Management of concomitant hepatocellular carcinoma and chronic hepatitis C: a review
Abstract
Our comprehensive review focuses on the treatment of hepatitis C virus in the context of hepatocellular carcinoma and vice versa, highlighting the ongoing complexity of this clinical scenario. There remain multiple unanswered questions when considering the management of these complex patients and, with a rapidly-changing treatment landscape for both chronic hepatitis C and hepatocellular carcinoma, these questions are only going to grow. Treatment timing, interactions and the impact of one disease condition on the other are vitally important, though guidance generally remains non-specific, suggesting that we make these decisions on a case-by-case basis. We focus on the current evidence for managing these cases, depending on disease stage and treatment type.
Keywords
Background
Hepatitis C virus (HCV) accounts for a third of all hepatocellular carcinoma (HCC) cases worldwide, with a 1%-8% annual risk of HCC development in cirrhotic HCV-infected patients[1-4]. The presence of cirrhosis greatly increases the risk of HCC development in HCV-positive patients, with the prominent pro-fibrotic effect of the virus undoubtedly playing a role[5]. Involvement of a direct mutagenic mechanism in addition to this is purely speculative at this stage, though animal and human models have demonstrated a potentially increased risk of HCC development in the non-cirrhotic HCV-positive patient[6-8]. Though HCV is a RNA-virus that cannot integrate into the host genome, it produces gene products that have been shown to have mutagenic effects in ex vivo human models, with further work in human models required to establish how this translates to in vivo processes[9,10].
Following HCV clearance, patients see a reduction in liver-related morbidity and death[11,12]. This has also been shown in HCV-cirrhotic patients with successfully treated HCC, in which hepatic decompensation has been found to be the major driver of death, highlighting the importance of preserving liver function in this group[13]. Understanding how HCV clearance might impact upon the pro-carcinogenic environment remains uncertain, though this will likely become clearer as our understanding of the post-SVR liver progresses.
The timing and duration of HCV treatment in patients with HCC is becoming increasingly important, though guidance generally remains that we should make these decisions on a case-by-case basis. Another consideration is the interaction between HCC and HCV treatments, particularly with the swelling tide of HCC treatments waiting to break.
HCV treatment regimens
HCV evokes a strong T cell-mediated reaction in the acute phase that successfully clears the virus in 30% of patients. In the remaining 70% of patients, multiple viral escape mechanisms - including inactivation of pathways that induce interferon - overwhelm the immune system, resulting in chronic infection[14]. Endogenous interferons are part of our natural arsenal against viruses, which explains the previous successes of exogenous interferon (IFN) in the treatment of HCV. Prior to 2011, prolonged courses of IFN were the mainstay of treatment outside of clinical trials for those infected with HCV, with or without concomitant ribavirin, with success rates ranging between 5%-50% depending on duration of therapy, stage of liver disease and genotype[15-17]. The exact mechanism by which ribavirin targets HCV is not completely understood, but is thought to have an effect on viral replication[18]. The addition of ribavirin improved outcomes but these regimens were poorly tolerated by many and improved alternatives were desperately sought.
The management of HCV has transformed over the past decade, with sustained virologic response (SVR) rates in excess of 95% following treatment with newer directly-acting antiviral agents (DAAs)[18]. Mechanistically, DAAs inhibit viral replication by inhibiting certain non-structural viral proteins, ultimately resulting in viral clearance[19]. DAA use has become more widespread and, with that, our understanding of their interaction with other treatments will improve.
Prior to the use of DAAs, IFN-based regimens were used in certain subgroups of patients, with significant histopathological improvements seen following successful treatment. It is more difficult to assess post-SVR histopathological changes as we are no longer required to perform pre-treatment biopsies as we were in the IFN-era. However, when assessing histopathology within 2 years of treatment, though there is suggestion of fibrosis regression, persistent inflammatory activity has been observed despite the absence of the virus[20].
Interferon-based therapies and HCC
Historical treatment with IFN-based therapies targeted patients with little or no fibrosis; a low-risk group in terms of HCC development[21]. In patients with advanced fibrosis or cirrhosis that were treated with maintenance pegylated interferon (PEG-IFN) in the HALT-C trial, it was noted that maintenance therapy did not reduce the risk of HCC development[22,23], though this and other studies have shown that reduction of HCV RNA correlated to a reduction in HCC risk, which reduced further still in cases of HCV eradication[23-26]. Further to this, IFN-based treatment may decrease the HCC recurrence rate in successfully treated HCC patients following curative therapy[17]. A speculative link has been drawn between the inhibitory effect of IFN on HCC proliferation, which may have an additional impact on HCC outcomes to the antiviral effect of IFN[17,27].
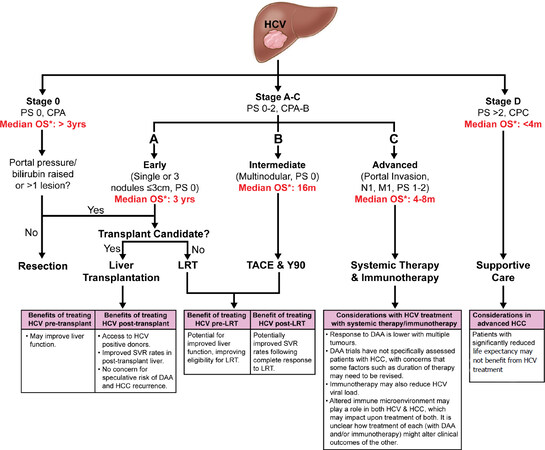
Current HCC treatment guidance is widely dictated by the Barcelona Clinic Liver Cancer (BCLC) criteria, which stratifies liver cancer cases into stages based on tumour burden, liver disease and performance status, allocating treatments accordingly[28]. Curative treatments include resection, locoregional therapy (LRT) or liver transplantation for those that fall within Milan criteria. Outside of this, palliative LRTs, targeted systemic therapies or immunotherapy are the recommendation in advanced HCC.
