Can radiotherapy finally “go live” in the management of liver metastases?
Abstract
Liver metastases can present synchronously or at different time points. While systemic therapy continues to be the mainstay of treatment for patients with liver metastases, it is unlikely to completely eradicate the disease. Surgical “metastectomy” for patients with limited metastatic burden, particularly from colorectal cancers, has been shown to improve survival. However, owing to medical co-morbidities or tumour location, not all patients are eligible for surgical resection. In recent years, there has been an increase in the use of non-surgical techniques, including high dose radiation using stereotactic body radiotherapy, or brachytherapy, to ablate liver metastases. The purpose of this narrative review is to describe the role of radiotherapy in the management of liver metastases, both for local ablation and symptom palliation. We will elaborate on the techniques used, patient selection process, expected outcomes and toxicities based on the current literature.
Keywords
Introduction
The liver is one of the most common sites for metastases from primary cancers of the colon, pancreas, breast, and lung. Liver metastases are associated with considerable morbidity and shortened survival. While systemic therapy is still the mainstay of treatment, most tumour responses are short-lived. Moreover, the response to systemic therapy can be mixed, with some tumours regressing and others remaining stable or progressing. Aggressive local therapy (such as surgical resection) can be considered for patients with oligometastatic disease. For example, surgical resection is recommended for patients with isolated liver metastases from colorectal primaries, with the potential of long-term disease control[1]. Early studies demonstrated a 30% 5-year survival in patients who underwent “metastectomy” for one to three liver metastases[2]. Factors that determine patient eligibility for resection include the size, number, and location of lesions, and hepatic reserve. While surgical techniques have improved, not all patients are good surgical candidates because of surgical factors and patient co-morbidities. Thus, such patients may be considered for non-surgical liver-directed therapies. These include invasive techniques such as radio-frequency ablation (RFA) and non-invasive techniques such as stereotactic body radiotherapy (SBRT).
Traditionally, the role of radiation therapy in liver metastases has been purely for palliation, as the tolerance of whole liver to radiation is limited to 30 Gy (in 2 Gy fractions)[3], and sustained tumor control is very unlikely at such doses. Technological advances with improvements in target localization, patient immobilization, motion management, and delivery of conformal radiation have allowed the use of high doses of radiation to ablate liver metastases. Moreover, mounting evidence shows that high doses of radiation can be delivered to small targets within the liver without causing toxicity[3]. In the context of SBRT, doses ranging from 45 to 60 Gy, over three to five fractions (given over 1-2 weeks), is delivered conformally to the target while sparing normal liver parenchyma.
The purpose of this narrative review is to describe the role of radiotherapy in liver metastases - both in the setting of ablative treatment (including SBRT and brachytherapy) for patients with oligometastatic disease, and in the setting of symptom palliation in patients with uncontrolled liver metastases. We will elaborate on the treatment technique, patient selection, expected outcomes and treatment-related toxicities.
Use of radiotherapy in patients with oligometastatic liver disease
Hellman and Weichselbaum were the first to introduce the concept of oligometastatic disease, which represented an intermediate state in the spectrum between locally confined and widely metastatic cancer[4]. They proposed that the process of metastatic disease occurs in a step-wise manner, and patients with limited disease should be managed aggressively. In more recent years, advances in systemic and targeted therapy have rendered a greater number of patients with upfront widely metastatic disease to a state of limited volume metastatic disease. In these patients, aggressive management of drug-resistant clones may improve cancer outcomes. However, to date, there is no universally accepted definition of oligometastasis with regards to the number of lesions involved. The most accepted number of metastatic lesions is considered to be 5 or less (with up to 3 metastases in any one organ).
Although surgery and RFA have a longer history of being used in management of oligometastatic disease involving the liver, there are no trials directly comparing these to SBRT. However, the use of SBRT has been reinvigorated by a recently published randomized phase II trial (SABR-COMET) which investigated the use of SBRT in patients with oligometastatic disease (including liver metastases). They compared SBRT to standard of care palliative treatment, and showed an overall survival benefit with SBRT[5].
