Intrahepatic cholangiocarcinoma: review and update
Abstract
Cholangiocarcinoma (CCA) is a heterogeneous group of malignancies that could develop at any level from the biliary tree. CCA is currently classified into intrahepatic (iCCA), perihilar and distal on the basis of its anatomical location. Of note, these three CCA subtypes have common features but also important inter-tumor and intra-tumor differences that can affect the pathogenesis and outcome. A unique feature of iCCA is that it recognizes as origin tissues, the hepatic parenchyma or large intrahepatic and extrahepatic bile ducts, which are furnished by two distinct stem cell niches, the canals of Hering and the peribiliary glands, respectively. The complexity of iCCA pathogenesis highlights the need of a multidisciplinary, translational and systemic approach to this malignancy. This review will focus on the advances of iCCA epidemiology, histo-morphology, risk factors, molecular pathogenesis, revealing the existence of multiple subsets of iCCA.
Keywords
Introduction
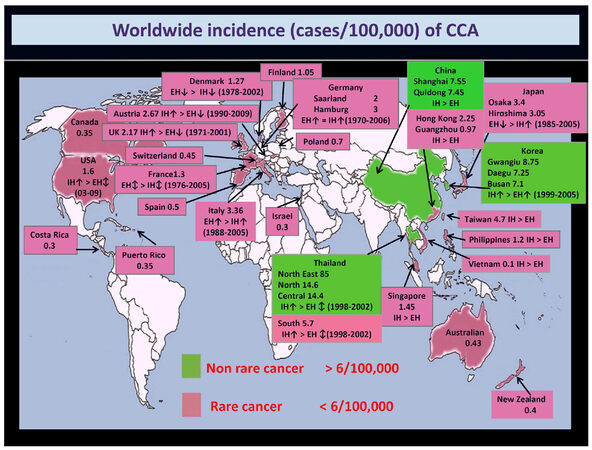
Cholangiocarcinoma (CCA) is a heterogeneous group of malignancies emerging at any level from the biliary tree[1-3][Figure 1]. CCA is classified into intrahepatic (iCCA), perihilar (pCCA) and distal (dCCA) based on its anatomical location[1-3]. Of note, these three CCA subtypes have common features but also important inter-tumor and intra-tumor differences that can affect the pathogenesis and outcome[4-9]. The complexity of the pathogenesis and the pronounced heterogeneity affected in particularly iCCAs had impeded clinical goals in iCCA[10]. This review will focus on the advances of iCCA epidemiology, classifications and histo-morphology, risk factors, molecular pathogenesis and clinical presentation revealing the existence of multiple subtypes of iCCA.
Figure 1. Worldwide incidence (cases/100,000) of cholangiocarcinoma (CCA). Data refer to the period 1971-2009. Green colour identifies areas with lower incidence (< 6/100,000 cases, rare cancer), while pink colour indicates countries where CCA is not a rare cancer (> 6/100,000 cases). Diagnoses have been classified according to the International Classification of Diseases (ICD-O-1, ICD-O-2, ICD-O-3, ICD-10, ICD-V9, ICD-V10, ICD-O). Where available, the more incident form [intrahepatic (IH) vs. extrahepatic (EH) CCA] and the temporal trend of incidence (↑increasing trend; ↕stable trend; ↓decreasing trend) have been reported. This figure was modified from Banales et al.[3] with permission
The burden of iCCA
The epidemiologic trend of CCA shows a constant and dramatic increase in incidence and mortality worldwide[1-3], clearly depicting CCA relevance among others types of cancer. A progressive increase in intrahepatic CCA incidence was reported, while the incidences of both perihilar CCA and distal CCA seem to be stable[1-3]. The incidence of CCA in European countries ranges from 1 to more than 4 cases/100,000[1-3][Figure 1]. However, the difficulties with classification coding for CCA, and with the various terminology that is used, determined an underestimation of CCA burden. In a recent report, the four ICD-10 (International Classification of Diseases) sub codes were agreed on for CCA and used[11]. This report showed that in England alone (not the whole of the UK), in 2013, 1965 new CCAs were diagnosed with an incidence rate of 3.65 per 100,000 population, while, 2161 deaths and a mortality rate of 4.01 per 100,000 population were registered. The number of deaths per 100,000 population for the CCA in the period from 2010 to 2013 in England tragically surpassed the ones for the hepatocellular carcinoma (HCC), with 7743 vs. 6899 deaths in 2013 for CCA and HCC respectively[11]. The trend in iCCA incidence is paralleled also by the fact that mortality for primary liver cancer