Some work has been done to assess the role of IFN as an adjuvant agent in post-resection cases, with promising early results in terms of mortality[29-32]. In the DAA era, the use of IFN-based regimens has declined drastically and so, even in the absence of evidence for a similar role for DAAs, using IFN in this context is unlikely to be recommended. Some debate continues over timing of HCV treatment in this subset of patients; these medications are in their infancy and many questions remain that may take time to address.
DAAs and HCC
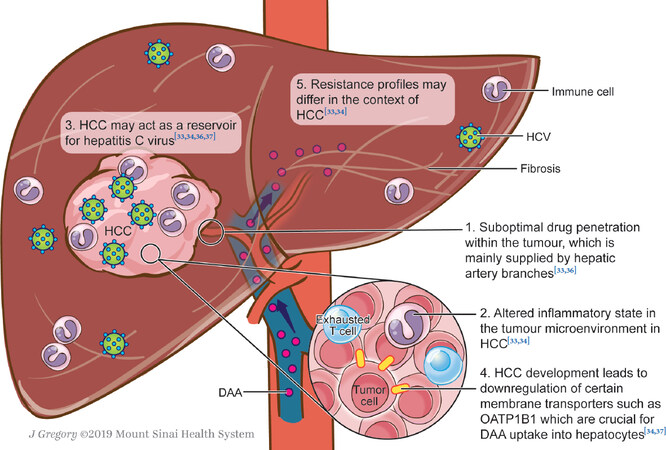
DAAs have revolutionised HCV treatment with SVR rates exceeding 95%. In the presence of HCC, SVR rates are lower at 60%-90%[33], the reasons for which are current sources of speculation [Figure 1][33-35]. Certainly, the tumour microenvironment expertly creates multiple mechanisms of immune escape in order to survive and so it stands to reason that HCV-infected cells within the tumour may evade antiviral treatment in the same vein. In addition to this, it has been proposed that penetration of DAAs to the HCV-infected HCC tissue is suboptimal, not only due to altered architecture but also as tumour blood supply is from the hepatic arterial branches as opposed to the portal venous system[35][Figure 1]. As original trials for newer DAAs often exclude patients with HCC from their eligibility criteria, data on this cohort is limited. Subsequent data has shown a decrease in SVR rates, though how this might shape our treatment regimens - be it duration or drug combination - is not yet clear.
Some of the discordance between studies may be due to the fact that some of the studies that have shown association between active HCC and lower SVRs included DAA regimens with sub-optimal combinations (e.g., SOF/RBV). It will be important to assess SVR rates in this population with the new generation of DAAs.
DAA regimen adaptations for HCV in the context of HCC
Recent data on efficacy of DAAs in patients that have concurrent HCC suggests that SVR rates are lower than those in the absence of HCC[35-40]. This includes both patients that respond and then relapse, as well as primary non-responders. It is, therefore, unclear whether these lower SVR rates are due to inadequate duration of therapy, treatment resistance or a combination thereof [Figure 1]. With this in mind, further trials are required to guide our treatment approach in this cohort of patients, be it prolonged courses of DAA therapy or treatment combinations.
HCC post-DAA treatment
Some initial concerns regarding allegedly high rates of HCC after DAA-induced SVR compared with IFN-induced SVR have caused controversy[41]. This could be explained by altered immune surveillance in the post-DAA liver environment, which may alter T cell responses and therefore have an impact on cancer evasion from the host immune system[41,42]. On reassessment of the data, the apparent increase in HCC seen in the post-DAA population is at least in part thought attributable to bias within the patient cohorts[33,35,43]. A recent systematic review by Waziry et al.[44] was unable to find evidence that DAA therapy is associated with subsequent HCC development when compared with IFN therapy, though the reviewed studies were small, observational and sometimes lacking in useful clinical detail with significant inter-trial heterogeneity also noted. Furthermore, when assessing overall incidence of HCC rather than recurrence alone, the risk of developing HCC reduces by 71% in DAA-induced SVR compared with treatment failure[45].
We eagerly await the outcome of ongoing clinical trials that are studying this potential association, which aim to assess recurrence rate of HCC as well as mapping the behaviour of HCC during and after DAA treatment of HCV[46-50]. Further research and debate are ongoing and in depth discussion on this topic is beyond the scope of this review.
Hepatitis C DAA treatment considerations based on HCC therapy
DAAs and locoregional therapies
LRT is used with curative intention in the early stages of HCC (for example microwave ablation, radiofrequency ablation, ethanol injection) and as palliative interventions in the intermediate/advanced stages [for example chemoembolization, selective internal radiation therapy (SIRT), stereotactic body radiotherapy (SBRT)][51]. Multiple factors should be considered when deciding whether or not to prescribe DAAs in patients with HCC amenable to LRTs, and when.
Firstly, because LRTs are recommended only in patients with well-compensated liver disease[51], achieving SVR may significantly improve a given patient’s clinical liver function, making them eligible for a therapeutic procedure. A recent multicentre study showed that 24% of the 122 patients with decompensated cirrhosis could be delisted due to improvement after HCV eradication[52]. Three patients with HCC that were originally listed for liver transplantation improved such that they were able to undergo resection or SIRT after achieving SVR.