The role of SBRT in oligometastatic liver disease
Technique
Stereotactic radiosurgery was first applied for intracranial targets, and similar concepts have been adapted to treat extracranial targets. SBRT involves the use of high doses of radiation delivered to a well-defined target whilst minimizing radiation to surrounding healthy tissue. The American College of Radiology and American Society for Radiation Oncology defines SBRT as the use of very large doses of radiation, defined as more than 6 Gy per fraction, given over few (up to 5) fractions[6]. This is in contrast to conventional external beam radiotherapy which is usually given in 1.8-2 Gy fractions, leading to protracted treatments.
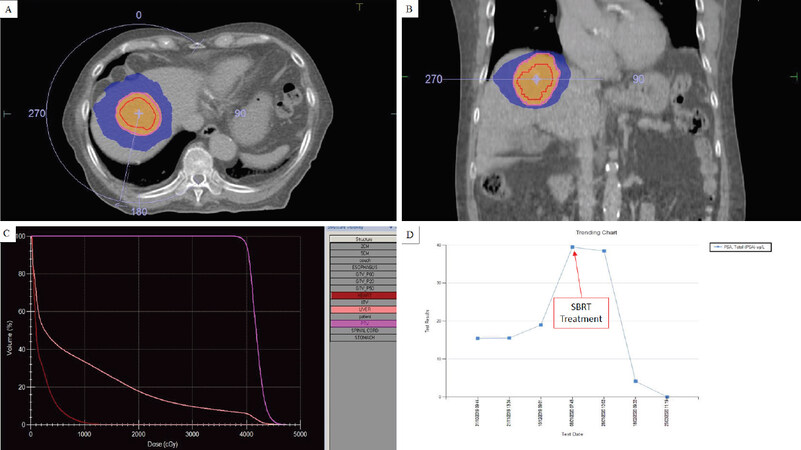
SBRT is administered via a linear accelerator (LINAC) delivering ionizing radiation in the form of mega-voltage photons. The radiation dose is highly conformal to the target, leading to a rapid dose fall off outside the target. This is achieved using multi-directional beams or arc therapy and modulating the intensity of each beam. Patient immobilisation is essential, and generally whole-body vacuum bags are utilised. As each treatment session can last up to 30 min, patient comfort and reproducibility are important for the accurate delivery of SBRT. The liver moves as much as 2-3 cm in the cranio-caudal direction. Motion management strategies are therefore critical in SBRT. Several methods exist such as 4D CT scanning with abdominal compression, breath holding in the form of active breathing control or voluntary breath holding, respiratory gating (synchronizing delivery of RT with specified respiratory phases) and real-time tumour tracking systems using radio-opaque fiduciary markers. On-board imaging must be incorporated prior the delivery of SBRT. This allows for the online correction of patient position. Several solutions exist in modern linear accelerators, such as integrated cone-beam computed tomography or magnetic resonance imaging (MR-LINAC). An example of an SBRT plan is shown in Figure 1.
Figure 1. Example of an SBRT plan (40 Gy in 5 fractions) for a single hepatic metastasis in a patient with a castration-resistant prostate primary who had progressed after abiraterone. Axial image; red line showing gross tumour volume, peach line showing planning target volume, orange colourwash showing the 95% isodose line, and blue colourwash showing the 50% isodose line (A) coronal image; showing the 50% isodose line away from the heart (B) dose-volume histogram of the SBRT plan (C) dramatic PSA response following SBRT treatment (D). SBRT: stereotactic body radiotherapy
Patient selection
Patient selection for SBRT is critical and should take into account factors such as age, performance status, disease burden and patient preferences. In addition, tumour factors such as the volume of hepatic disease, location of metastases (particularly proximity to critical structures such as bowel, biliary tract, and heart), number of lesions (less than 3), size (preferably each less than 6 cm, and combined less than 15 cm) should be considered. In addition, sufficient hepatic reserve (ideally total liver volume more than 1000 mL, with at least 700 mL spared from doses more than 15 Gy) is essential, to mitigate toxicities, as will be explained below.
