Pathomolecular characterization of HCC in non-cirrhotic livers
Abstract
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and usually arises in cirrhotic livers. Increasingly, it is diagnosed in non-cirrhotic livers. A variety of risk factors and etiologies can trigger the development of HCC in non-fibrotic and non-cirrhotic backgrounds. The most important causes are metabolic syndrome and hepatitis B virus infection. Postulated pathogenetic mechanisms are direct carcinogenesis, chronic liver injury and repair cycles, and genetic/epigenetic aberrations. Histopathology has a very important role in the diagnosis of non-cirrhotic HCC. Gross features of non-cirrhotic HCC are quite different from HCC originating in a cirrhotic background. Microscopic characteristics are similar to a classical HCC. However, certain histological variants show a predilection to occur in non-cirrhotic livers. These encompass fibrolamellar, scirrhous, steatohepatitic and mixed hepato-cholangiocarcinoma subtypes. Due to the non-cirrhotic background, adenoma, metastasis and most of the other non-neoplastic and neoplastic conditions enter the differential diagnosis. Genomic studies and morpho-molecular classifications of HCC provide further understanding of the molecular pathogenesis of non-cirrhotic HCC. This group however, has rarely been exclusively studied. This review offers an update of etiology, patho-molecular characteristics and differential diagnosis of HCC arising in non-cirrhotic backgrounds.
Keywords
Introduction
Hepatocellular carcinoma (HCC) usually arises in a cirrhotic liver and is characterized by tremendous phenotypic and molecular heterogeneity[1]. Around 20% of HCCs arise in non-cirrhotic livers[2-5]. The underlying etiologies, risk factors, pathogenetic mechanisms, gross specimen features, histological variants and the differential diagnosis of non-cirrhotic HCCs is quite distinct from HCCs arising in cirrhotic backgrounds. However, these aspects have not been adequately studied and further analysis of the molecular genetics and pathological characteristics in non-cirrhotic HCCs is necessary. In this article, an overview of the clinical, etiological and etiopathogentic, patho-molecular characteristics and differential diagnosis of HCCs arising in non-cirrhotic backgrounds, are presented.
Demographic and clinical characteristics
HCC is the most common primary malignant liver tumor and usually arises in cirrhotic livers. The occurrence of HCC in non-cirrhotic livers varies across different geographic regions of the world with a prevalence ranging from 7% to 54%[6]. Most series, however, have reported a prevalence of around 15%-20%[2-5].
The existence of HCC in non-cirrhotic livers has a bimodal age distribution, with peaks in the second and seventh decades. Non-cirrhotic HCCs have a lower male to female sex ratio (1.3-2.1) in comparison to cirrhotic HCCs where the ratio is 3.2 to 8:1[6]. HCC in non-cirrhotic patients manifest with non-specific symptoms or a clinically silent course in the initial period. This is due to the lack of surveillance imaging and higher hepatic reserves, leading to a delay in diagnosis until an advanced stage with a larger tumor burden has been reached[7]. Extrahepatic metastasis is already present in more than 25% of these patients at the time of presentation[8].
Non-cirrhotic HCCs usually display similar imaging features to cirrhotic HCCs on computed tomography (CT) and MRI, with arterial phase hyper-enhancement followed by washout on portal venous and/or delayed phase imaging[9,10]. However, due to the non-prototypical background, the development of HCCs in non-cirrhotic livers is one of the most important indications for tissue diagnosis. Imaging features illustrate a solitary mass with or without satellite lesions[11]. Similar to imaging, serum α-fetoprotein measurements are similar for HCCs arising from both cirrhotic and non-cirrhotic backgrounds[7].
The prognosis for non-cirrhotic HCCs is usually better than that for cirrhotic HCCs[7]. Non-cirrhotic HCCs are more amenable to hepatic resection due to the lower risk of liver failure. Patients without cirrhosis have longer survival (postoperative overall survival and recurrence-free survival) than patients with cirrhosis[7,12]. However, the recurrence rate of HCC in non-cirrhotic livers is very high after surgical resection[11,13,14].
Etiologic considerations
A variety of conditions can be a risk factor for developing non-cirrhotic HCC.
Infections
Hepatitis B virus (HBV) infection is one of the most common underlying etiologies, especially in high incidence areas. Up to 30% of HBV-related HCCs arise in non-cirrhotic livers[15,16]. HBV infection can directly trigger liver carcinogenesis by integration of the HBV genome into the host hepatocyte DNA. This can cause secondary chromosomal rearrangement and genomic instability, or produce genototoxins such as the HBx protein, resulting in HCC development in non-cirrhotic backgrounds[7,17]. In addition, the X protein of HBV, through its interaction with p53, interferes with tumor-suppressor activity. This oncogenic impact of HBV can remain even in treated cases or after seroconversion and resolution of HBV[18,19].
Although relatively less common, chronic HCV-infection is also a risk factor for the development of HCC in non-cirrhotic livers[8,20,21]. HCV carcinogenesis is likely mediated by viral factors and the host immune response[7]. Oxidative stress in hepatocytes with sustained necro-inflammatory processes leads to cell injury, repeated cell divisions leading to genetic alterations in stem cells, and cause transformation into dysplastic and malignant phenotypes[16,22,23]. HCV does not integrate into the host genome, but its direct hepatocarcinogenic potential is attributable to certain HCV gene products (core, NS3, NS4B and NS5A) and has been reported in murine fibroblast culture studies. However, the carcinogenic potential of HCV is much lower than HBV, and HCCs related to HCV mostly develop in cirrhotic livers[24]. A study reported the annual incidence of HCV-associated HCC to be 0.8% among non-cirrhotic patients, and 2%-8% among cirrhotic patients[21].
Metabolic syndrome and alcohol related liver disease
Metabolic syndrome is the most frequent cause of HCCs in non-cirrhotic backgrounds[8]. NAFLD, with or without NASH, is the hepatic manifestation of metabolic syndrome and a common risk factor for HCC development[25]. 39%-49% of NAFLD-related HCC cases arise in non-cirrhotic livers[26]. Amongst various etiologic links, patients with NAFLD most frequently develop HCCs in non-cirrhotic backgrounds[27]. Alcohol related hepatocarcinogenicity is almost exclusively due to the development of cirrhosis[28,29]. However, excessive alcohol intake in the setting of chronic HCV and diabetes mellitus may potentiate oxidative stress and free radical damage. This can lead to rapid progression to HCC even in non-cirrhotic livers[8,30]. As shown in one study, 15% of non-cirrhotic HCC patients had alcohol-HCV infection synergism[8].
The aforementioned cause-effect relationships for HCC development can be due to direct carcinogenic action. Nonetheless, the role of chronic liver inflammation leading to repeated cycles of cell injury and regeneration, and subsequent genetic and epigenetic alterations in hepatocytes, is equally plausible[31].
Hepatocyte injury resulting from microbial or sterile etiologies activates resident liver immune cells and later, facilitates the recruitment of nonresident immune cells to the liver, thereby mounting a strong inflammatory response. Persistent inflammation as a result of hepatitis virus or microbial attack resulting from breaches of the gut–liver axis lead to the production of proinflammatory cytokines such as IL-6 TNF-α, IL-1 and IL-18 through inflammasome-independent or -dependent pathways. Activated transcription factors make the hepatic milieu a fertile zone for cellular transformation[32]. Tregs coupled to the activation of Notch and TGF-β are involved in perpetuation of the inflammatory response and HCC development in patients chronically infected with the HBV[33].
Other liver disorders and tumors
Inherited metabolic and congenital diseases, in particular hereditary hemochromatosis, α-1-antitrypsin deficiency, Wilson disease, type I glycogen storage disease, porphyria, hypercitrullinemia, Alagille syndrome, and congenital hepatic fibrosis have predisposition to developing HCC in non-cirrhotic livers[6]. Mild iron accumulation is found in background liver parenchyma in non-cirrhotic HCCs, indicating the role of excess iron within hepatocytes as a genotoxic cocarcinogen factor[34].