has become more uniform across Europe over recent years with an evident decline of HCC mortality, but, in contrast, intrahepatic CCA mortality has substantially increased for the most part of Europe[12,13]. Over recent years intrahepatic CCA accounted for over a fourth of all liver cancer deaths in men and 50% in women[12]. Liver cancer mortality rates are expected to rise by 58% in the UK between 2014 and 2035, i.e., to 16 deaths per 100,000 people by 2035[14]. Considering epidemiology trend in primary liver cancer, half of deaths for primary liver cancer will be determined by intrahepatic CCA[12-14]. Furthermore, when the mortality rates for all malignancies are considered, the untargeted problem of CCA emerged clearly. Indeed, while a reduction of the mortality rate from 19 malignancies (comprising breast, lung, colon, etc.) was shown from 1990 to 2009 (US data), the mortality rate for malignancies of liver and bile ducts increased by more than 40% and 60% in females and males, respectively[15]. Finally, it is noteworthy to mention that CCA is the most frequent cause of metastasis of unknown origin, and thus further highlights how we still do not know the real burden of CCA[16].
New insights into iCCA classifications
A huge number of different classifications have been proposed for CCA[1-10,17]. The most updated one, but still discussed, identify on the basis of the anatomical localization the iCCA, the pCCA and the dCCA[1-3]. However, being a topographic classification it suffers several pitfalls, and, of course, it does not reflect different biological features. Firstly, it should be noted that, the diagnosis of CCA frequently occurs at an advanced stage, where, the differentiation between the intra-hepatic or extra-hepatic location results is very difficult, and sometimes impossible[1-3]. Since, small bile ducts and ductules are also present in the perihilar liver parenchyma, then, pCCA as iCCA, may originate either from these smaller ducts and this cannot be discriminated based on gross morphology. Similarly, the iCCA may originate from larger or smaller portion of intrahepatic biliary tree. Third, recent studies demonstrated how, from a pathological and molecular point of view, differences between pCCA and the iCCA originated from larger bile ducts ceased to exist and, therefore, the distinction between these two forms of CCA is losing relevance[4,9]. Taking into consideration the macroscopic pattern of growth, iCCA has been classified in mass-forming (MF), periductal infiltrating (PI), and intraductal growing (IG)[2,3]. As far as pCCA and dCCA are concerned, either a PI or IG pattern has been recognized. For pCCA a nodular + PI growth pattern predominates (> 80%)[2,5,17,18].
On the histological level, while, the vast majority of pCCA and dCCA are mucinous adenocarcinomas, iCCAs are highly heterogeneous tumors and several classifications have been proposed[4,5,9,19]. The small bile duct type (mixed) iCCAs display an almost exclusively MF growth pattern[4,5,9,19], and are frequently associated with chronic liver diseases (viral hepatitis or cirrhosis)[4,5,9,19,20]. Notably, this subtype shares clinic-pathological similarities with cytokeratin (CK) 19-positive hepatocarcinoma (HCC)[4,21]. On the other hand, large bile duct type (mucinous) iCCAs may grossly appear as MF, PI or IG types; they are more frequently associated with PSC and can be preceded by pre-neoplastic lesions such as biliary intraepithelial neoplasm (BiIN) or intraductal papillary neoplasm (IPNB)[4,5,9,19]. Interestingly, the large bile duct type (mucinous) iCCAs share phenotypic traits with pCCA and pancreatic cancers[4].
In our opinion, this histological subtyping should be taken into serious consideration because it underlines different risk factors, molecular profile, and clinical management[3,4,9,14,22-28].