Outcomes
Local control of hepatic metastases with SBRT are generally encouraging with most studies achieving approximately 80% at 2 years (range 32% to 91%) [Table 1]. This is mostly influenced by size of tumour, prior treatment, and biologically equivalent dose delivered. Median overall survival after SBRT can vary from 26% to 75% at 2 years [Table 1]. However, it is recognised that the patient’s overall prognosis may be related to extra-hepatic metastases, thus reinforcing the need for multimodal treatment with effective systemic therapy as opposed to monotherapy with either alone. In most studies concerning the outcomes of SBRT for liver metastases, patients would have received systemic therapy (e.g., chemotherapy, targeted therapy) before and/or after SBRT. This reinforces the need for both effective local and systemic therapy.
Outcomes of SBRT to liver metastases from selected recent studies
| Authors | Study design | n | Primary tumor | Dose/fractionation (#) | Median Followup in months | 2 year LC (%) | 2 year OS (%) |
|---|---|---|---|---|---|---|---|
| Scorsetti et al.[7] 2015 | Prospective (Phase 2) | 42 | CRC | 75 Gy/3 # | 24 | 91 | 65 |
| Goodman et al.[8] 2016 | Retrospective | 81 | CRC 66.6%
Breast 7.4% Lung 3.7% Ovarian 3.7% GI 13.6% Others 4.9% | 32-60 Gy/3-5 # | 33 | 90.5 | 68.6 |
| McPartlin et al.[9] 2017 | Prospective (Phase 1 & 2) | 60 | CRC | 22.7-62.1 Gy/6 # | 28.1 | 32 | 26 |
| Joo et al.[10] 2017 | Retrospective | 70 | CRC | 45-60 Gy/3-4 # | 34.2 | 73 | 75 |
| Mahadevan et al.[11] 2018 | Retrospective | 427 | CRC 44.3%
Lung 12.2% Breast 9.8% GI 7.7% Gynae 5.9% Pancreas 4.9% Other 15.2% | Median 45 (12-60) Gy/median 3 (1-5) # | 14 | 72 | 49 |
Toxicity
Radiation-induced liver disease (RILD) is a feared complication which can be hard to manage[12]. RILD typically presents 4-8 weeks after completion of radiotherapy (RT). The occurrence of RILD is related to the volume of liver irradiated, pre-existing hepatic functional reserve, and patient co-morbidities. Classic RILD symptoms include fatigue, abdominal pain, anicteric ascites and hepatomegaly. RILD however is more common in whole liver RT (WLRT), although it can occur with SBRT[13]. Collateral damage to nearby structures is known to occur, including biliary obstruction and stricture formation (for lesions near the porta hepatis), and gastro-intestinal injury (resulting in bleeding, perforation or strictures). With adherence to known dose limits, the risks of these complications can be reduced to below 5%.
Comparison of SBRT with RFA
The most common technique of thermal ablation is radiofrequency ablation (RFA). RFA uses a high frequency alternating electric current which produces ionic agitation and frictional heating, thereby heating tumour tissue to over 60 degrees Celsius. Tumour heating causes extracellular and intracellular dehydration, resulting in tissue destruction by coagulative necrosis. RFA can be performed percutaneously, laparoscopically, or during open surgery[14]. RFA is usually limited, however, by proximity to the biliary tree as well as to blood vessels because of the “heat sink” effect. Stang et al.[15] reported that local recurrence rates were 5% to 42% after RFA and that the dominant factor affecting local failure rates were the size of the lesion, particularly those larger than 3 cm. Jackson et al.[16]. also reported similar efficacy of SBRT and RFA for lesions smaller than 2 cm, however SBRT achieved better local control comparatively for lesions larger than 2 cm[16]. As such, RFA and SBRT are complementary modalities. SBRT is preferred for lesions near blood vessels or the dome of the liver, and for larger lesions.