Other genotoxic factors such as aflatoxin B1, produced by the fungus Aspergillus flavus, can contaminate cereals, legumes, spices and fruits[35]. It is metabolized by the P450 enzyme in the liver to generate an epoxide, which binds to DNA and leads to the development of non-cirrhotic HCC via p53 mutation[35,36] and also, amplifies the risk of HCC development among patients with HBV infection through this mutation[37]. Exposure to microcystins (metabolites of cyanobacterial blooms) through water and aquatic food is also implicated in hepatocarcinogenesis[38]. Chemical industrial carcinogens such as pesticides, vinyl chloride, arsenic, tobacco combustion derivatives, and radioactive elements such as Thorotrast, can also cause liver cancer[6].
Other liver lesions such as hepatocellular adenoma occur in non-cirrhotic backgrounds and can undergo malignant transformation in around 15% of cases[39]. Patients taking anabolic C17-alkylated androgenic steroids are also predisposed to HCC development in a non-cirrhotic background[40].
Pathological features
Macroscopic evaluation of non-cirrhotic HCC
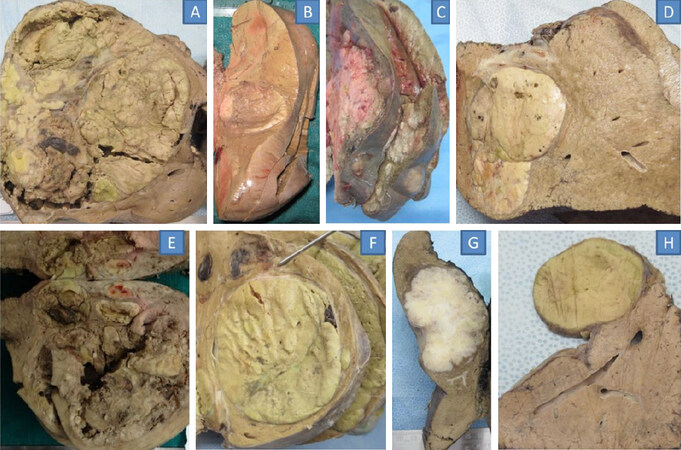
Gross examination of a non-cirrhotic HCC frequently displays a large solitary mass or a dominant mass with small satellite nodules. This is in contrast to HCCs in cirrhosis, which has either a single nodule or multiple small nodules[6,24,41]. In a study based on retrospective analysis of the gross specimens of 242 solitary and resected primary HCCs, the absence of cirrhosis was recorded in 45%. Various gross subtypes including expanding nodular, multinodular confluent, nodular with perinodular extension were almost equally prevalent in both cirrhotic and non-cirrhotic HCCs; the infiltrative type however, was far more common in cirrhotic patients[42][Figure 1].
Figure 1. Gross specimens: HCC in a non-cirrhotic background: massive solitary HCC in a patient with NASH (A); single HCC in HBV related liver disease (B); multinodular HCC in a patient infected with HBV (C); steatohepatitic variant of HCC in a patient with NASH (D); single large HCC in a patient infected with HCV (E); large HCC with prominent cholestasis and pseudoglandular pattern on microscopy (F); combined HCC-CC in a patient infected with HBV (G); fibrolamellar HCC (H). HCC: hepatocellular carcinoma; HBV: hepatitis B virus
Non-cirrhotic HCCs are more likely to develop intratumoral hemorrhage. It shows tumour heterogeneity with variegated appearances due to necrosis and hemorrhage[24]. Intracellular fat accumulation is more frequently seen in well-differentiated, non-cirrhotic HCCs[24]. Encapsulated tumors occur significantly more in patients without cirrhosis[12]. However other studies have reported lack of encapsulation in this group[43]. Altogether, non-cirrhotic HCCs are markedly different from cirrhotic HCCs in terms of lesion number, dimensions, fat content, intratumoral hemorrhage, and encapsulation [7].
The published literature indicate more frequent metastasis, direct invasion of adjacent organs and macroscopic portal vein or hepatic vein invasion in non-cirrhotic HCC, which is probably related to delayed diagnosis or inherent biological aggressiveness[41].
Fibrolamellar HCCs are a distinct variant of HCCs known to occur in non-cirrhotic livers[44]. Up to one third of HCCs developing in noncirrhotic backgrounds are of the fibrolamellar type[2,45]. This variant forms a heterogeneous and well-circumscribed mass[46]. On cut sections, prominent fibrous septa subdividing the mass into a central zone of scarring and calcifications may be observed[47].
Histopathology of HCCs arising in non-cirrhotic backgrounds
The histological evaluation of HCC specimens plays a key role in tumor staging and in distinguishing HCC from its precursor lesions or other liver nodules[48]. Tumor biopsy based diagnosis is recommended for all nodules occurring in non-cirrhotic livers[1,49,50].
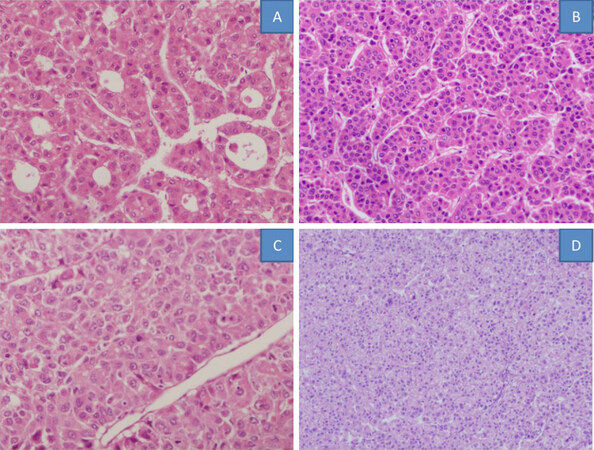
The pathological characteristics are similar to those of an HCC developing in a cirrhotic background. Thickened tumor cell plates, malignant cytology, capillarization of sinusoids and evidence of invasion constitute the principal diagnostic microscopic features. The four major histological patterns in HCC are microtrabecular, compact, macrotrabecular and pseudoglandular. Of these, the trabecular form is the most common histological pattern of HCC, both in cirrhotic and non-cirrhotic livers (41%-76%)[51][Figure 2].