Multiple risk factors reveal iCCA subtype-specific pathogenesis
Although CCA is a rare cancer (incidence < 6/100,000) in most countries, its incidence may reach an extremely high in some populations of Chile, Bolivia, South Korea and North Thailand[29][Figure 1]. The different prevalence of risk factor in geographic areas may explain the variation in incidence rates of CCA. For example, in Thailand regions, the very high incidence of CCA is closely related to the incidence of liver flukes[30-32].
In order to review literature on risk factors associated with iCCA we have searched for case series of iCCA or case series with appropriate topographic classification of histologically verified iCCA. The risk factors of iCCA (diagnosed according the current recognized criteria, i.e. European RARECARE[33]) could be classified on the basis of the tissue or the cell which is primarily targeted by diseases or conditions and therefore likely involved in the carcinogenic process as cell or tissue of origin. For instance, biliary diseases as cholangitis/PSC, secondary biliary cirrhosis, choledocholithiasis, hepatolithiasis, cholecystitis, and liver flukes are pathologic conditions primarily affecting large intra-hepatic bile ducts [Table 1][34-46], and are risk factors for both iCCA and p/dCCA. Parenchymal liver diseases include chronic viral and non-viral liver diseases, recognize the interlobular bile ducts, bile ductules and the canals of Hering as the primary targets. Accordingly, these conditions are specific risk factors for iCCA [Table 1].
Summary of risk factors significantly associated to iCCA* as assessed by case control studies (odd ratios by multivariate analyses)
| Risk factors for iCCA | Odds ratios for increased risk |
|---|---|
| Bile duct diseases and conditions | |
| Cholecystitis[36] | 8.5 |
| Cholelithiasis[35,40] | 10.23-13.5 |
| Hepatolithiasis[37,39,40,43,77§] | 50.0-4.8; 6.7§ |
| Choledochal cysts[36,37,44,59] | 10.7-43.03; 36.9 |
| Choledocholithiasis[35,43] | 4.17-33.35 |
| Cholangitis/primary sclerosing cholangitis[36,44] | 64.2-75.23 |
| Biliary cirrhosis/PBC[36,44] | 17.08-19.8 |
| Cholecystectomy[36,39] | 3.6-5.4 |
| Digestive diseases | |
| Inflammatory bowel diseases[36,58] | 1.72-3.95 |
| Crohn's disease[ 36,44] | 1.68-2.4 |
| Ulcerative colitis[36,44] | 3.3-4.5 |
| Duodenal ulcer[36] | 3.4 |
| Chronic pancreatitis[36] | 5.9 |
| Liver flukes | |
| Clonorchis sinensis infection[38,42] | 8.6-13.6 |
| Endocrine disorders | |
| Thyrotoxicosis[36] | 1.5 |
| Diabetes mellitus type II[37-39,43,75,86] | 1.8-3.2 |
| Metabolic conditions and general risks | |
| Obesity[36,44] | 1.7-1.71 |
| Alcohol intake > 80 g/day[37,39,75] | 1.52-5.21 |
| Smoking[36,44] | 1.3-2.1 |
| Metabolic syndrome[44#] | 1.32-1.83 |
| Dyslipoproteinemia[44] | 1.65 |
| Hypertension[44] | 1.63 |
| Chronic liver diseases | |
| Alcoholic liver disease[36,44] | 3.1-5.69 |
| Non specific cirrhosis[36,37,43,44,75] | 18.24-28.79 |
| Hemochromatosis[36] | 2.6 |
| Hepatic schitsomias[43] | 11 |
| Non alcoholic liver disease[36] | 3 |
| Unspecified viral hepatitis[44] | 7.66 |
| HCV infection[36-40,44,75,77§] | 2.41-9.71; 9.7§ |
| HCV infection plus cirrhosis[40] | 8.53 |
| HBsAg positive[35,37-40,44,75,81°] | 2.3-9.7; °2.35-4.3 |
| HBsAg positive plus cirrhosis[35,40,41] | 13-18 |
| HBsAg negative/HBcAb positive[45,81°] | 1.09-1.81° |
| Occupational exposure | |
| Occupational exposure to asbestos[46] | 4.81 |
Other risk factors, like several toxic and environmental factors; amongst them nitrosamine-contaminated food, asbestos, dioxins, vinychlorides, and thorotrast as was always the case in the past[47], which hit multiple cellular targets, are considered risk factors associated to all CCA subtypes.