The role of interstitial brachytherapy for oligometastatic liver disease
Technique
Although brachytherapy has a long history in oncology, it was not until the early 1980s when it was used for liver tumours. Dritschilo et al.[17] described the percutaneous implantation of interstitial brachytherapy applicators under sonographic guidance in 1986. Subsequently, intraoperative catheter placement (under direct visualisation and/or sonography-assisted) has also been described[18]. Later, in 2004, Ricke et al.[19] published a phase II trial using CT-guidance for interstitial high dose-rate brachytherapy (HDRBT) of liver tumours which were unsuitable for thermal ablation.
The choice of image guidance for interstitial liver HDRBT application is operator-specific. In general, the use of CT, or a hybrid method combining ultrasound and CT, is the preferred choice. CT guidance allows better visualization of gastrointestinal structures, major vessels and intrahepatic bile ducts. The use of ultrasound, even when combined with CT, can reduce radiation exposure of the healthcare personnel involved.
The procedure is performed under sterile conditions using local anaesthesia and mild to moderate sedation. The skin puncture site is identified based on CT images. Under CT-guidance, the applicator is advanced past the liver capsule, into the target, in the same phase of breathing. The applicators are advanced at least 5 mm beyond the target, to account for breathing motion. After insertion of the applicators, CT or MRI with contrast is usually performed with thin axial cuts (2 mm) for applicator reconstruction. Doses in the range of 15-25 Gy in a single fraction are usually prescribed depending on the histology and organs-at-risk tolerance. Colorectal and sarcoma metastases tend to be radioresistant, and are usually treated with 25 Gy. In view of the clear dose-response reported by Ricke et al.[20], higher doses should be used when possible.
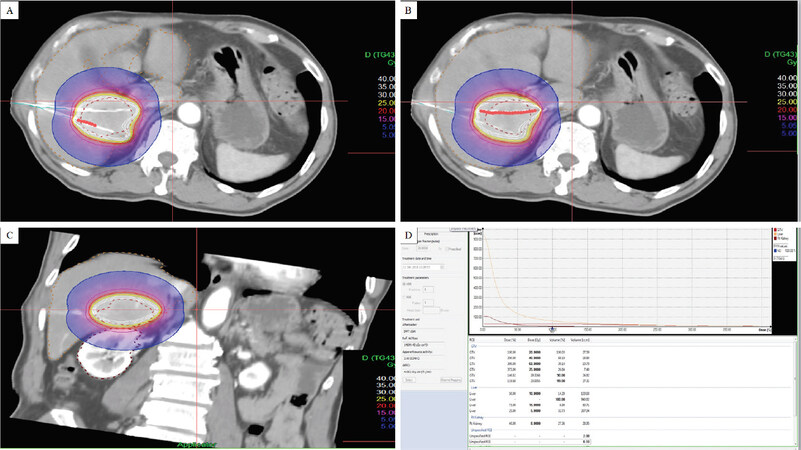
Applicators are removed immediately after completing the treatment. An example of a CT HDRBT plan is shown in Figure 2.
Figure 2. Example of a CT HDRBT plan for a single hepatic metastasis. axial images; the patient has 2 after-loading catheters advanced into the lesion. Dose distribution is adjusted by 3D treatment planning. The planned minimal enclosing dose was 20 Gy (red line) (A, B), coronal image (C), dose-volume histogram (D). HDRBT: high dose-rate brachytherapy
Patient selection
Most centres adopt the selection criteria used by Mohnike et al.[21], with some variation. These include Child-Pugh score of B8 or less, platelet counts > 50,000, prothrombin time < 1.5X. Generally, chemo- and radio-sensitive primaries (such as lymphomas and germ-cell tumours) are excluded. Chemotherapy should be withheld one week pre- and post-HDRBT. The time-interval from prior therapies need to be considered: for RFA at least 1 month, Yttrium-90 at least 6 months, and brachytherapy to the same site at least 3 months. Lesion size and number have no specific cut-off, provided that not more than one third of normal liver parenchyma receives more than 5 Gy. The requirement is more stringent for patients with cirrhotic-appearing livers, with an aim for not more than half of non-target liver tissue to receive more than 5 Gy. Proximity of the target to major vessels or the target adjacent to the hilum is also not a contraindication[22,23].