Secondly, with some studies showing decreased SVR rates in the presence of active HCC, consideration should be given to treating the HCC with LRTs prior to DAA initiation. One retrospective study demonstrated that failure to achieve SVR rates was higher in patients with active HCC when compared to patients with inactive or resected HCCs or in patients with no HCC[37]. Similarly, a large prospective national multicentre study showed that successfully treated HCCs (resection, ablation, or chemoembolization) do not influence subsequent SVR rates with DAA therapy. DAA therapy was given at least 6 months after successful treatment (i.e., complete response) of the HCC[53]. As radiological response following LRT does not always accurately predict pathologic necrosis - and in some cases this may be overestimated - this underscores the importance of this time window before pursuing HCV treatment. Conversely, preliminary data from the HCV-TARGET study comparing SVR rates of cirrhotic patients with HCC with those of patients without HCC, again showed significantly inferior SVR rates in the former, but also showed no difference in SVR rates between those patients with active HCC versus those with complete response to LRTs[38]. In another study of 62 patients, who were started on DAAs just after radiological documentation of complete response to treatment (mainly radiofrequency ablation, TACE, microwave ablation, and percutaneous ethanol injection), the SVR rate was only 64.5%[54]. Importantly, 42% had HCC recurrence, and in most cases within the following 6 months after initiation of DAAs, suggesting the presence of residual HCC despite documentation of radiological response. Hence, in this case the presence of viable HCC could have contributed to the low SVRs.
Finally, in cases where LRTs fail to achieve complete necrosis of the tumour, DAA metabolite distribution to viable HCC areas may be compromised through multiple mechanisms. Impaired blood supply will impair penetration into the HCV-infected tumour tissue, particularly with procedures that include vascular embolization such as TACE. Altered tissue architecture may also have an impact on tissue penetration, as LRTs induce fibrosis, which seems to be particularly accentuated with SIRT[37,55]. There may, therefore, be a role for re-treatment of HCCs in an attempt to achieve a complete response before initiating DAAs. In reality, however, many physicians commence HCV treatment prior to HCC treatment, with an unmet need in research into this area.
In summary, the evidence is variable and further trials in this area may help to confirm the best approach where an HCC in chronic HCV cases is amenable to LRT. Until more evidence is available, it may be prudent to treat an active HCC with LRTs and achieve a complete and sustained response before initiating DAAs, in order to improve SVR rate. Where a patient is anatomically a candidate for LRT but is not suitable due to poor liver function, one might consider treating the HCV in order to improve the patient’s clinical condition.
DAAs and liver transplantation
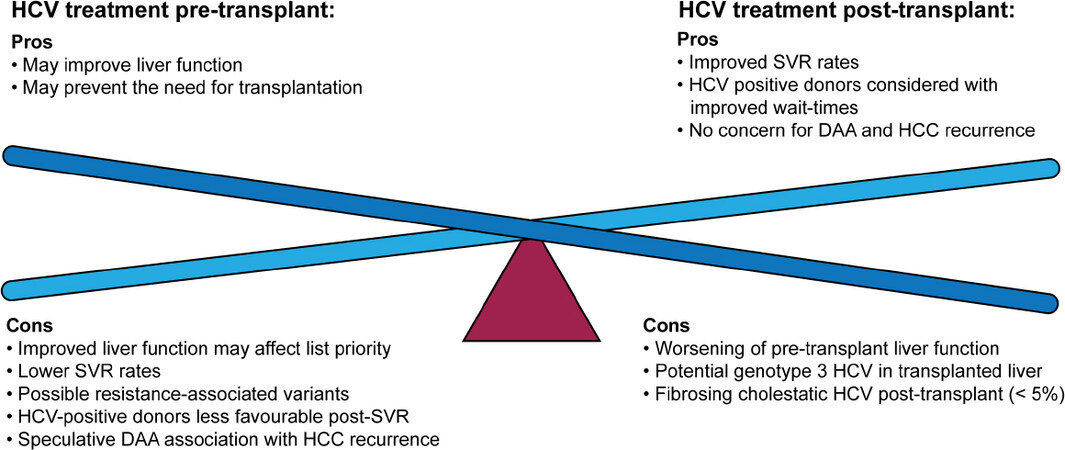
There is also much speculation regarding timing of HCV treatment in patients with HCC, particularly in those for which liver transplantation is being considered[35][Table 1]. Where no guidelines exist that prevent the transplantation of HCV-viraemic organs into HCV-negative recipients, limited data is available into this practice and so it is not generally accepted. In liver transplantation specifically, outcomes of HCV-viraemic organs into HCV-positive recipients do not appear to negatively impact patient or graft survival, therefore many centres have adopted this practice[35]. Treatment of HCV prior to transplantation may therefore pose a disadvantage in terms of wait-list time, thus allowing potential for tumour progression[35]. This is particularly relevant in locations with high volumes of HCV-positive liver donors[35][Figure 2].