PSC, a disease affecting both intra-hepatic and extra-hepatic bile ducts, represents the strongest independent risk factor both for iCCA and for pCCA [Table 1]. Most of the studies evaluated the cumulative risk of CCA in PSC patients, but not the discrete risk of iCCA and/or pCCA to PSC[48-51]. The cumulative incidence of CCA in PSC patients ranges from 5% to 10%[52-55]. Clinical and pathological observations suggested that PSC is specifically associated with the development of bile duct (mucinous) type CCA[4,56]. Data on the role of inflammatory bowel diseases (IBD), associated with or preceding PSC, in affecting the risk of CCA are controversial. The coexistence and duration of IBD significantly increased the risk of CCA in PSC patients[51]. In IBD patients the RR estimated was 2.61 for iCCA vs. 1.47 for pCCA[57]. Crohn’s disease (CD) seemed to have a lower risk of CCA than ulcerative cholitis (UC)[57,58]. In contrast, in a study carried out in the USA, neither IBD nor its duration confers additional risk of CCA in PSC patients[59].
In a study, Welzel et al.[36] described that duodenal ulcer disease was significantly more common among pCCA and iCCA cases than controls. Many studies have demonstrated associations between CCA and H. pylori but the correlation remains controversial and a direct cause-and-effect relationship has not been established[60-66]. In particular, in East-Asia, where iCCA represents a large proportion of primitive liver cancers, a strong association exists between liver fluke infestation (Ophistorchis viverrini and Clonorchis sinensis) and the development of CCA [Table 1][67,68]. Several epidemiological studies estimated the relationship between type II diabetes and CCA [Table 1][36,69-71]. Notably, a possible explanation of this association is attributable to a recent demonstration that in a diabetes model and in human subjects affected by type II diabetes, PBGs underwent proliferation and expansion in relation to hyperglycemia[72]. It’s worthy to note that metformin reduced the risk of iCCA in diabetic patients by a significant margin up to 60%[73,74]. A recent meta-analysis confirmed that, in addition to type II diabetes, even obesity, alcohol use and smoking, have an association with iCCA[75].
It is becoming increasingly evident that metabolic conditions predispose to the development of primary liver cancers[3,44,76]. Nonalcoholic fatty liver disease/non alcoholic steato-hepatitis (NAFLD/NASH) resulted in independent predictors of iCCA (not of pCCA development), even if with a less strong association compared with other risk factors (viral hepatitis, cirrhosis) [Table 1][76]. Hemochromatosis resulted in an independent predictor of iCCA development, and it failed to predict pCCA [Table 1].
It has long been known that the presence of cirrhosis increases the risk of iCCA[36,37,40,44,75]. HBV- and HCV-related liver diseases have been identified as definitive risk factors for CCA, with a stronger association for iCCA than pCCA[77,78]. A meta-analysis by Palmer and Patel[75] concerning 8 case control studies indicated that HCV was associated with an overall OR of 4.84 for iCCA. Where the prevalence of the HBV infection is higher, the association with iCCA and HBV is more significant (e.g. Asian countries)[79,80]. The range of the OR in the HbsAg positive subjects goes from 2.3 to 9.7 [Table 1][81]. The presence of cirrhosis increases the risk of CCA [Table 1] even more by 2.5 fold (95% CI: 1.2-5.1; P = 0.02) in HBV, and 3.2 fold (95% CI: 1.231-8.148, P = 0.017) in HCV patients[41].
The burden of HCV in the last decades has been associated with the specific increase of the iCCA as well as the HCC[81]. Accordingly, clinical and pathological observations suggested that liver cirrhosis is specifically associated with the development of small bile duct (mixed) type iCCA[4]. Ductular reaction is a marker strongly associated with the evolution of chronic liver disease in cirrhosis. The origin of the small bile duct type iCCA may be associated with the chronic proliferative activation of hepatic stem cells and mature hepatocytes senescence in chronic liver diseases[12,82]. Since cirrhosis, chronic hepatitis B and C, alcohol use, diabetes, and obesity are major risk factors for iCCA and HCC[75], a common pathogenesis of primary intrahepatic epithelial cancers has been suggested. The parallel worldwide reduction of mortality of HCC[12], which is highly correlated to viral infection and cirrhosis, and on the pandemic of metabolic disorders, suggests that metabolic risk factors are responsible for the rising clinical impact of iCCA. Interestingly we provided the pathologic basis of this epidemiology phenomenon since we demonstrated DM-induced proliferation of PBG cells[72].