Outcomes
Local control rates are highly dependent on the isodose lines covering the target’s periphery. In a prospective trial of three single fraction HDRBT dose levels, Ricke et al.[20] (2010) reported a recurrence in only 1 out of 33 lesions (3%) in the 25 Gy group, in contrast to 34 out of 98 lesions (35%) recurring in the 15 Gy group.
Overall, local control rates with HDRBT appear favourable, as shown in Table 2.
Outcomes of CT-HDRBT to liver metastases from selected recent studies
| Authors | Study design | n | Primary tumor | Dose/fractionation (#) | Median follow up in months | 1 year LC (%) | 1 year OS (%) |
|---|---|---|---|---|---|---|---|
| Ricke et al.[20] 2010 | Prospective (Phase III) | 73 | Colorectal | 15-25 Gy/1 # | 15.2 | 74.9 | NR |
| Wieners et al.[24] 2011 | Prospective (Phase II) | 41 | Breast | 15-25 Gy/1 # | 18 | 93.5 | 79 |
| Collettini et al.[25] 2012 | Prospective | 37 | Breast | 15-20 Gy/1-4 # | 11.6 | 97.4 | 80 |
| Sharma et al.[26] 2013 | Prospective | 10 | Breast 30%
CRC 20% GB 20% Stomach 20% Others 10% | 20 Gy/1 # | 9 | 75% | NR |
| Kieszko et al.[27] 2018 | Retrospective | 61 | GI 75.4%
Breast 11.5% Lung 8.2% Others 4.9% | 15-25 Gy/1 # | 11 | 70.7 | 79.6 |
| Omari et al.[28] 2019 | Retrospective | 14 | Renal | 16 (6.5-27.4) Gy/1-5 # | 10 | 92.6 (at median 10.2 months) | NR |
Toxicity
Interstitial HDRBT in the liver is a well-tolerated procedure. Post-procedural fever is very common and is related to cytokine-release. Nausea and vomiting may also occur, which is usually related to the volume of treatment. Prophylactic anti-emetics may be used to counteract these effects. Pain is also a common complaint which may be treated with appropriate analgesia.
Procedure-related toxicity, such as bleeding, is usually limited to the subcapsular space and rarely requires transfusion. Potentially serious, but rare, complications include intra-hepatic biliary occlusion, liver abscess, gastrointestinal ulceration, and non-classic RILD, occurring in less than 1% of cases[21].
Comparison between Brachytherapy, SBRT and RFA
Unlike SBRT, interstitial HDRBT to the liver delivers highly lethal doses of radiation from inside to out. As such, the physical property of HDRBT plays to its advantage, as the central part of the tumour is often radio-resistant due to tumour hypoxia. Hass et al.[29] performed a dosimetric comparison and demonstrated HDRBT to be superior to SBRT in terms of tumour coverage, whilst reducing the dose received by the unaffected liver parenchyma. However, SBRT has better dose manoeuvring options as compared to HDRBT, especially in non-oval shaped targets. SBRT has the advantage of being a non-invasive procedure which reduces the procedure-associated risks, such as bleeding and infection.
Compared to RFA, the outcomes of interstitial HDRBT liver are not affected by the proximity to the great vessels or vascularized tumour (“heat sink effect”). Lesions near the main intra-hepatic biliary ducts are better treated with HDRBT compared to RFA[22]. Similarly, lesions near segment VII/VIII, and those near the diaphragm, are technically difficult to treat with RFA. RFA is limited by lesion size, as discussed earlier, whilst in HDRBT, large lesions can be treated with the use of multiple applicators. RFA does have the advantage of real-time manoeuvrability, whereby under ultrasound guidance the operator can keep the RFA probe away from non-static structures such as the stomach and small intestine.