| First author | Study characteristics | Patient characteristics | DAA therapy | Outcome | |||
|---|---|---|---|---|---|---|---|
| Genotype | Cirrhosis | HCC vs HCV Treatment timing | Treatment Regimen | Treatment Duration | |||
| Saberi et al.[35],2017 | Retrospective study assessing pre transplant HCV HCC, n = 21 with both HCC & HCV | 71% genotype 1 | All cirrhotic, 52% CPA | Pre-transplant patients, some had received HCC treatment prior to HCV treatment though exact timings unclear | Multiple regimens used | 12-32 weeks | 33.3% HCV relapse prior to transplant |
| Pascasio et al.[52],2017 | Retrospective study assessing delisting of patients post DAA therapy, n = 116 with HCC & HCV (also n = 122 HCV without HCC) | 73% genotype 1 | All cirrhotic, 72% decompensated with or without HCC, 28% compensated with HCC | Pre-transplant patients at the start of HCV therapy, no bridging HCC therapy given prior to HCV treatment | Variable | Not specified | 86% SVR across all groups 92% SVR in compensated cirrhosis with HCC vs 83% in decompensated cirrhosis with or without HCC |
| Curry et al.[78], 2015 | Prospective study assessing efficacy of Sof/Riba in pre-transplant HCV HCC, n = 61 with HCC & HCV (also n = 122 HCV without HCC) | 73% genotype 1 | All CPA cirrhotic | Pre-transplant patients, no comment on prior or subsequent bridging HCC therapy | Sofosbuvir + Ribavirin | Up to 24 weeks or liver transplant, whichever came first. Latterly protocol changed from 24 to 48 weeks due to observed relapses. | 70% SVR12 achieved Of all 61 patients, 49% maintained post-transplant SVR 93% of the 46 patients that underwent liver transplant had HCV RNA < 25IU/mL at the time of transplant, but 30% of these relapsed. |
| Prenner et al.[37], 2017 | Retrospective study comparing SVR in HCV with vs without HCC, n = 135 with HCC & HCV (also n = 284 HCV without HCC) | 85% genotype 1 | All cirrhotic, 81% CPA | Mixture of treated and untreated HCC, with 43% of patients with active tumour at the time of DAA treatment, exact timings unclear | Multiple regimens used | Not specified; the authors classified regimens as adequate or inadequate | 15% treatment failure, with 93% of these cases having active tumour 54% SVR in cases with active tumour, 97% SVR in treated HCC |
| Chang et al.[79],2017 | Retrospective study assessing SVR and AEs in SOF-based DAA treatment of HCV in Asian Americans, n = 17 with HCC & HCV (also n = 93 HCV without HCC) | 64.5% genotype 1 | 50% cirrhotic | HCC treatment given either prior or subsequent to HCV treatment - not specified how many in each category | Sofosbuvir-based regimens | 8-24 weeks | SVR12 93% overall, but 82% in patients with concomitant HCC - this did not reach statistical significance. |
| Beste et al.[36], 2017 | Retrospective study assessing SVR in HCV HCC patients, n = 624 with HCC & HCV (also n = 16,863 HCV without HCC) | 73% genotype 1 | 85% cirrhotic | Post-transplant (n = 142) or post HCC therapy (n = 482), exact timings unclear | Multiple regimens used | 8-24 weeks | 94% SVR post-transplant, 74% SVR pre-transplant 91.9% SVR in non-HCC patient Highest SVR in genotype 1 patients, lowest in genotype 3 patients |
| Radhakrishnan et al.[38], 2017 | Retrospective study comparing SVR in cirrhotic HCV with vs without HCC, n = 133 with HCC & HCV (also n = 891HCV without HCC) | 78% genotype 1 | All cirrhotic | Assessed complete response vs partial or non-response to HCC treatment, up to 6 months prior to DAAs | Not specified | Variable | Non-significant trend toward improved SVR rates in treated HCC vs active HCC 83.1% SVR in HCC (vs 90.3% non-HCC) |
| Revuelta-Herrero et al.[69], 2018 | Prospective study assessing patients on a specific DAA regimen in combination with sorafenib, n = 3 with HCC & HCV | 100% genotype 1b | Unclear, at least 1 non-cirrhotic patient | Patients on sorafenib, all had previous HCC treatments in the years preceding DAA treatment including LRT and resection | Ombitasvir/ paritaprevir/ ritonavir and dasabuvir | 12 weeks | 100% SVR24 Adverse effects of sorafenib reported after DAA Patients all discontinued sorafenib with no recurrence at average 16.6m follow up |
In addition to the potential impact on HCC outcomes, an impact on HCV outcomes has been demonstrated in patients receiving DAA therapy pre- vs. post-transplant [Figure 2]. One recent large retrospective study demonstrated a difference in SVR rates between pre- and post-transplant treated patients, with the latter seeing improved clearance[34]. In terms of liver transplantation, there are certainly advantages and disadvantages of treating HCV prior to HCC [Figure 2], which should be considered on an individual basis.
The evidence to date offers a compelling argument for considering treatment of the HCC before treatment of HCV. These findings require further data in order to make concrete recommendations in terms of HCV treatment timing, and each case should still be reviewed on an individual basis.
DAAs and systemic therapies
There is a paucity of data regarding concomitant use of DAAs and the systemic agents used in advanced HCC. Sorafenib was the breakthrough targeted therapy first used in the treatment of advanced HCC and although its effect on median overall survival does not extend life expectancy beyond one year, it is yet to be superseded a decade after the seminal SHARP trial[56-58]. Sorafenib is a multi-kinase inhibitor with a potent inhibitory effect on c-Raf[59]. NS5a - a non-structural protein produced by HCV that is integral in viral replication - has been shown to bind to cRaf[60] and, studied in vitro, inhibition of cRaf by sorafenib effectively blocks HCV replication[59]. Multiple other mechanisms of sorafenib inhibition of HCV replication, such as alteration of the viral entry step, the production of viral particles and Claudin-1 downregulation, have been demonstrated[61-63]. Though the antiviral effect of sorafenib in human studies to date have been disappointing, this association has not yet been excluded[56,64,65]. Interestingly, Sorafenib has been shown to provide a greater benefit in overall survival in HCV patients when compared to other aetiologies of liver disease[66]. Newer drugs including lenvatinib, a multi-kinase inhibitor, in the front-line and regorafinib, cabozantinib (both multi-kinase inhibitors) and ramucirumab (an antiVEGFR mAb) in the second-line have been incorporated into new guidelines and are now increasing in use[67], but their potential interactions with DAA regimens have been explored still less.
As many trials for the new DAA regimens excluded patients with HCC, there is a little data on the interaction between targeted therapies and DAAs[68]. One small case series noted that there were no deleterious effects in combining ombitasvir/paritaprevir/ritonavir and dasabuvir, either in terms of anti-neoplastic effect or SVR rate[69], but more studies are required to assess these interactions.