Molecular profiling and the identification of multiple iCCA subsets
Although there exist enormous geographic and racial differences[3,83], generally, the prominent genetic alterations described in CCAs affect TP53 (DNA repair)[84-86], tyrosine kinase (KRAS, BRAF, SMAD4 and FGFR2)[8,84-88], protein tyrosine phosphatase (PTPN3)[89], deregulated WNT/CTNNB1[90] and Notch pathways, epigenetic (IDH1 and IDH2)[28,84,88,91,92], and chromatin-remodeling factors (MLLs, ARID1A, PBRM1 and BAP1)[84-86,88,91].
Chronic bile duct inflammation characterizes CCA risk factors[93-95]. Accordingly, it was demonstrated that the enzyme cyclooxygenase-2 (COX-2) is induced in CCA by both bile acids and oxysterols, the oxidation products of cholesterol that are increased in the bile during biliary inflammation[96,97]. Inflammatory cytokines may also upregulate the expression of inducible nitric oxide synthase (iNOS) in CCA. Notably, nitric oxide (NO) promotes DNA damage directly by inhibiting DNA repair mechanisms, thus promoting carcinogenesis[98,99]. Moreover, iNOS activation stimulates further the expression of COX-2[100]. Notably, the tumoral stroma seems to have a peculiar role in the amplification of the inflammation. While the tumor epithelium was defined by deregulation of the HER2 network and frequent over-expression of EGFR, the hepatocyte growth factor receptor (HGF/MET), pRPS6, and Ki67, the stroma was enriched in inflammatory cytokines[101].
In the chronic inflammation milieu of CCA emerging in hepatitis infection[88], recurrent genetic variants in the promoter of the human telomerase reverse transcriptase (TERT) were described[88]. This could be correlated with the pivotal role of this “longevity” enzyme in controlling stem cells. These cells are extremely challenged in these conditions because the senescence of the mature hepatocytes determines the secondary stem proliferative activation (e.g. ductular reaction)[12].
A dissection of the molecular heterogeneity of iCCA, conducted by the evaluation of gene expression profile (transcriptome), clinic-pathological traits, and patient outcomes in iCCA cases, has allowed the identification of 2 main biological classes of iCCA. The first inflammation class (38% of IH-CCA), characterized by activation of inflammatory signaling pathways, overexpression of cytokines, and STAT3 activation and; the second proliferation class (62% of IH-CCA), characterized by activation of oncogenic signaling pathways (i.e. RAS, MAP-kinase and HGF/MET), DNA amplifications at 11q13.2, deletions at 14q22.1, mutations in KRAS and BRAF, and gene expression signatures previously associated with poor outcomes for patients with HCC[7].
Molecular studies of human iCCA associated with liver flukes demonstrated over-expression of genes involved in xenobiotic metabolism (UGT2B11, UGT1A10, CHST4, SULT1C1). Whereas non-OV-associated iCCA showed enhanced expression of genes related to growth factor signaling (TGFBI, PGF, IGFBP1, IGFBP3)[32,102]. Possible mechanism associated with liver flukes carcinogenesis may emerge from the discovery of the draft genome of Clonorchis sinensis and transcriptomes of Clonorchis Sinensis and OV[103,104]. For instance, the evaluation of the putative signature of liver flukes associated CCA could help in screening and surveillance, with the perspective of an early diagnosis of infestation in subjects[102]. A putative role of liver fluke infestation in modulating epigenetic has been suggested by the demonstration of promoter hypermethylation in a handful of target genes in a large cohort of iCCA (n = 102) associated with liver fluke infection[105].
CCA genetic susceptibility has been investigated in geographic areas where liver flukes are endemic. In these studies, specific haplotypes of COX2-coding gene (PTGS2) or IL8RB have been recently associated with a significant risk of CCA development[106].