The use of radiotherapy for palliation of symptoms
In cases of uncontrolled hepatic metastases, patients may consequently experience abdominal pain (from capsular stretch), nausea and vomiting, jaundice, and constitutional symptoms such as weight loss or night sweats. In general, systemic therapy for palliation can be used for such patients, although a large number will eventually be refractory in end-stage disease. WLRT or partial liver irradiation (PLRT) has been shown to effectively palliate such patients, thereby improving quality of life[30-32]. Examples of regimens include 8Gy/1 fraction, 21Gy/7 fractions, and 30Gy/15 fractions. Bydder et al.[33] reported prospectively between 53% to 66% improvement in symptoms at 2 weeks. Edyta et al.[34] reported a 100% improvement in symptoms at 1 month in a retrospective study. The results of these studies are shown in further detail in Table 3.
Outcomes of recent palliative whole or partial liver irradiation
| Authors | Primary tumor | n | Treatment | Dose/fractionation (#) | Outcome | Toxicities |
|---|---|---|---|---|---|---|
| Bydder et al.[33] 2003 | CRC 39%
NSCLC 4% Esophageal 11% SCLC 7% Other 29% | 28 | WLRT/PLRT | 10 Gy/2 # | 54% partial or complete symptomatic response | 2 patients with Grade 3 vomiting and diarrhoea |
| Yin et al.[35] 2014 | CRC | 19 | WLRT + tumor boost + concurrent chemotherapy | 53.4 Gy (including boost)/# NR | 52.6% overall response | 2 patients with Grade 3 elevated bilirubin |
| Edyta et al.[34] 2015 | Colon 59%
Stomach 26% Pancreas 15% | 27 | WLRT | Mean 17 Gy/
5-12 # | 40% partial or complete symptomatic response | 1 patient with Grade 3 vomiting and diarrhoea |
Palliative liver radiotherapy is delivered using a simple method of conventional radiotherapy. Patients are positioned supine and treated with 2 to 3 portals, including most of the liver. Treatment is generally well-tolerated and serious adverse events are rare. Most patients may experience grade 1 to 2 anorexia, and nausea and vomiting following treatment with radiotherapy, and these can be managed symptomatically. Dexamethasone and anti-emetics are useful to counter radiotherapy-induced nausea.
Conclusion
Alongside surgical resection of hepatic metastases, local ablative therapies in the form of SBRT and CT-HDRBT have a role in the management of oligometastatic disease. Prospective randomized trials comparing the various modalities are needed to elucidate comparative long-term outcomes of RT specifically. Dose selection is currently arbitrary, based on lesion size, location and liver function. However, we acknowledge that the spectrum of primary tumours may have varying radio-sensitivity, and an attempt to tailor the dose (biologically-guided treatment), according to the different primaries, should be investigated[36]. In addition, the patient and disease should be considered holistically. As such, multidisciplinary discussion and collaboration between surgeons, interventional radiologists and oncologists is crucial. Treatment options should be personalized, with the pros and cons of each therapy balanced against the risk of disease progression. On the other end of the palliative spectrum, low-dose whole or partial liver radiotherapy may be used for patients with high disease burden and severe symptoms.
Declarations
Authors’ contributionsMade substantial contributions to the conception and design of the work: John RG, Vellayappan BA
Drafted the manuscript: John RG, Appalanaido GK, Vellayappan BA
Reviewed the manuscript and provided approval for publication of the content: John RG, Ho F, Appalanaido GK, Chen D, Tey J, Soon YY, Vellayappan BA
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2020.
REFERENCES
1. Van Cutsem E, Nordlinger B, Adam R, Köhne CH, Pozzo C, et al. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer 2006;42:2212-21.
2. Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309-18.
3. Pan CC, Kavanagh BD, Dawson LA, Li XA, Das SK, et al. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys 2010;76:S94-100.
5. Palma DA, Olson R, Harrow S, Gaede S, Louie AV, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet 2019;393:2051-8.
6. Potters L, Kavanagh B, Galvin JM, Hevezi JM, Janjan NA, et al. American society for therapeutic radiology and oncology (ASTRO) and American college of radiology (ACR) practice guideline for the performance of stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2010;76:326-32.
7. Scorsetti M, Comito T, Tozzi A, Navarria P, Fogliata A, et al. Final results of a phase II trial for stereotactic body radiation therapy for patients with inoperable liver metastases from colorectal cancer. J Cancer Res Clin Oncol 2015;14:543-53.