DAAs and immunotherapy
Though markedly different pathological processes are involved in chronic hepatitis C and the development of HCC, there are some similarities between the two when considering the role of the immune system, with T cell exhaustion implicated in both[70]. CD8+ T cells are integral in targeting and destroying both tumour cells and cells infected with HCV. T cell exhaustion exists to protect from tissue damage due to persistent and overzealous immunological response to antigens and is driven by upregulation of negative co-stimulatory pathways. With these pathways in action, key T cell effector processes are disrupted, they become tolerant of antigenic stimuli and ultimately apoptose[70]. T cell exhaustion is particularly efficient in T cells that are activated in the liver, causing the immunotolerant state required in an organ that encounters many antigenic threats. Chronic inflammation and development of cancer have both been shown to be associated with T cell exhaustion. These negative co-stimulatory pathways are multiple, including PD1, CTLA4, Tim3 and LAG3 - targets that are under scrutiny for potential new pharmacological options in the treatment of HCC. Inhibition of these targets aims to unlock the potential of these T cells to reinvigorate the immune cells. Though PD1 inhibitors in particular are now commonly associated with the treatment of HCC, they have also been trialled in the treatment of HCV in the past, with some success[71]. More studies assessing the impact of anti-PD1 immunotherapy on active HCV infection are required to fully understand its role in viral response. Multiple ongoing clinical trials using anti-PD1 antibodies are currently allowing patients with untreated HCV to enrol, which may go some way to answering this question. Nivolumab, a human monoclonal IgG4 antibody against PD1, is showing promise as a treatment in advanced HCC[72]. It has also been trialled in chronic HCV infection, showing a persistent suppression of HCV RNA in a subgroup of patients[71]. Pembrolizumab, another anti-PD1 monoclonal antibody, has also recently been granted accelerated FDA approval for the treatment of advanced HCC, with promising results in the Keynote-224 study[73].
In addition to the impact of immunotherapy treatment of HCC on DAA treatment of HCV, we must also consider the opposite, as there is growing research showing the impact of DAAs on immune cells both within the liver and peripherally[74,75]. From this, we might extrapolate that this in turn may impact on the immune surveillance in this population, thus may affect HCC treatment outcomes. Currently the clinical impact of the immune environment and altered immune surveillance is not clear, but insight into these processes in the post-DAA liver is improving, which may be crucial in how we shape our treatment[74].
Increased research into the immune environment in the post-DAA treated liver is vital to understand the potential impact viral clearance may have on HCC treatment response and vice versa. The effect of HCC-targeted immunotherapy on DAA treatment of HCV is not well-studied, but it would be interesting to see the impact on HCV treatment, and vice versa, be it synergistic, deleterious or non-existent.
Timing of HCV treatment in advanced HCC
As previously discussed, timing of HCV treatment when considering curative options has been the source of some controversy, as the decreased efficacy of DAAs seen in the context of HCC offers a compelling argument for treating HCV after treatment of the tumour. In advanced HCC, the chance of cure is marginal and so delaying treatment of HCV for this reason is not practical. In patients where life-expectancy is significantly limited, the risk vs benefit of treating HCV at all must be considered. AASLD guidance recommends that patients with limited life expectancy within 12 months are unlikely to benefit from HCV eradication and therefore palliative measures should take precedence in this setting[76]. This will include patients with decompensated liver disease and advanced hepatocellular carcinoma. For those with a better prognosis, HCV eradication prior to sorafenib treatment of HCC may prolong post-progression survival and improve overall survival[77]. Decisions regarding treatment timings should be considered on an individual basis, taking into consideration the advantages and disadvantages of treatment order [Figure 3].
Figure 3. Modified from BCLC criteria for treatment of HCC[28], with considerations for each treatment option outlined beneath
Conclusion
In summary, there is a paucity of clinical data surrounding the co-management of patients with both active HCV infection and HCC. The guidance for this challenging clinical scenario is to treat patients on a case-by-case basis, with conflicting evidence as to which condition to treat first. In cases where liver transplantation may be an option, there are advantages and disadvantages for treating one condition before the other, which should be considered on a case-by-case basis to enhance patient outcomes depending on individual clinical factors. Treatment of HCC through LRTs prior to HCV treatment may confer individual benefit in terms of SVR rates, but viral clearance conversely may improve liver function to allow more advanced treatment options. Again, assessment on an individual patient basis may be the most appropriate advice in the absence of robust clinical trials exploring this. For more advanced cases that are only eligible for systemic therapies, there are interesting parallels in the underlying immune processes that may have a significant impact on our management, though further trials into this are required before robust recommendations can be made. With newer treatments rapidly emerging for both conditions, this is an exciting area of hepatology that no doubt will be at the forefront of research in the coming decade.
Key points
There is a paucity of clinical data surrounding the co-management of patients with both active HCV infection and HCC.
As many trials for the new DAA regimens excluded patients with HCC, there is a little data on the interaction between targeted HCC therapies and DAAs, though there are interesting parallels in the underlying immune processes for HCV and HCC.
For patients with potentially curable HCC, deciding which pathology to treat first is complex and the data is conflicting. Improving liver function following SVR could enable the patient to undergo more favourable therapeutic HCC procedures. However SVR rates are significantly lower in patients with active HCC. In the absence of formal guidance and with conflicting evidence, we suggest this should be managed on an individual patient basis.
For patients awaiting liver transplantation, the ability to transplant an HCV-viraemic organ may improve waitlist times and thus guide decisions, but concrete data is lacking and so in the absence of formal guidance, we suggest this should be managed on a case-by-casebasis.
Declarations
Authors’ contributionsStudy concept and design, literature search, drafting of the manuscript: Harrod E, Moctezuma-Velazquez C, Gurakar A, Ala A, Dieterich D, Saberi B
Critical revision of the manuscript for important intellectual content: Gurakar A, Ala A, Dieterich D, Saberi B
Study supervision: Saberi B
Availability of data and materialsNot applicable.
Financial support and sponsorshipCMV is a recipient of a grant from Gilead, Canada.
Conflicts of interestDieterich D: Gilead, Merck, AbbVie.