Figure 2. Histological patterns of hepatocellular carcinoma in non-cirrhotic livers: pseudoglandular (A), microtrabecular (B), macrotrabecular (C), compact (D)
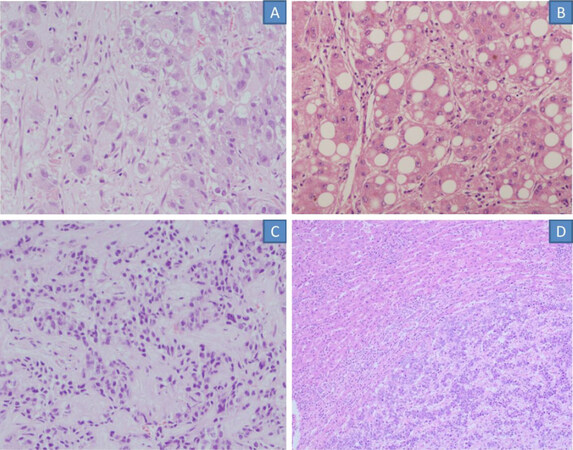
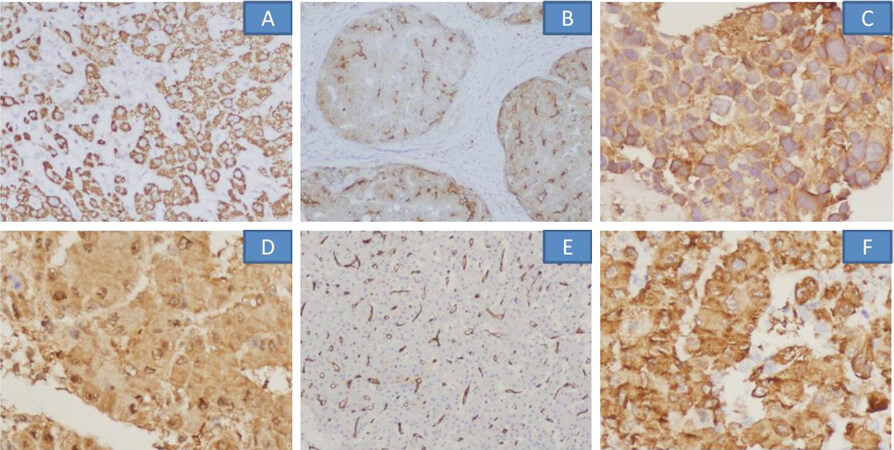
There are several histological subtypes of HCC such as fibrolamellar, steatohepatitic, lymphoepithelioma-like carcinoma, combined hepatocholangiocarcinoma, clear cell HCC, sarcomatoid HCC and many others[52]. These HCC histological subtypes have distinct morphological features, some of which have prognostic importance; recently, several have also been reported to have dysregulation of specific molecular pathways with implications with respect to molecular targeted therapies[1]. All of the subtypes can be found in cirrhotic and non-cirrhotic livers but fibrolamellar carcinoma is found almost exclusively in non-cirrhotic livers[52][Figure 3]. FLC typically occurs as a single tumor in non-cirrhotic livers in younger individuals[53]. Scirrhous HCCs are often located beneath the liver capsule and are most common in non-cirrhotic livers[54,55]. Mixed HCC-cholangiocarcinoma subtype also occurs more frequently in non-cirrhotic livers[6]. The steatohepatitic variant of HCC also often occurs in non-cirrhotic backgrounds. Cirrhotomimetic HCC typically arises in cirrhotic livers but in rare cases, can occur in non-cirrhotic livers[52]. Well-differentiated HCCs are frequent in non-cirrhotic livers and have microscopic fat. Immunohistochemical panel comprising glypican 3 (GPC3), heat shock protein (HSP70) and glutamine synthetase (GS) may assist in diagnosis[56].
Figure 3. Microscopic sections of histological variants of hepatocellular carcinoma occuring in non-cirrhotic backgrounds: fibrolamellar (A), steatohepatitic (B), scirrhous (C), mixed hepatocellular-cholangiocarcinoma (D)
HCC is characterised by cytologic features which have prognostic importance and add heterogeneity to the tumour phenotype[1]. The occurrence of steatosis, clear cells, cholestasis, giant cells, and other miscellaneous features in HCCs in non-cirrhotic backgrounds is comparable with that in cirrhotic livers. However, a few studies have shown that giant cells, multinucleate cells, local hepatic venous invasion by tumor, and Mallory bodies were 2.4, 2.0, 1.6 and 1.5 times more likely, respectively, to be found in tumors occurring in patients with cirrhosis than those in patients without cirrhosis[12].
There is conflicting data on the occurrence of prognostic histological indices in non-cirrhotic HCCs. Nzeako et al.[12] reported that cirrhotic patients, as compared to non-cirrhotic individuals, have a risk of harbouring grade 3-4 tumors and venous invasion of 1.7 and 1.6 times respectively. Whereas extrahepatic extension was reported to be greater in HCC arising in non-cirrhotic liver (20.5% vs. 6.5%)[41]; others have reported comparable tumor differentiation and portal tree invasion between cirrhotic and non-cirrhotic HCCs[41,57].
Pathology of background liver
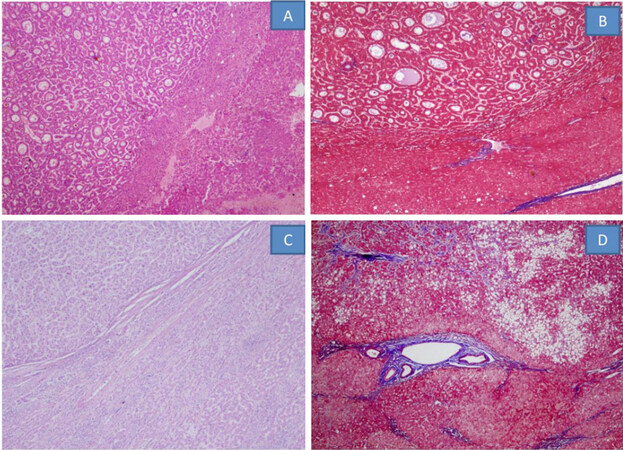
HCC may develop without advanced liver fibrosis or even in normal livers. The non-tumoral liver exhibits features of chronic hepatitis, varying degrees of fibrosis, steatosis, iron overload or other metabolic disorders[58]. In actuality, very few cases have an absolutely normal liver. However, in a study, 21/87 HCCs occurred in non-cirrhotic livers, and histopathological evaluation showed that nearly a third had no fibrosis in the liver[8]. Another study of 1,221 patients revealed that 238 (19%) had no cirrhosis yet the grade of fibrosis was ≤ F2 in 62% of all non-cirrhotic patients, and F3 in 8%. In another series of surgical resections of HCC, a high rate of non-cirrhotic livers was identified (F0-F1 38%, F2-F3 33%, F4 29%)[59]. Other features such as liver cell dysplasia, more often the large cell type, was found in 27%-40% of cases[60,61] and these figures decreased to 6%-20% in the subgroup of non-fibrotic livers[62,63]. The presence of NAFLD (12% vs. 28%) is more common in patients without cirrhosis than in those with cirrhosis[64][Figure 4].
Figure 4. HCC in a non-cirrhotic background: HCC with pseudoglandular pattern in a background with F0-F1 fibrosis (A, HE stain; B Masson Trichrome stain), HCC with microtrabecular pattern in F0 fibrosis (C, HE stain), steatohepatitic HCC with F3 fibrosis in background liver (D, MT stain). HCC: hepatocellular carcinoma
Differential diagnosis of HCCs arising in non-cirrhotic livers
There is a plethora of inflammatory, immunologic, neoplastic and infective lesions that occur in a non-cirrhotic liver background. However, from the point of view of histopathological interpretation, the most important are preneoplastic and neoplastic conditions, HCC variants and tumor metastasis.
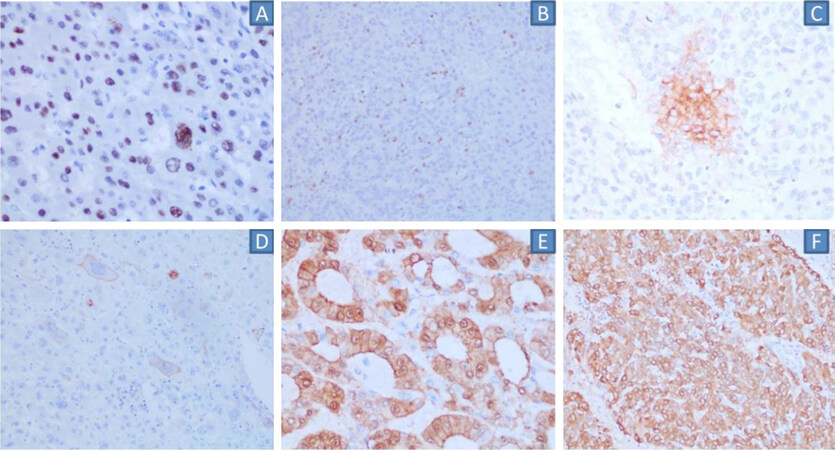
Dysplastic nodules are premalignant lesions, which are well-defined and circumscribed. These lesions mostly arise from a background of chronic liver disease and more often, cirrhosis[65]. These are important for diagnosis as often, they come under the differential diagnosis of well-differentiated HCCs. Immunohistochemical panels comprising glypican 3, HSP-70, glutamine synthetase, CD34 and CK7, are particularly useful in such scenarios[18][Figure 5].