8. Goodman BD, Mannina EM, Althouse SK, Maluccio MA, Cárdenes HR. Long-term safety and efficacy of stereotactic body radiation therapy for hepatic oligometastases. Pract Radiat Oncol 2016;6:86-95.
9. McPartlin A, Swaminath A, Wang R, Pintilie M, Brierley J, et al. Long-term outcomes of phase 1 and 2 studies of SBRT for hepatic colorectal metastases. Int J Radiat Oncol Biol Phys 2017;99:388-95.
10. Joo JH, Park JH, Kim JC, Yu CS, Lim SB, et al. Local control outcomes using stereotactic body radiation therapy for liver metastases from colorectal cancer. Int J Radiat Oncol Biol Phys 2017;99:876-83.
11. Mahadevan A, Blanck O, Lanciano R, Peddada A, Sundararaman S, et al. Stereotactic body radiotherapy (SBRT) for liver metastasis - clinical outcomes from the international multi-institutional RSSearch(R) patient registry. Radiat Oncol 2018;13:26.
12. Ingold JA, Reed GB, Kaplan HS, Bagshaw MA. Radiation hepatitis. Am J Roentgenol Radium Ther Nucl Med 1965;93:200-8.
13. Hoyer M, Roed H, Traberg Hansen A, Ohlhuis L, Petersen J, et al. Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncol 2006;45:823-30.
14. Decadt B, Siriwardena AK. Radiofrequency ablation of liver tumours: systematic review. Lancet Oncol 2004;5:550-60.
15. Stang A, Fischbach R, Teichmann W, Bokemeyer C, Braumann D. A systematic review on the clinical benefit and role of radiofrequency ablation as treatment of colorectal liver metastases. Eur J Cancer 2009;45:1748-56.
16. Jackson WC, Tao Y, Mendiratta-Lala M, Bazzi L, Wahl DR. Comparison of stereotactic body radiation therapy and radiofrequency ablation in the treatment of intrahepatic metastases. Int J Radiat Oncol Biol Phys 2018;100:950-8.
17. Dritschilo A, Grant EG, Harter KW, Holt RW, Rustgi SN, et al. Interstitial radiation therapy for hepatic metastases: sonographic guidance for applicator placement. AJR Am J Roentgenol 1986;147:275-8.
18. Thomas DS, Nauta RJ, Rodgers JE, Popescu GF, Nguyen H, et al. Intraoperative high-dose rate interstitial irradiation of hepatic metastases from colorectal carcinoma. Results of a phase I-II trial. Cancer 1993;71:1977-81.
19. Ricke J, Wust P, Wieners G, Beck A, Cho CH, et al. Liver malignancies: CT-guided interstitial brachytherapy in patients with unfavorable lesions for thermal ablation. J Vasc Interv Radiol 2004;15:1279-86.
20. Ricke J, Mohnike K, Pech M, Seidensticker M, Rühl R, et al. Local response and impact on survival after local ablation of liver metastases from colorectal carcinoma by computed tomography-guided high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys 2010;78:479-85.
21. Mohnike K, Wolf S, Damm R, Seidensticker M, Seidensticker R, et al. Radioablation of liver malignancies with interstitial high-dose-rate brachytherapy: complications and risk factors. Strahlenther Onkol 2016;192:288-96.
22. Tselis N, Chatzikonstantinou G, Kolotas C, Milickovic N, Baltas D, et al. Computed tomography-guided interstitial high dose rate brachytherapy for centrally located liver tumours: a single institution study. Eur Radiol 2013;23:2264-70.
23. Collettini F, Singh A, Schnapauff D, Powerski MJ, Denecke T, et al. Computed-tomography-guided high-dose-rate brachytherapy (CT-HDRBT) ablation of metastases adjacent to the liver hilum. Eur J Radiol 2013;82:e509-14.
24. Wieners G, Mohnike K, Peters N, Bischoff J, Kleine-Tebbe A, et al. Treatment of hepatic metastases of breast cancer with CT-guided interstitial brachytherapy - a phase II-study. Radiother Oncol 2011;100:314-9.