As far as CCA emerging in PSC, different molecular signatures of the high oncogenic risk were described in PSC patients. KRAS mutations were found in 30% of bile fluid of PSC patients without evidence of CCA[107]. Since KRAS mutations are frequently observed in CCA, and since the mutational profiling can be performed in cell-free DNA of bile supernatant, this early mutagenic event into the bile duct carcinogenesis could be evaluated for screening purposes in PSC patients[108]. The inflammatory microenvironment has also been associated with an aberrant DNA methylation profile in CCA emergence in PSC patients, which provides survival signals for the tumor[109]. Even, an inherited increase in the risk of CCA development in PSC patients was demonstrated by studies concerning the natural killer cell receptor G2D receptor, where specific genetic variants have been described in PSC patients[110].
Heterogeneity of molecular profile of CCA provides a demonstration of how somatic mutagenesis and epigenome features are highly cell/lineage type-specific, and are largely driven by the pre-neoplastic tissue pathologic milieu (see inflammation). Indeed, at a molecular level, distinct patterns of genetic mutations, methylation, and expression profiling may differentiate iCCA from pCCA. iCCAs were significantly more frequently bcl-2+ and p16+, whereas pCCAs were more often p53+[111]. Miller et al.[112] revealed 545 genes with altered expression in p/dCCA and 2354 in iCCA. Mutations in IDH1 and IDH2 were found only in iCCA (n = 9), but in none of the examined p/dCCA (n = 22) and gallbladder cancer (n = 75)[113]. Recent papers confirmed liver fluke negative iCCAs are enriched for IDH mutants[14,28]. A cross-platform comparison of iCCA with pancreatic cancer and HCC further emphasizes the presence of distinct tumor subsets, suggesting similarities of the IDH mutants CCAs with the HCCs rather than pancreatic cancers[28]. Conversely, mutations in KRAS by tumor site demonstrated predominance in pCCAs (53.3% of hilar vs. 6.7% of peripheral type)[7]. As far as epigenetic abnormalities are concerned, methylation of RASSF1A was more common in pCCA than in iCCA, while the opposite was demonstrated for methylation of GSTP gene[114]. Other reported alterations uniquely associated with iCCA, comprised fibroblast growth factor receptor (FGFR) pathways and ephrin type-A receptor 2 mutations[115].
Finally, the histopathological distinction of cholangiolocellular differentiation of iCCA has been correlated with molecular features[115]. iCCA with cholangiolocellular differentiation resembling an inflammation-related subtype revealed less aggressive histopathological features compared to iCCA without cholangiolocellular differentiation resembling a proliferation subtype. Accordingly, the former showed more favorable clinical outcomes, including overall survival, than iCCA without cholangiolocellular differentiation[116]. The emerging therapeutic approaches based on the molecular targets in CCA have been recently reviewed by Rizvi and Gores[117].
Variable clinical presentations and diagnostic features
Clinical presentation of CCA is largely influenced by anatomic location and pattern of growth, which ultimately belong from the cells of the origin. Accordingly, emerging concepts into CCA origins demonstrated that it comprises at least two separate entities which a distinct histology, progression and risk factors. These sub-types have been recently classified in large bile duct (mucinous) type CCAs and the small bile ducts or mixed-CCAs. According to different observations, pCCAs are more likely associated with pre-neoplastic lesions emerging in surface epithelium[2,3] and PBGs[118]. On the other hand, iCCAs show inter-tumor heterogeneity leading to the classification into two main different histological subtypes[4,119], with likely different cells of origin[4]: the CCAs of the small bile ducts or mixed-CCAs and the large bile duct (mucinous) type iCCAs[22,119]. The last iCCA subtype displays IHC, gene expression and clinic-pathological profile that can be superimposed on pCCA[4,120-122]. Small bile ducts or mixed-CCAs usually showed a peripheral localization and a mass forming growing pattern. Differently, the large bile duct (mucinous) type usually showed a peri-ductal infiltrating and/or mass forming growth pattern[4]. Importantly, these separate entities displayed different prognosis (being worst the one of the mucin-producing iCCAs) and different associated diseases[4,10,82,123]. Indeed, parenchymal liver diseases, including chronic viral and non-viral liver diseases and liver cirrhosis, characterize the clinical-pathologic background for mixed-iCCAs[4,10,82,123]. In contrast, chronic biliary diseases or pathologies and conditions affecting the intrahepatic medium-large and extrahepatic bile ducts characterize the clinical-pathologic background for mucin-producing iCCAs and pCCAs[4,10,82,123].