Harrod E, Moctezuma-Velazquez C, Gurakar A, Ala A, Saberi B: no conflicts of interest
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
CopyrightThe Author(s) 2019.
REFERENCES
1. Alazawi W, Cunningham M, Dearden J, Foster GR. Systematic review: outcome of compensated cirrhosis due to chronic hepatitis C infection. Aliment Pharmacol Ther 2010;32:344-55.
2. Fattovich G, Pantalena M, Zagni I, Realdi G, Schalm SW, et al. Effect of hepatitis B and C virus infections on the natural history of compensated cirrhosis: a cohort study of 297 patients. Am J Gastroenterol 2002;97:2886-95.
3. Kobayashi M, Ikeda K, Hosaka T, Sezaki H, Someya T, et al. Natural history of compensated cirrhosis in the Child-Pugh class A compared between 490 patients with hepatitis C and 167 with B virus infections. J Med Virol 2006;78:459-65.
4. Toshikuni N, Izumi A, Nishino K, Inada N, Sakanoue R, et al. Comparison of outcomes between patients with alcoholic cirrhosis and those with hepatitis C virus-related cirrhosis. J Gastroenterol Hepatol 2009;24:1276-83.
5. Wirth TC, Manns MP. The impact of the revolution in hepatitis C treatment on hepatocellular carcinoma. Ann Oncol 2016;27:1467-74.
6. Gaddikeri S, McNeeley MF, Wang CL, Bhargava P, Dighe MK, et al. Hepatocellular Carcinoma in the Noncirrhotic Liver. Am J Roentgenol 2014;203:34-47.
7. El-Refaie A, Savage K, Bhattacharya S, Khakoo S, Harrison TJ, et al. HCV-associated hepatocellular carcinoma without cirrhosis. J Hepatol 1996;24:277-85.
8. Yeh M, Daniel H, Torbenson M. Hepatitis c associated hepatocellular carcinomas in non- cirrhotic livers. Mod Pathol 2010;23:276-83.
9. Ray RB, Meyer K, Ray R. Hepatitic decompensation is the major driver of death in HCV-infected cirrhotic patients with successfully treated early hepatocellular carcinoma. Virology 2000;271:197-204.
11. McCombs J, Matsuda T, Tonnu-Mihara I, Saab S, Hines P, et al. The risk of long-term morbidity and mortality in patients with chronic hepatitis c : results from an analysis of data from a department of veterans affairs clinical registry. JAMA Intern Med 2014;174:204-12.
12. van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, et al. Association between sustained virological response and all cause mortality among patients with chronic hepatitis c and advanced hepatic fibrosis. J Am Med Assoc 2012;308:2584-93.
13. Cabibbo G, Petta S, Barbara M, Attardo S, Bucci L, et al. Hepatic decompensation is the major driver of death in hcv-infected cirrhotic patients with successfully treated early hepatocellular carcinoma. J Hepatol 2017;67:65-71.
15. McHutchison JG, et al. Interferon Alfa-2b Alone or in Combination with Ribavirin as Initial Treatment for Chronic Hepatitis C. N Engl J Med 1998;339:1485-92.
16. Manns M P, Wedemeyer H, Cornberg M. Treating viral hepatitis C: efficacy, side effects, and complications. Gut 2006;55:1350-9.
17. Hsu CS, Chao YC, Lin HH, Chen DS, Kao JH. Systematic review: impact of interferon-based therapy on hcv-related hepatocellular carcinoma. Sci Rep 2015;5:1-9.
18. Kohli A, Shaffer A, Sherman A, Kottilil S. Treatment of hepatitis C: a systematic review. J Am Med Assoc 2014;312:631-40.
19. Geddawy A, Ibrahim YF, Elbahie NM, Ibrahim MA. Direct acting anti-hepatitis C virus drugs: Clinical pharmacology and future direction. J Transl Intern Med 2017;5:8-17.
20. Putra J, Schiano T, Fiel MI. Histological assessment of the liver explant in transplanted hcv-patients achieving sustained virologic response with direct-acting antiviral agents. Histopathology 2018;72:990-6.
21. Ahmad J, Eng F, Branch AD. HCV and HCC : clinical update and a review of hcc-associated viral mutations in the core gene. Semin. Liver Dis 2011;31:347-55.
22. Lok AS, Seeff LB, Morgan TR, di Bisceglie AM, Sterling RK, et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis c-related advanced liver disease. Gastroenterology 2009;136:138-48.
23. Lok AS, Everhart JE, Wright EC, Di Bisceglie AM, Kim HYt, et al. Maintenance peginterferon therapy and other factors associated with hepatocellular carcinoma in patients with advanced hepatitis c. Gastroenterology 2011;140:840-9.
24. Shiffman ML, Morishima C, Dienstag JL, Lindsay KL, Hoefs JC, et al. Effect of HCV RNA suppression during peginterferon alfa-2a maintenance therapy on clinical outcomes in the HALT-C trial. Gastroenterology 2009;137:1986-94.
25. Morgan TR, Ghany MG, Kim HY, Snow KK, Shiffman ML, et al. Outcome of sustained virological responders with histologically advanced chronic hepatitis c. Hepatology 2010;52:833-44.
26. Cardoso AC, Moucari R, Figueiredo-Mendes C, Ripault MP, Giuily N, et al. Impact of peginterferon and ribavirin therapy on hepatocellular carcinoma: incidence and survival in hepatitis C patients with advanced fibrosis. J Hepatol 2010;52:652-7.
27. Kusano H, Akiba J, Ogasawara S, Sanada S, Yasumoto M, et al. Pegylated interferon-α2a inhibits proliferation of human liver cancer cells in vitro and in vivo. PLoS One 2013;8:1-10.