Figure 5. Immunohistochemical markers helpful for confirming hepatocytic origin and in confirming well-differentiated HCC: HepPar1 (A), polyclonal CEA (B), Glypican-3 (C), HSP70 (D), CD34 (E), glutamine synthetase (F). HCC: hepatocellular carcinoma
The differential diagnosis between hepatocellular adenoma (HCA) and well-differentiated HCC arising in non-cirrhotic livers is another challenging situation, especially the HCA subtype with cytological or architectural atypia[66]. Clinico-pathological correlation helps to sort this conundrum. Malignant transformation is one of the most important complications of HCA and is reported to occur in 4%-10% of HCA[67-69]. Awareness about the malignant potential and predisposing risk factors such as male gender, larger tumor size, and β-catenin activated subtype, is crucial[68].
Focal nodular hyperplasia (FNH) is another important differential consideration for HCCs occurring in non-cirrhotic backgrounds. FNH, a benign lesion resulting from the regenerative response to vascular abnormalities, has a characteristic histomorphology. A central scar with thick-walled vessels and nodular regeneration of hepatocytes, with marked ductular reaction and inflammatory infiltrate at the junction of fibrous bands and hepatocyte nodules, distinguishes FNH from HCC. A “map-like,” geographic pattern of GS staining of FNH is especially useful in diagnostic dilemmas[70-72].
Awareness of other benign and malignant epithelial, mesenchymal and vascular tumours is crucial. Cholangiocarcinoma is the second most common primary cancer and accounts for 15% of primary liver tumors[73]. Glands lying in desmoplastic stroma and immunohistochemical expression of mucin1, CK7 and CK19, while negative for hepatocytic origin, aids in the diagnosis[74]. Bile duct adenoma is often a difficult differential, particularly in intra-operative frozen section examination. A subcapsular location and a well-circumscribed appearance are important clues to the diagnosis. Vascular lesions such as hemangioma, epithelioid hemangioendothelioma, angiosarcoma, and angiomyolipoma are other mimics of HCC on imaging. The histopathology and immunohistochemical markers are quite distinct however, so the diagnosis is straightforward.
Certain HCC variants have a predilection to occur in non-cirrhotic livers. Fibrolamellar, scirrhous, steatohepatiic and mixed hepato-cholangiocarcinoma are the more frequent subtypes. Molecular pathways and morpho-molecular features are reviewed in the molecular pathology section.
The fibrolamellar subtype merits special mention. It was first described by Edmondson in 1980, and is a rare entity, accounting for less than 1% of all cases of primary liver cancer[44]. It is mostly encountered in the young population without any underlying chronic liver disease, or other known predisposing risk factors[75]. Its key histological features are the presence of lamellar stromal bands surrounding nests of large polygonal eosinophilic tumor cells, which have prominent nucleoli. The tumor cells display the presence of cytoplasmic inclusions - ground-glass pale bodies, eosinophilic cytoplasmic globules, and Mallory-Denk bodies. The immunohistochemical markers CK7 and CD68 are expressed by tumor cells and have a sensitivity of 100% and 96% respectively[76,77], which are important for confirmation[47].
Metastasis is the most common hepatic malignancy and occurs in non-cirrhotic livers. In patients without underlying liver disease, HCC accounts for only about 2% of malignant liver neoplasms[78,79]. The lung, colon, pancreas and breast are the most common primary sites that metastasize to the liver. In some cases, these mimic HCC, particularly clear-cell renal cell carcinoma, clear-cell adenocarcinoma of the female genital organs, adrenal carcinoma and hepatoid adenocarcinoma of the stomach[78,79].
Patho-molecular characterization of HCC in non-cirrhotic livers
The etiology and etiopathogenesis for a vast number of non-cirrhotic HCCs still remain unknown. Advancements in translational research have made it possible to analyse thousands of molecular targets in HCC using microarray-based technologies as well as next-generation sequencing. However, unlike the cirrhotic HCC and HCA, molecular pathways and classifications have not been exclusively studied in non-cirrhotic HCCs. Genomic studies in this particular group would be able to elucidate the similarities and distinctness of the underlying mechanisms and biology in comparison to the cirrhotic HCC. Despite the scarcity of the literature, recent studies on the molecular pathology of HCC, regardless of the background liver, provides vital information.
The most frequent mutations affect the TERT promoter (60%), which is associated with an increased expression of telomerase. TP53 and CTNNB1 are the next most prevalent mutations. These, combined with low-frequency mutated genes (e.g., AXIN1, ARID2, ARID1A, TSC1/TSC2, RPS6KA3, KEAP1, MLL2), represent the main deregulated pathways in HCC[80].
Different pathways of genetic alterations point towards different hepatocarcinogenetic mechanisms in HCC with and without cirrhosis. HCCs in non-cirrhotic livers are more often associated with higher β-catenin mutation, p21 expression, p14 inactivation, and global gene methylation, in contrast to higher p53 and Wnt/β-catenin pathway aberrations seen in cirrhotic HCCs[81,82]. However, one study showed nuclear p53 labeling in 30% of non-cirrhotic HCCs[83]. Other studies have also suggested that tumor suppressor genes play an important role in the development of HCC in the absence of cirrhosis[84][Figure 6].
Figure 6. Immunohistochemical markers representative of phenotypic correlates of dysregulated molecular pathways: TP53 (A), PD1 in immune cells (B), PDL1 in tumour cells (C), CK19 in tumour cells (D), β-catenin nuclear positivity (E), glutamine synthetase diffuse expression (F). PD1: Programmed death-1; PD-L1: programmed death-ligand 1
Analysis of miRNAs holds great promise for improving diagnosis and prognosis, along with the therapeutic management of HCCs originating in non-cirrhotic backgrounds. miRNA, particularly hsa-mir-149 expression, is considered an independent risk factor for the poor prognosis of non-cirrhotic HCCs but not for cirrhotic HCCs[85]. Early and unique changes in circulating miRNA in the serum could allow it to be a biomarker for the early detection of non-cirrhotic HCCs. A similar role of a three mi-RNA panel comprising of miR-92-3p, miR-107, and miR-3126-5p in early HCC was shown by Zhang et al.[86] and Koh et al.[87] reported the difference in expression of 16 miRNAs between non-tumor and HCC tissues in non-cirrhotic livers[87].
Few studies have pointed towards the role of altered mismatch repair genes in hepatocarcinogenesis. A study to explore the presence of microsatellite instability (MSI) in 37 non-cirrhotic HCC patients with a histologically normal liver, low alcohol intake and absence of HBV and HCV infection[88], demonstrated that 26 (43%) had MSI (16% of high grade). However, other authors did not find MSI to be significant in non-cirrhotic HCCs[88,89].
Angiogenesis related characteristics are similar between cirrhotic and non-cirrhotic HCCs[90]. Favorable outcomes in the fibrolamellar subtype might be partly due to the low number of cytogenetic aberrations in this type of tumor[91]. Stemness marker expression analysis revealed that 88% of non-cirrhotic HCCs were keratin-19 negative. Expression of K19 in this study demonstrated correlation with less tumor encapsulation and with the presence of p53 mutation[39].
Certain etiology specific studies have reported important molecular alterations in non-cirrhotic HCCs.
STAT signaling pathways have an important role in HCC-NASH[92], especially STAT-3 signaling with regard to NASH associated HCC occurring in non-cirrhotic backgrounds[93]. The literature advocates targeting of the STAT-1 signaling pathway in steatohepatitic HCC with cirrhosis or severe fibrosis and NASH, whereas NASH-HCC without cirrhosis or fibrosis is mediated through the STAT-3 signaling pathway[92]. Tumor suppressor genes play an important role in the development of steatosis, liver cell damage and HCC development in the absence of cirrhosis in fatty liver disease[27,84,94]. Patients whose tumors showed heterogeneous staining patterns of glutamine synthetase in non-cirrhotic livers were more commonly overweight or obese[39].