As far as the mixed type-mass-forming iCCA is concerned, the clinical presentation is similar to other intrahepatic liver malignancies, but different from that of pCCA[4,10,82,123]. iCCAs are usually asymptomatic in early stages (20%-25% of cases are incidental finding). Malaise, cachexia, abdominal pain, night sweats, fatigue and/or jaundice, associated or not with systemic manifestations, represent the clinical onset of symptomatic iCCA[4,10,82,123]. In contrast, a typically painless jaundice is the most frequent clinical onset in pCCA[4,10,82,123]. Regarding patients with PSC, CCA may present as the development of a rapid deterioration of clinical conditions or a dominant stricture during follow-up[3]. In general, the MF type represents the most frequent macroscopic presentation of iCCA (> 90%) appearing, at imaging, as a nodule[3,123]. In the context of cirrhotic liver, the first diagnostic challenge is the differential diagnosis of iCCA vs. HCC. In the cirrhotic liver it was demonstrated that by contrast, enhanced MRI iCCAs showed constantly a lack of HCC hallmarks; however, by CT, this occurs only in large nodules (> 3 cm)[124-126]. Although, the HCC diagnosis belong from the demonstration of the typical contrast agent uptake, the identification of HCC with stem cell features (CK19+-HCC), combined HCC-CCA, cholangiolocellular carcinoma and bile duct mixed type iCCA, by imaging procedures, still remains an unsolved challenge[3,4,10,123,127,128]. Biopsy is, therefore, necessary after excluding HCC in cirrhosis, or in the context of a nodule in non-cirrhotic liver[3,129]. From a histological point of view, differential diagnosis of iCCA vs. HCC or metastasis represents an unsolved problem[2,3,129,130], also due to the lack of validation of specific markers.
Radiologically, iCCA may appear as a dominant stricture in the context of PSC or in patients without a documented specific hepato-biliary disease. This is a typical presentation of the pCCA. When a dominant stricture of the intrahepatic biliary tree is suspected, the MRI + MRCP represents the imaging procedure with the highest diagnostic accuracy for localizing and sizing the stricture[3]; the challenge being the definitive demonstration of malignancy[3]. In this respect, ERCP enables a number of procedures in order to obtain a microscopic confirmation, comprising, cytology, brushing, FISH-polisomy, biopsy, or further innovative techniques[3]. However, all these techniques show an unsatisfactory sensitivity[54,130-133], and even, the FISH-polisomy in detecting CCA in PSC patients demonstrated a low sensitivity in a meta-analysis[133].
In substance, diagnosis of CCA still requires a combination of clinical, radiologic and non-specific histologic/biochemical markers (see review by Banales et al.[3]).
As already mentioned, no specific serum, urine, biliary or histological biomarkers are currently available for the diagnosis of CCA and a proposal by our group which has been recently refreshed by new confirmation, identifies biliary IGF1 as specific markers of CCA. However, the very promising role of biliary IGF1 has been confirmed only in CCA without PSC. Recently, Arbelaiz et al.[134] evaluated the serum concentration of extracellular vesicles (EVs) and performed a careful analysis of the protein content in patients with CCA, PSC, and HCC. Proteomic signatures found in serum EV of CCA, PSC, and HCC patients show potential usefulness as diagnostic tools. As noted previously, the EV cargo in the two distinct EV populations (i.e., basolateral and apical) is evidently different as a large difference exists between the protein content of EVs released by normal cholangiocytes and cholangiocytes involved in chronic inflammation (i.e., PSC) or neoplastic transformation (i.e., CCA)[135]. Further validation studies will be necessary to bring this important scientific advance into the clinical approach of CCA differential diagnosis.