25. Collettini F, Golenia M, Schnapauff D, Poellinger A, Denecke T, et al. Percutaneous computed tomography-guided high-dose-rate brachytherapy ablation of breast cancer liver metastases: initial experience with 80 lesions. J Vasc Interv Radiol 2012;23:618-26.
26. Sharma DN, Thulkar S, Sharma S, Gandhi AK, Haresh KP, et al. High-dose-rate interstitial brachytherapy for liver metastases: first study from India. J Contemp Brachytherapy 2013;5:70-5.
27. Kieszko D, Cisek P, Kordzińska-Cisek I, Grzybowska-Szatkowska L. Treatment of hepatic metastases with computed tomography-guided interstitial brachytherapy. Oncol Lett 2018;15:8717-22.
28. Omari J, Heinze C, Damm R, Hass P, Janitzky A, et al. Radioablation of hepatic metastases from renal cell carcinoma with image-guided interstitial brachytherapy. Anticancer Res 2019;39:2501-8.
29. Hass P, Mohnike K, Kropf S, Brunner TB, Walke M, et al. Comparative analysis between interstitial brachytherapy and stereotactic body irradiation for local ablation in liver malignancies. Brachytherapy 2019;18:823-8.
30. Yeo SG, Kim DY, Kim TH, Kim SY, Hong YS, et al. Whole-liver radiotherapy for end-stage colorectal cancer patients with massive liver metastases and advanced hepatic dysfunction. Radiat Oncol 2010;5:97.
31. Cunningham SC, Choti MA, Bellavance EC, Pawlik TM. Palliation of hepatic tumors. Surg Oncol 2007;16:277-91.
32. Greco C, Catalano G, Di Grazia A, Orecchia R. Radiotherapy of liver malignancies. From whole liver irradiation to stereotactic hypofractionated radiotherapy. Tumori 2004;90:73-9.
33. Bydder S, Spry NA, Christie DR, Roos D, Burmeister BH, et al. A prospective trial of short-fractionation radiotherapy for the palliation of liver metastases. Australas Radiol 2003;47:284-8.
34. Edyta WR, Jakub L, Jerzy W. Whole liver palliative radiotherapy for patients with massive liver metastases. Asian Pac J Cancer Prev 2015;16:6381-4.
35. Yin H, Lu K, Qiao WB, Zhang HY, Sun D, et al. Whole-liver radiotherapy concurrent with chemotherapy as a palliative treatment for colorectal patients with massive and multiple liver metastases: a retrospective study. Asian Pac J Cancer Prev 2014;15:1597-602.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
John RG, Ho F, Appalanaido GK, Chen D, Tey J, Soon YY, Vellayappan BA. Can radiotherapy finally “go live” in the management of liver metastases?. Hepatoma Res 2020;6:56. http://dx.doi.org/10.20517/2394-5079.2020.37
AMA Style
John RG, Ho F, Appalanaido GK, Chen D, Tey J, Soon YY, Vellayappan BA. Can radiotherapy finally “go live” in the management of liver metastases?. Hepatoma Research. 2020; 6: 56. http://dx.doi.org/10.20517/2394-5079.2020.37
Chicago/Turabian Style
John, Reggie G., Francis Ho, Gokula K. Appalanaido, Desiree Chen, Jeremy Tey, Yu Yang Soon, Balamurugan A. Vellayappan. 2020. "Can radiotherapy finally “go live” in the management of liver metastases?" Hepatoma Research. 6: 56. http://dx.doi.org/10.20517/2394-5079.2020.37
ACS Style
John, RG.; Ho F.; Appalanaido GK.; Chen D.; Tey J.; Soon YY.; Vellayappan BA. Can radiotherapy finally “go live” in the management of liver metastases?. Hepatoma. Res. 2020, 6, 56. http://dx.doi.org/10.20517/2394-5079.2020.37
About This Article
Copyright
Data & Comments
Data
 Cite This Article 12 clicks
Cite This Article 12 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.