HBV infection has direct oncogenic potential and the accumulation of mutations in basal core promoters and a high viral load is considered an independent predictor of HCC development. These mutational patterns have the potential to identify those at risk of HCC development. HCC development in HCV infected patients is mainly attributable to sustained necro-inflammatory processes and therefore, occurs on a background of advanced liver fibrosis or frank cirrhosis. Studies have shown that several HCV gene products (core, NS3, NS4B and NS5A) possess transformation potential in murine fibroblast culture, suggesting that HCV also has direct hepatocarcinogenic potential[16,22,23]. Approximately 46% of HCV-related HCCs exhibit CTNNB mutations[95]. Of these, the majority arise in the absence of underlying cirrhosis.
Overrepresentation of T>C at ApTpX with transcription strand bias, a pattern known to be strongly associated with genotoxic injury, was reported in HCC developing in non-cirrhotic patients with high alcohol and tobacco consumption[96]. A variety of congenital and acquired conditions also induce the development of HCC without underlying cirrhosis, often through alterations in cell cycle regulation, oxidative stress, and increased levels of tumorigenic growth factors.
Dysregulation of molecular pathways in histological variants of HCC
Macrotrabecular massive HCC (MTM-HCC), a novel distinct subtype of HCC, is characterized by the presence of a macrotrabecular pattern of more than 6 cells thick recorded in > 50% of the tumor as described by Ziol et al.[97]. In another recent study, taking a cut off of > 30% macrotrabecular pattern, MTM-HCC was found to be less often associated with cirrhosis[98]. This variant shows genetic aberrations, which are related to cell cycle activation, chromosomal instability, the G3 transcriptomic subgroup, FGF19 amplifications and TP53 mutation[59,99]. MM-HCC is also characterized by the high expression of two key regulators of neoangiogenesis and vascular remodeling, angiopoietin 2 and vascular endothelial growth factor A (VEGFA)[59,100]. Endothelial-specific molecule 1 (ESM1) was identified as a biomarker for this variant[59].
The steatohepatitic subtype is characterized by prominent steatotic changes in the tumor cells, namely fat accumulation, ballooning degeneration, the presence of Mallory-Denk bodies and peri-cellular fibrosis[48,101]. SH-HCC also often occurs in non-cirrhotic backgrounds as shown in a recent study that reported SH-HCC in 20% of the 96 HCC cases reviewed[102]. IL6/JAK/STAT pathway activation, wild type CTNNB1 and TP53 are the molecular pathways implicated in the pathogenesis[59,103]. However, the literature reports that CTNNB1 mutations (beta catenin pathway alterations) are less frequent in steatohepatitic HCCs compared to conventional HCCs[103].
Lymphocyte-rich HCCs are characterized by an immune rich stroma and have been demonstrated to have cirrhosis in only 46% of cases in a recent comprehensive review[104]. Molecular studies have revealed that mutations of CTNNB1, AXIN1, APC, NOTCH1 and NOTCH2 were less frequently observed in lymphocyte-rich HCCs than conventional HCCs[105], suggesting a relation between these pathways and immune exclusion. In lymphoepithelioma-like HCC, oncogenes expressed from chromosome 11q13.3 (CCND1FGF19, and FGF4) are strongly associated with the immune checkpoint signature (CD274, PDCD1, BTLA, CTLA4, HAVCR2, IDO1, and LAG3). Such differences in genetic aberrations from classical HCCs provide insight into the need for therapeutic strategies that evade immune surveillance seen in classical HCCs[105,106].
Scirrhous HCC is another variant, which is less often associated with liver cirrhosis when compared with conventional HCC[55]. Genetic studies have highlighted TGF-β signaling, TSC1/TSC2 mutations and the expression of stem cell markers as the main derangements[59,107].
Fibrolamellar HCC is characterized by a chimeric transcript, found as a result of a deletion in the chromosome 19 - DNAJB1-PRKACA chimeric protein[108]. This genetic signature is not reported in other tumors, which indicates that the mutation plays a key role in FL-HCC tumorigenesis[46,109]. Interaction between the fusion kinase and b-catenin[110] also contribute to the pathogenesis of FLC. Comparative genomic hybridization studies have demonstrated that in contrast to the classical HCC, the TP53, Wnt/β-catenin, or surviving pathways, are not mutated in FL-HCC[91,111].
Combined hepatocellular-cholangiocarcinoma (cHCC-CCA) also occurs in the non-cirrhotic liver background. Genomic sequencing shows a profile similar to conventional HCCs[112]. Studies have shown that the same oncogenic drivers delivered to hepatocytes could generate tumors with either a hepatocellular or biliary phenotype, and such differentiation is mainly dependent upon the microenvironment created by the oncogenic process[113]. Recurrent alterations in TERT, TP53, cell cycle genes, receptor tyrosine kinase/Ras/PI3K pathway genes, chromatin regulators etc. were identified in cHCC-CCA, while IDH1, IDH2, FGFR2 and BAP1 mutations were absent[97]. CCND1, MET and ERBB2 amplifications are present at higher frequencies in CHCs compared with HCCs, whereas Wnt pathway alterations are relatively less frequent[114]. The literature shows that in comparison to HCCs, TP53 mutations occur twice as frequently in cHCC-CCAs. TP53 mutations in HCC are usually associated with a worse prognosis and poorly differentiated histomorphology[59,115]. This suggests that cHCC-CCA is more similar to poorly differentiated HCCs and explains its worse prognosis [Figure 7].
Conclusion
HCCs arising in non-cirrhotic livers are distinct from cirrhotic HCCs in many ways. The risk factors, etiologies, pathogenesis, histopathology and the differential diagnosis, along with genomic pathways, differ from the typical cirrhotic HCC. Current knowledge of phenotypic-molecular alterations are based on studies on HCC irrespective of the liver background. Studies devoted exclusively to non-cirrhotic HCCs are scarce. In the era of molecular targeted therapies, there is an urgent need to analyze the carcinogenetic mechanisms and pathology based phenotypic correlates of specific molecular aberrations in HCCs originating in non-cirrhotic livers.
Declarations
AcknowledgmentsProf. (Dr.) SK Sarin, Senior Professor (Dept. of Hepatology) & Director, Institute of Liver & Biliary Sciences, Delhi, for constant guidance & support. Dr. Gayatri Ramakrishna, Additional Professor, Department of Molecular & Cellular Medicine, for creating figure of molecular pathways.
Authors’ contributionsThe author contributed solely to the article.
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestThe authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2020.
REFERENCES
1. Rastogi A. Changing role of histopathology in the diagnosis and management of hepatocellular carcinoma. World J Gastroenterol 2018;24:4000-13.
2. Gaddikeri S, McNeeley MF, Wang CL, Bhargava P, Dighe MK, et al. Hepatocellular carcinoma in the noncirrhotic liver. Am J Roentgenol 2014;203:W34-47.
4. Wörns MA, Bosslet T, Victor A, Koch S, Hoppe-Lotichius M, et al. Prognostic factors and outcomes of patients with hepatocellular carcinoma in non-cirrhotic liver. Scand J Gastroenterol 2012;47:718-28.
5. Schütte K, Kipper M, Kahl S, Bornschein J, Götze T, et al. Clinical characteristics and time trends in etiology of hepatocellular cancer in Germany. Digestion 2013;87:147-59.
6. Trevisani F, Frigerio M, Santi V, Grignaschi A, Bernardi M. Hepatocellular carcinoma in non-cirrhotic liver: a reappraisal. Dig Liver Dis 2010;42:341-7.
7. Zhang Y, Wang C, Xu H, Xiao P, Gao Y. Hepatocellular carcinoma in the noncirrhotic liver: a literature review. Eur J Gastroenterol Hepatol 2019;31:743-8.