28. Llovet JM, Fuster J, Bruix J. The barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transplant 2004;10:115-20.
29. Zhang YJ, Liu YT, Yu XS. Effect of interferon therapy on outcomes after hepatic resection for hepatitis C virus-related hepatocellular carcinoma: a meta-analysis. Int J Clin Exp Med 2016;9:1675-83.
30. Tanimoto Y, Tashiro H, Aikata H, Amano H, Oshita A, et al. Impact of pegylated interferon therapy on outcomes of patients with hepatitis c virus-related hepatocellular carcinoma after curative hepatic resection. Ann Surg Oncol 2012;19:418-25.
31. Singal AK, Freeman DH Jr, Anand BS. Meta-analysis: interferon improves outcomes following ablation or resection of hepatocellular carcinoma. Aliment Pharmacol Ther 2010;32:851-8.
32. Miao RY, Zhao HT, Yang HY, Mao YL, Lu X, et al. Postoperative adjuvant antiviral therapy for hepatitis B/C virus-related hepatocellular carcinoma: a meta-analysis. World J. Gastroenterol 2010;16:2931-42.
33. Kushner T, Dieterich D, Saberi B. Direct-acting antiviral treatment for patients with hepatocellular carcinoma. Curr Opin Gastroenterol 2018;34:1-8.
34. Prenner SB, Kulik L. Hepatocellular carcinoma in the wait-listed patient with hepatitis C virus. Curr Opin Organ Transplant 2018;23:237-43.
35. Saberi B, Dadabhai AS, Durand CM, Philosophe B, Cameron AM, et al. Challenges in treatment of hepatitis c among patients with hepatocellular carcinoma. Hepatology 2017;66:661-3.
36. Beste LA, Green PK, Berry K, Kogut MJ, Allison SK, et al. Effectiveness of hepatitis c antiviral treatment in a USA cohort of veteran patients with hepatocellular carcinoma. J Hepatol 2017;67:32-9.
37. Prenner SB, VanWagner LB, Flamm SL, Salem R, Lewandowski RJ, et al. Hepatocellular carcinoma decreases the chance of successful hepatitis C virus therapy with direct-acting antivirals. J Hepatol 2017;66:1173-81.
38. Radhakrishnan K, Di Bisceglie Adrian M., Reddy K. Rajender, Lim Joseph K., Levitsky Josh, et al. Impact of hepatocellular carcinoma (HCC) and tumor treatment on sustained virologic response (SVR) rates with direct-acting antiviral (DAA) therapy for hepatitis C: HCV-TARGET results. Hepatology 2017;66:755-6.
39. Soria A, Fabbiani M, Lapadula G, Gori A. Unexpected viral relapses in hepatitis C virus-infected patients diagnosed with hepatocellular carcinoma during treatment with direct-acting antivirals. Hepatology 2017;66:992-4.
40. Ji F, Yeo YH, Wei MT, Wei B, Dang S, et al. Hepatocellular carcinoma decreases the effectiveness of hepatitis C antiviral treatment: Do direct-acting antiviral regimens matter? Hepatology 2018;67:1180-2.
41. Reig M, Mariño Z, Perelló C, Iñarrairaegui M, Ribeiro A, et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol 2016;65:719-26.
42. Guarino M, Sessa A, Cossiga V, Morando F, Caporaso N, et al. Direct-acting antivirals and hepatocellular carcinoma in chronic hepatitis C: a few lights and many shadows. World J Gastroenterol 2018;24:2582-95.
43. El-Serag HB, Kanwal F, Richardson P, Kramer J. Risk of hepatocellular carcinoma after sustained virological response in veterans with hepatitis c virus infection. Hepatology 2016;64:130-7.
44. Waziry R, Hajarizadeh B, Grebely J, Amin J, Law M, et al. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: A systematic review, meta-analyses, and meta-regression. J Hepatol 2017;67:1204-12.
45. Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol 2018;68:25-32.
46. Recurrence Rate of Hepatocellular Carcinoma After Treatment of Chronic Hepatits C Patients With Direct Acting Antivirals: Randomized Controlled Phase 3 Trial. Available from: https://clinicaltrials.gov/ct2/show/NCT03551444 [Last accessed on 27 June 2019].
47. Incidence of de Novo Hepatocellular Carcinoma After Antiviral Agents for HCV. Available from: https://clinicaltrials.gov/ct2/show/NCT03551444 [Last accessed on 27 June 2019].
48. Long-term Outcomes of Chronic Hepatitis C Patients Post Sofosbuvir-based Treatment (LONGHEAD). Available from: https://clinicaltrials.gov/ct2/show/NCT03551444 [Last accessed on 27 June 2019].
49. Effect of DAAs on Behavior of HCC in HCV Patients. Available from: https://clinicaltrials.gov/ct2/show/NCT03551444 [Last accessed on 27 June 2019].
50. Direct Acting Antiviral-Post Authorization Safety Study. Available from: https://clinicaltrials.gov/ct2/show/NCT03551444 [Last accessed on 27 June 2019].
52. Pascasio JM, Vinaixa C, Ferrer MT, Colmenero J, Rubin A, et al. Clinical outcomes of patients undergoing antiviral therapy while awaiting liver transplantation. J Hepatol 2017;67:1168-76.
53. Persico M, Aglitti A, Aghemo A, Rendina M, Lleo A, et al. High efficacy of direct-acting anti-viral agents in hepatitis C virus-infected cirrhotic patients with successfully treated hepatocellular carcinoma. Aliment Pharmacol Ther 2018;47:1705-12.
54. Hassany M, Elsharkawy A, Maged A, Mehrez M, Asem N, et al. Hepatitis C virus treatment by direct-acting antivirals in successfully treated hepatocellular carcinoma and possible mutual impact. Eur J Gastroenterol Hepatol 2018;30:876-81.
55. Bas A, Samanci C, Gulsen F, Cantasdemir M, Kabasakal L, et al. Evaluation of liver stiffness after radioembolization by real-time shearwave TM elastography : preliminary study. Cardiovasc Intervent Radiol 2014;38:957-63.
56. LLovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90.
57. Llovet JM, Ducreux M, Lencioni R, Di Bisceglie AM, Dufour JF, et al. EASL - EORTC Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2012;56:908-43.
58. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358-80.
59. Himmelsbach K, Sauter D, Baumert TF, Ludwig L, Blum HE, et al. New aspects of an anti-tumour drug: Sorafenib efficiently inhibits HCV replication. Gut 2009;58:1644-53.
60. Bürckstümmer T, Kriegs M, Lupberger J, Pauli EK, Schmittel S, et al. Raf-1 kinase associates with Hepatitis C virus NS5A and regulates viral replication. FEBS Lett 2006;580:575-80.
61. Zona L, Lupberger J, Sidahmed-Adrar N, Thumann C, Harris HJ, et al. HRas signal transduction promotes hepatitis c virus cell entry by triggering assembly of the host tetraspanin receptor complex. Cell Host Microbe 2013;13:302-13.
62. Menzel N, Fischl W, Hueging K, Bankwitz D, Frentzen A, et al. MAP-Kinase Regulated Cytosolic Phospholipase A2 Activity Is Essential for Production of Infectious Hepatitis C Virus Particles. PLoS Pathog 2012;8:1-17.
63. Descamps V, Helle F, Louandre C, Martin E, Brochot E, et al. The kinase-inhibitor sorafenib inhibits multiple steps of the Hepatitis C Virus infectious cycle in vitro. Antiviral Res 2015;118:93-102.
64. Cabrera R, Limaye AR, Horne P, Mills R, Soldevila-Pico C, et al. The antiviral effect of sorafenib in hepatitis c-related hepatocellular carcinoma. Aliment Pharmacol Ther 2013;37:91-7.
65. Ji F, Li Z. Letter : the antiviral activity of sorafenib in patients with hepatitis C-related hepatocellular carcinoma. Aliment Pharmacol Ther 2013;37:372-3.
66. Bruix J, Cheng AL, Meinhardt G, Nakajima K, De Sanctis Y, et al. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: analysis of two phase III studies. J Hepatol 2017;67:999-1008.
67. Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol 2018;15:599-616.
68. Liverpool U. of. HEP Drug Interaction checker.
69. Revuelta-Herrero JL, Giménez-Manzorro A, Matilla-Peña A, Herranz-Alonso A, Sanjurjo-Sáez M. Concomitant use of sorafenib with ombitasvir/paritaprevir/ritonavir and dasabuvir: Effectiveness and safety in clinical practice. J Clin Pharm Ther 2018;43:906-9.
70. Moreno-Cubero E, Larrubia JR. Specific CD8+ T cell response immunotherapy for hepatocellular carcinoma and viral hepatitis. World J Gastroenterol 2016;22:6469-83.
71. Gardiner D, Lalezari J, Lawitz E, DiMicco M, Ghalib R, et al. A randomized, double-blind, placebo-controlled assessment of bms-936558, a fully human monoclonal antibody to programmed death-1 (PD-1), in patients with chronic hepatitis c virus infection. PLoS One 2013;8:1-11.
72. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492-502.
73. Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib ( KEYNOTE-224 ): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018;940-52.
74. Mazouz S, Boisvert M, Shoukry NH, Lamarre D. Reversing immune dysfunction and liver damage after direct-acting antiviral treatment for hepatitis C. Can Liver J 2018;1:78-105.
75. Meissner EG, Kohli A, Higgins J, Lee YJ, Prokunina O, et al. Rapid changes in peripheral lymphocyte concentrations during interferon-free treatment of chronic hepatitis c virus infection. Hepatol Commun 2017;1:586-94.
76. AASLD & IDSA. AASLD/IDSA HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. Clin Liver Dis (Hoboken) 2018;12:117.
77. Kawaoka T, Aikata H, Teraoka Y, Inagaki Y, Honda F, et al. Impact of hepatitis c virus eradication on the clinical outcome of patients with hepatitis c virus-related advanced hepatocellular carcinoma treated with sorafenib. Oncology 2017;92:335-46.
78. Curry MP, Forns X, Chung RT, Terrault NA, Brown R Jr, et al. Sofosbuvir and ribavirin prevent recurrence of hcv infection after liver transplantation: an open-label study. Gastroenterology 2015;148:100-7.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Harrod E, Moctezuma-Velazquez C, Gurakar A, Ala A, Dieterich D, Saberi B. Management of concomitant hepatocellular carcinoma and chronic hepatitis C: a review. Hepatoma Res 2019;5:28. http://dx.doi.org/10.20517/2394-5079.2019.15
AMA Style
Harrod E, Moctezuma-Velazquez C, Gurakar A, Ala A, Dieterich D, Saberi B. Management of concomitant hepatocellular carcinoma and chronic hepatitis C: a review. Hepatoma Research. 2019; 5: 28. http://dx.doi.org/10.20517/2394-5079.2019.15
Chicago/Turabian Style
Harrod, Elizabeth, Carlos Moctezuma-Velazquez, Ahmet Gurakar, Aftab Ala, Douglas Dieterich, Behnam Saberi. 2019. "Management of concomitant hepatocellular carcinoma and chronic hepatitis C: a review" Hepatoma Research. 5: 28. http://dx.doi.org/10.20517/2394-5079.2019.15
ACS Style
Harrod, E.; Moctezuma-Velazquez C.; Gurakar A.; Ala A.; Dieterich D.; Saberi B. Management of concomitant hepatocellular carcinoma and chronic hepatitis C: a review. Hepatoma. Res. 2019, 5, 28. http://dx.doi.org/10.20517/2394-5079.2019.15
About This Article
Copyright
Data & Comments
Data
 Cite This Article 39 clicks
Cite This Article 39 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.