J Hepatol 2017;67:727-38.
19. Shin JH, Kim CJ, Jeon EJ, Sung CO, Shin HJ, et al. Overexpression of C-reactive protein as a poor prognostic marker of resectable hepatocellular carcinomas. J Pathol Transl Med 2015;49:105-11.
20. Deniz K, Moreira RK, Yeh MM, Ferrell LD. Steatohepatitis-like changes in focal nodular hyperplasia, a finding to distinguish from steatohepatitic variant of hepatocellular carcinoma. Am J Surg Pathol 2017;41:277-81.
21. Liu Y, Zen Y, Yeh MM. Steatohepatitis-like changes in hepatocellular adenoma. Am J Clin Pathol 2020;154:525-32.
22. Wood LD, Heaphy CM, Daniel HD, et al. Chromophobe hepatocellular carcinoma with abrupt anaplasia: a proposal for a new subtype of hepatocellular carcinoma with unique morphological and molecular features. Mod Pathol 2013;26:1586-93.
23. Terada T, Nakanuma Y, Hoso M, Saito K, Sasaki M, Nonomura A. Fatty macroregenerative nodule in non-steatotic liver cirrhosis. A morphologic study. Virchows Arch A Pathol Anat Histopathol 1989;415:131-6.
24. Kutami R, Nakashima Y, Nakashima O, Shiota K, Kojiro M. Pathomorphologic study on the mechanism of fatty change in small hepatocellular carcinoma of humans. J Hepatol 2000;33:282-9.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Torbenson MS, Yeh MM. Steatohepatitic hepatocellular carcinoma. Hepatoma Res 2021;7:38. http://dx.doi.org/10.20517/2394-5079.2021.08
AMA Style
Torbenson MS, Yeh MM. Steatohepatitic hepatocellular carcinoma. Hepatoma Research. 2021; 7: 38. http://dx.doi.org/10.20517/2394-5079.2021.08
Chicago/Turabian Style
Torbenson, Michael S., Mathew M. Yeh. 2021. "Steatohepatitic hepatocellular carcinoma" Hepatoma Research. 7: 38. http://dx.doi.org/10.20517/2394-5079.2021.08
ACS Style
Torbenson, MS.; Yeh MM. Steatohepatitic hepatocellular carcinoma. Hepatoma. Res. 2021, 7, 38. http://dx.doi.org/10.20517/2394-5079.2021.08
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 11 clicks
Cite This Article 11 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.