8. Schütte K, Schulz C, Poranzke J, Antweiler K, Bornschein J, et al. Characterization and prognosis of patients with hepatocellular carcinoma (HCC) in the non-cirrhotic liver. BMC Gastroenterol 2014;14:117.
9. Lafitte M, Laurent V, Soyer P, Ayav A, Balaj C, et al. MDCT features of hepatocellular carcinoma (HCC) in non-cirrhotic liver. Diagn Interv Imaging 2016;97:355-60.
10. Di Martino M, Saba L, Bosco S, Rossi M, Miles KA, et al. Hepatocellular carcinoma (HCC) in non-cirrhotic liver: clinical, radiological and pathological findings. Eur Radiol 2014;24:1446-54.
11. Desai A, Sandhu S, Lai JP, Sandhu DS. Hepatocellular carcinoma in non-cirrhotic liver: a comprehensive review. World J Hepatol 2019;11:1-18.
12. Nzeako UC, Goodman ZD, Ishak KG. Hepatocellular carcinoma in cirrhotic and noncirrhotic livers. A clinico-histopathologic study of 804 North American patients. Am J Clin Pathol 1996;105:65-75.
13. Adam R, Bismuth H, Castaing D, Waechter F, Navarro F, et al. Repeat hepatectomy for colorectal liver metastases. Ann Surg 1997;225:51-60. discussion 60-2
14. Hadjittofi C, Athanasopoulos PG, Koti RS, Konstantinidou SK, Davidson BR. Long-term survival with repeated resections of recurrent hepatocellular carcinoma in a non-cirrhotic liver: case report and brief review of the literature. Ann Transl Med 2016;4:112.
15. el-Refaie A, Savage K, Bhattacharya S, Khakoo S, Harrisson TJ, et al. HCV-associated hepatocellular carcinoma without cirrhosis. J Hepatol 1996;24:277-85.
16. Ray RB, Meyer K, Ray R. Hepatitis C virus core protein promotes immortalization of primary human hepatocytes. Virology 2000;271:197-204.
17. Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer 2006;6:674-87.
18. Sherman M. Hepatocellular carcinoma: epidemiology, risk factors, and screening. Semin Liver Dis 2005;25:143-54.
19. Wong GL, Chan HL, Chan HY, Tse PC, Tse YK, et al. Accuracy of risk scores for patients with chronic hepatitis B receiving entecavir treatment. Gastroenterology 2013;144:933-44.
20. Conti F, Buonfiglioli F, Scuteri A, Crespi C, Bolondi L, et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV related cirrhosis treated with direct-acting antivirals. J Hepatol 2016;65:727-33.
21. Bruix J, Sherman M; American association for the study of liver diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2.
22. Park JS, Yang JM, Min MK. Hepatitis C virus nonstructural protein NS4B transforms NIH3T3 cells in cooperation with the Ha-ras oncogene. Biochem Biophys Res Commun 2000;267:581-7.
23. Sakamuro D, Furukawa T, Takegami T. Hepatitis C virus nonstructural protein NS3 transforms NIH 3T3 cells. J Virol 1995;69:3893-6.
24. Lee DH, Lee JM. Primary malignant tumours in the non-cirrhotic liver. Eur J Radiol 2017;95:349-61.
25. Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J Hepatol 2012;56:1384-91.
26. Thompson SM, Garg I, Ehman EC, Sheedy SP, Bookwalter CA, et al. Non-alcoholic fatty liver disease-associated hepatocellular carcinoma: effect of hepatic steatosis on major hepatocellular carcinoma features at MRI. Br J Radiol 2018;91:20180345.
27. Ertle J, Dechêne A, Sowa J, Penndorf V, Herzer K, et al. Non-alcoholic fatty liver disease progressesto hepatocellular carcinoma in the absence of apparent cirrhosis. Int J Cancer 2011;128:2436-43.
28. Adami HO, Hsing AW, Mclaughlin JK, Trichopoulos D, Hacker D, et al. Alcoholism and liver cirrhosis in the etiology of primary liver cancer. Int J Cancer 1992;51:898-902.
29. London WT, McGlynn KA. Liver cancer. In: Schottenfeld D, Fraumeni Jr JF, editors. Cancer epidemiology and prevention. New York: Oxford University Press; 1996. pp. 772-93.
30. Burra P, Zanetto A, Germani G. Liver transplantation for alcoholic liver disease and hepatocellular carcinoma. Cancers (Basel) 2018;10:46.
31. Jiang HY, Chen J, Xia CC, Cao LK, Duan T, et al. Noninvasive imaging of hepatocellular carcinoma: from diagnosis to prognosis. World J Gastroenterol 2018;24:2348-62.
32. Ramakrishna G, Rastogi A, Trehanpati N, Sen B, Khosla R, et al. From cirrhosis to hepatocellular carcinoma: new molecular insights on inflammation and cellular senescence. Liver Cancer 2013;2:367-83.
33. Trehanpati N, Shrivastav S, Shivakumar B, Khosla R, Bhardwaj S, et al. Analysis of notch and TGF-β signaling expression in different stages of disease progression during hepatitis B virus infection. Clin Transl Gastroenterol 2012;3:e23.
34. Turlin B, Juguet F, Moirand R, Le Quilleuc D, Loréal O, et al. Increased liver iron stores in patients with hepatocellular carcinoma developed on a noncirrhotic liver. Hepatology 1995;22:446-50.
35. Ozakyol A. Global epidemiology of hepatocellular carcinoma (HCC epidemiology). J Gastrointest Canc 2017;48:238-40.
36. Liu CJ, Chen BF, Chen PJ, Lai MY, Huang WL, et al. Role of hepatitis B virus precore/core promoter mutations and serum viral load on noncirrhotic hepatocellular carcinoma: a case-control study. J Infect Dis 2006;194:594-9.
38. Zheng C, Zeng H, Lin H, Wang J, Feng X, et al. Serum microcystin levels positively linked with risk of hepatocellular carcinoma: a case-control study in southwest China. Hepatology 2017;66:1519-28.
39. Liu TC, Vachharajani N, Chapman WC, Brunt EM. Noncirrhotic hepatocellular carcinoma: derivation from hepatocellular adenoma? Clinicopathologic analysis. Mod Pathol 2014;27:420-32.
40. Farrell GC, Joshua DE, Uren RF, Baird PJ, Perkins KW, et al. Androgen-induced hepatoma. Lancet 1975;22:430-2.
41. Trevisani F, D’Intino PE, Caraceni P, Pizzo M, Stefanini GF, et al. Etiologic factors and clinical presentation of hepatocellular carcinoma. Differences between cirrhotic and noncirrhotic Italian patients. Cancer 1995;75:2220-32.
42. Lee Y, Park H, Lee H, Cho JY, Yoon YS, et al. The clinicopathological and prognostic significance of the gross classification of hepatocellular carcinoma. J Pathol Transl Med 2018;52:85-92.
43. Kojiro M. Pathology of hepatocellular carcinoma. Hoboken: Wiley; 2009. pp. 1-184.
44. Craig JR, Peters RL, Edmondson HA, Omata M. Fibrolamellar carcinoma of the liver: a tumor of adolescents and young adults with distinctive clinico-pathologic features. Cancer 1980;46:372-9.
45. Houben KW, McCall JL. Liver transplantation for hepatocellular carcinoma in patients without underlying liver disease: a systematic review. Liver Transpl Surg 1999;5:91-5.
46. Lin CC, Yang HM. Fibrolamellar carcinoma: a concise review. Arch Pathol Lab Med 2018;142:1141-5.
47. Van Eyken P, Sciot R, Brock P, Casteels-Van Daele M, Ramaekers FC, et al. Abundant expression of cytokeratin 7 in fibrolamellar carcinoma of the liver. Histopathology 1990;17:101-7.
48. Cheuk-Lam Lo R. An update on the histological subtypes ofhepatocellular carcinoma. Hepatoma Res 2019;5:41.
49. International consensus group for hepatocellular neoplasia. Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology 2009;49:658-64.
50. Agni RM. Diagnostic histopathology of hepatocellular carcinoma: a case-based review. Semin Diagn Pathol 2017;34:126-37.
51. Bosman FTCF, Hruban RH, Theise ND. World Health Organization classification oftumours: pathology and genetics of tumours of the digestive system. Lyon, France: IARC Press; 2010.
52. Torbenson MS. Morphologic Subtypes of hepatocellular carcinoma. Gastroenterol Clin North Am 2017;46:365-91.
53. Ang CS, Kelley RL, Choti MA, Cosgrove DP, Chou JF, et al. Clinicopathologic characteristics and survivaloutcomes of patients with fibrolamellar carcinoma: data from the fibrolamellar carcinoma consortium. Gastrointest Cancer Res 2013;6:3-9.
54. Fujii T, Zen Y, Harada K, Harada K, Niwa HN, Masuda S, et al. Participation of liver cancer stem/progenitor cells in tumorigenesis of scirrhous hepatocellular carcinoma-human and cell culture study. Hum Pathol 2008;39:1185-96.
55. Lee JH, Choi MS, Gwak GY, Gwak GY, Lee JH, et al. Clinicopathologic characteristics and long-term prognosis of scirrhous hepatocellular carcinoma. Dig Dis Sci 2012;57:1698-707.
56. Trojan J, Zangos S, Schnitzbauer AA. Diagnostics and treatment of hepatocellular carcinoma in 2016: standards and developments. Visc Med 2016;32:116-120.
57. Chang CH, Chau GY, Lui WY, Tsay SH, King KL, et al. Long-term results of hepatic resection for hepatocellular carcinoma originating from the noncirrhotic liver. Arch Surg 2004;139:320-5.
58. Paradis V, Zalinski S, Chelbi E, Guedj N, Degos F, et al. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology 2009;49:851-9.
59. Calderaro J, Couchy G, Imbeaud S, Amaddeo G, Letouzéet E, et al. Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification. J Hepatol 2017;67:727-38.
60. Okuda K, Nakashima T, Kojiro M, Kondo Y, Wada K. Hepatocellular carcinoma without cirrhosis in Japanese patients. Gastroenterology 1989;97:140-6.
61. Shimada M, Rikimaru T, Sugimachi K, Hamatsu T, Yamashita Y, et al. The importance of hepatic resection for hepatocellular carcinoma originating from nonfibrotic liver. J AmColl Surg 2000;191:531-7.
62. Grando-Lemaire V, Guettier C, Chevret S, Beaugrand M, Trinchet JC. Hepatocellular carcinoma without cirrhosis in the West: epidemiological factors and histopathology of the non-tumorous liver. Groupe d’Etude et de Traitement du Carcinome Hépatocellulaire. J Hepatol 1999;31:508-13.
63. Bralet MP, Régimbeau JM, Pineau P, Dubois S, Loas G, et al. Hepatocellular carcinoma occurring in nonfibrotic liver: epidemiologic and histopathologic analysis of 80 French cases. Hepatology 2000;32:200-4.
64. van Meer S, van Erpecum KJ, Sprengers D, Coenraad MJ, Klümpen HJ, et al. Hepatocellular carcinoma in cirrhotic versus noncirrhotic livers: results from a large cohort in the Netherlands. Eur J Gastroenterol Hepatol 2016;28:352-9.
65. Pittman ME, Brunt EM. Anatomic pathology of hepatocellular carcinoma: histopathology using classic and new diagnostic tools. Clin Liver Dis 2015;19:239-59.
66. Evason KJ, Grenert JP, Ferrell LD, Kakar S. Atypical hepatocellular adenoma-like neoplasms with β-catenin activation show cytogenetic alterations similar to well-differentiated hepatocellular carcinomas. Hum Pathol 2013;44:750-8.
67. Dokmak S, Paradis V, Vilgrain V, Sauvanet A, Farges O, et al. A single-center surgical experience of 122 patients with single and multiple hepatocellular adenomas. Gastroenterology 2009;137:1698-705.
68. Bioulac-Sage P, Laumonier H, Couchy G, Bail BL, Cunha A, et al. Hepatocellular adenoma management and phenotypic classification: the Bordeaux experience. Hepatology 2009;50:481-9.
69. Deneve JL, Pawlik TM, Cunningham S, Clary B, Reddy S, et al. Liver cell adenoma: a multicenter analysis of risk factors for rupture and malignancy. Ann Surg Oncol 2009;16:640-8.
70. Roncalli M, Sciarra A, Tommaso LD. Benign hepatocellular nodules of healthy liver: Focal nodular hyperplasia and hepatocellular adenoma. Clin Mol Hepatol 2016;22:199-211.
71. Libbrecht L, Cassiman D, Verslype C, Maleux G, Van Hees D, et al. Clinicopathological features of focal nodular hyperplasia-like nodules in 130 cirrhotic explant livers. Am J Gastroenterol 2006;101:2341-6.
72. Bioulac-Sage P, Laumonier H, Rullier A, Cubel G, Laurent C, et al. Over-expression of glutamine synthetase in focal nodular hyperplasia: a novel easy diagnostic tool in surgical pathology. Liver Int 2009;29:459-65.
73. Nakanuma Y, Sripa B, Vatanasapt B, Leong A, Ponchon T, et al. Intrahepatic cholangiocarcinoma. In: Hamilton SR, Aaltonen LA, editors. WHO Classification of Tumors, Pathology and Genetics Tumors of the Digestive System. Lyon: IARC Press; 2000. pp. 173-80.
75. El-Serag HB, Davila JA. Is fibrolamellar carcinoma different from hepatocellular carcinoma? A US population-based study. Hepatology 2004;39:798-803.
76. Ward SC, Huang J, Tickoo SK, Thung SN, Ladanyi M, et al. Fibrolamellar carcinoma of the liver exhibits immunohistochemical evidence of both hepatocyte and bile duct differentiation. Mod Pathol 2010;23:1180-90.
77. Ross HM, Daniel HD, Vivekanandan P, Kannangai R, Yeh MM, et al. Fibrolamellar carcinomas are positive for CD68. Mod Pathol 2011;24:390-5.
78. Schlageter M, Terracciano LM, D’Angelo S, Sorrentino P. Histopathology of hepatocellular carcinoma. World J Gastroenterol 2014;20:15955-64.
79. Melato M, Laurino L, Mucli E, Valente M, Okuda K. Relationship between cirrhosis, liver cancer, and hepatic metastases. An autopsy study. Cancer 1989;64:455-9.
80. Zucman-Rossi J, Villanueva A, Nault JC, Llovet JM. Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology 2015;149:1226-39.e4.
81. Tretiakova MS, Shabani-Rad MT, Guggisberg K, Hart J, Andera RA, et al. Genomic and immunophenotypical differences between hepatocellular carcinoma with and without cirrhosis. Histopathology 2010;56:683-93.
82. Tannapfel A, Wittekind C. Genes involved in hepatocellular carcinoma: deregulation in cell cycling and apoptosis. Virchows Arch 2002;440:345-52.
83. Sanyal AJ, Yoon SK, Lencioni R. The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist 2010;15 Suppl 4:14-22.
84. Herzer K, Hofmann TG, Teufel A, Schimanski CC, Moehler M, et al. IFN-alpha-induced apoptosis in hepatocellular carcinoma involves promyelocytic leukemia protein and TRAIL independently of p53. Cancer Res 2009;69:855-62.
85. Mei Y, You Y, Xia J, Gong JP, Wang YB. Identifying differentially expressed microRNAs between cirrhotic and non-cirrhotic hepatocellular carcinoma and exploring their functions using bioinformatic analysis. Cell Physiol Biochem 2018;48:1443-56.
86. Zhang Y, Li T, Qiu Y, Zhang T, Guo P, et al. Serum microRNA panel for early diagnosis of the onset of hepatocellular carcinoma. Medicine (Baltimore) 2017;96:e5642.
87. Koh YS, Kim JH, Cai H, Li LH, Kim HS, et al. Dysregulated microRNAs in non-cirrhotic hepatocellular carcinoma. Genes Genom 2013;35:759-65.
88. Chiappini F, Gross-Goupil M, Saffroy R, Azoulay D, Emile JF, et al. Microsatellite instability mutator phenotype in hepatocellular carcinoma in non-alcoholic and non-virally infected normal livers. Carcinogenesis 2004;25:541-7.
89. Togni R, Bagla N, Muiesan P, Miquel R, O'Grady J, et al. Microsatellite instability in hepatocellularcarcinoma in non-cirrhotic liver in patients older than 60 years. Hepatol Res 2009;39:266-73.
90. Zeng W, Gouw AS, van den Heuvel MC, Molema G, Poppema S, et al. Hepatocellular carcinomas in cirrhotic and noncirrhotic human livers share angiogenic characteristics. Ann Surg Oncol 2010;17:1564-71.
91. Kakar S, Chen X, Ho C, Burgart LJ, Sahai V, et al. Chromosomal changes in fibrolamellar hepatocellular carcinoma detected by array comparative genomic hybridization. Mod Pathol 2009;22:134-41.
92. Grohmann M, Wiede F, Dodd GT, Gurzov EN, Ooi GJ, et al. Obesity drives STAT-1-dependent NASH and STAT-3-dependent HCC. Cell 2018;175:1289-306.e20.
93. Takakura K, Oikawa T, Nakano M, Saeki C, Torisu Y, et al. Recent insights into the multiple pathways driving non-alcoholic steatohepatitis-derived hepatocellular carcinoma. Front Oncol 2019;9:762.
94. Cazanave SC, Mott JL, Elmi NA, Bronk SF, Werneburg NW, et al. JNK1-dependent PUMA expression contributes to hepatocyte lipoapoptosis. J Biol Chem 2009;284:26591-602.
95. Shanbhogue AK, Prasad SR, Takahashi N, Vikram R, Sahani DV. Recent advances in cytogenetics and molecular biology of adult hepatocellular tumors: implications for imaging and management. Radiology 2011;258:673-93.
96. Schulze K, Imbeaud S, Letouze E, Alexandrov LB, Calderaro J, et al. Exome sequencing of hepatocellular carcinoma identifies new mutations signatures and potential therapeutic targets. Nat Genet 2015;47:505-11.
97. Ziol M, Pote N, Amaddeo G, Laurent A, Nault JC, et al. Macrotrabecular-massive hepatocellular carcinoma: a distinctive histological subtype with clinical relevance. Hepatology 2018;68:103-12.
98. Jeon Y, Benedict M, Taddei T, Jain D, Zhang X. Macrotrabecular hepatocellular carcinoma: an aggressive subtype of hepatocellular carcinoma. Am J Surg Pathol 2019;43:943-8.
99. Boyault S, Rickman DS, de Reynies A, Balabaud C, Rebouissou S, et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology 2007;45:42-52.
100. Zhang L, Yang N, Park JW, Katsaros D, Fracchioli S, et al. Tumor-derived vascular endothelial growth factor up-regulates angiopoietin-2 in host endothelium and destabilizes host vasculature, supporting angiogenesis in ovarian cancer. Cancer Res 2003;63:3403-12.
101. Salomao M, Yu WM, Brown RS Jr, Emond JC, Lefkowitch JH. Steatohepatitic hepatocellular carcinoma (SH-HCC): a distinctive histological variant of HCC in hepatitis C virus-related cirrhosis with associated NAFLD/NASH. Am J Surg Pathol 2010;34:1630-6.
102. Tan PS, Nakagawa S, Goossens N, Venkatesh A, Huang T, et al. Clinicopathological indices to predict hepatocellular carcinoma molecular classification. Liver Int 2016;36:108-18.
103. Ando S, Shibahara J, Hayashi A, Fukayama M. β-catenin alteration is rare in hepatocellular carcinoma with steatohepatitic features: immunohistochemical and mutational study. Virchows Arch 2015;467:535-42.
104. Labgaa I, Stueck A, Ward SC. Lymphoepithelioma-like carcinoma in liver. Am J Pathol 2017;187:1438-44.
105. Chan AW, Zhang Z, Chong CC, Tin EK, Chow C, et al. Genomic landscape of lymphoepithelioma-like hepatocellular carcinoma. J Pathol 2019;249:166-72.
106. Han Y, Wu Y, Yang C, Huang J, Guo Y, et al. Dynamic and specific immune responses against multiple tumor antigens were elicited in patients with hepatocellular carcinoma after cell-based immunotherapy. J Transl Med 2017;15:64.
107. Seok JY, Na DC, Woo HG, Roncalli M, Kwon SM, et al. A fibrous stromal component in hepatocellular carcinoma reveals a cholangiocarcinoma-like gene expression trait and epithelial-mesenchymal transition. Hepatology 2012;55:1776-86.
108. Honeyman JN, Simon EP, Robine N, Chiaroni-Clarke R, Darcy DG, et al. Detection of a recurrent DNAJB1-PRKACA chimeric transcript in fibrolamellar hepatocellular carcinoma. Science 2014;343:1010-4.
109. Riggle KM, Turnham R, Scott JD, Yeung RS, Riehle KJ. Fibrolamellar hepatocellular carcinoma: mechanistic distinction from adult hepatocellular carcinoma. Pediatr Blood Cancer 2016;63:1163-7.
110. Kastenhuber ER, Lalazar G, Houlihan SL, Tschaharganeh DF, Baslan T, et al. DNAJB1-PRKACA fusion kinase interacts with beta-catenin and the liver regenerative response to drive fibrolamellar hepatocellular carcinoma. Proc Natl Acad Sci U S A 2017;114:13076-84.
111. Wilkens L, Bredt M, Flemming P, Kubicka S, Klempnauer J, et al. Cytogenetic aberrations in primary and recurrent fibrolamellar hepatocellular carcinoma detected by comparative genomic hybridization. Am J Clin Pathol 2000;114:867-74.
112. Joseph NM, Tsokos CG, Umetsu SE, Shain AH, Kelley RK, et al. Genomic profiling of combined hepatocellular-cholangiocarcinoma reveals similar genetics to hepatocellular carcinoma. J Pathol 2019;248:164-78.
113. Seehawer M, Heinzmann F, D’Artista L, Harbig J, Roux PF, et al. Necroptosis microenvironment directs lineage commitment in liver cancer. Nature 2018;562:69-75.
114. Cancer Genome Atlas Research Network. Comprehensive and inte-grative genomic characterization of hepatocellular carcinoma. Cell 2017;169:1327-41.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Rastogi A. Pathomolecular characterization of HCC in non-cirrhotic livers. Hepatoma Res 2020;6:47. http://dx.doi.org/10.20517/2394-5079.2020.35
AMA Style
Rastogi A. Pathomolecular characterization of HCC in non-cirrhotic livers. Hepatoma Research. 2020; 6: 47. http://dx.doi.org/10.20517/2394-5079.2020.35
Chicago/Turabian Style
Rastogi, Archana. 2020. "Pathomolecular characterization of HCC in non-cirrhotic livers" Hepatoma Research. 6: 47. http://dx.doi.org/10.20517/2394-5079.2020.35
ACS Style
Rastogi, A. Pathomolecular characterization of HCC in non-cirrhotic livers. Hepatoma. Res. 2020, 6, 47. http://dx.doi.org/10.20517/2394-5079.2020.35
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 11 clicks
Cite This Article 11 clicks










Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.