New advances into CCA therapy
Surgery with complete resection, including liver transplantation in highly selected cases, is the only curative therapy for CCA. In patients with unresectable tumours, several types of loco regional therapy or chemotherapy (such as trans arterial chemoembolization, trans arterial radio embolization or radiofrequency ablation) can be considered. In substance, CCAs must be managed by dedicated centres with multidisciplinary expertise in which personalized diagnostic work‑up and management can be performed, as clearly stated by a European Consensus (see review by Banales et al.[3]).
Recently two important advances have been reached in therapy of iCCA. On one hand, the first clinical trial of adjuvant therapy has been concluded[136]. In this clinical trial, 447 surgically resected patients were randomly assigned to capecitabine for 6 months or observation (> 80% of the patients were followed for at least 3 years). Interestingly, results showed a survival of 51 vs. 36 months in capecitabine arm vs. observation, and median time to cancer recurrence of 25 vs. 18 months, respectively. In 430 patients who received treatment per study protocol, capecitabine is associated with a 25% lower chance of death than observation[136]. On the other, the first report of a molecular target therapy in chemotherapy-refractory CCA appeared. BGJ398 was a first-in-class FGFR kinase inhibitor with manageable toxicities showing meaningful clinical activity against chemotherapy-refractory CCA containing FGFR2 fusions. This promising antitumor activity supports continued development of BGJ398 in this highly selected patient population[137]. Emerging therapeutic approaches based on the molecular targets are still in early phase of clinical study and have been recently reviewed by Rizvi and Gores[117].
Perspectives
A unique feature of CCA is that it recognizes as origin tissues, the hepatic parenchyma or large bile ducts, which are furnished by two distinct stem cell niches, the canals of Hering and the peribiliary glands (PBGs), respectively[138].
Stem cells have been identified as cells of origin of different cancer types, comprising primary liver cancers, both in experimental studies and in humans[139-147]. Based on the grade of maturation of the cells of origin within the two lineages of the liver (hHpSC-derived and hBTSC-derived lineages), we have proposed that CCAs could be classified as:
• Primary liver parenchymal CCA: cholangiolo-carcinoma, small bile duct type (mixed) CCA. These tumors emerge within the liver parenchyma from canals of Hering, bile ductules and interlobular bile ducts and indeed originate from hHpSCs, immature NCAM+ cholangiocytes, or mature (NCAM-) interlobular cholangiocytes. A rigourous study, based on an integrative genomic analysis of HCC-CCAs, demonstrated that cholangiolo-carcinoma represents a distinct biliary-derived entity compared with the mixed/combined HCC-CCA, which, on the other side, comprised the stem-cell type, with an aggressive nature and poor outcome, and the classical type, with common cell lineage for both the HCC and the iCCA component[148].
• Primary biliary CCA: dCCA, pCCA, and large bile duct (mucinous) type iCCA. These tumors emerge from extra-hepatic biliary tree and larger intra-hepatic bile ducts and originate from PBGs or surface epithelium of corresponding bile ducts.
Thus, facing the origin of iCCA, a physiopathology concept should be considered, instead of the cell of origin, the lineage of origin[10,12,13,138]. An iCCA classification based on the cell-lineages-of origin is more coherent with current knowledge on the epidemiology and risk factors and may have important clinical implications for the definition of specific therapeutic targets. Moreover, it highlights a lineage dependency of the chronic liver diseases and related molecular carcinogenesis[12]. Being somatic mutagenesis and epigenome features highly cell/lineage type-specific[149], and largely driven by the pre-neoplastic tissue pathologic milieu (see inflammation), finally, the multiple lineages of origin plus the related diseases may explain the intertumoral heterogeneity observed at any level in iCCA, comprising molecular profiling, with clear implication into preventive strategies in patient with clinical or subclinical underlining hepatic or biliary diseases, therapy and in near future approaches of personalized medicine in iCCA patients.
Declarations
Authors’ contributionsConcept, design, definition of intellectual content, literature search, data acquisition and analysis, statistical analysis, manuscript preparation, editing and review: Cardinale V, Bragazzi MC, Carpino G
Data acquisition and analysis: Di Matteo S, Overi D, Nevi L
Manuscript editing and review: Gaudio E
Definition of intellectual content, literature search, manuscript preparation, editing and review: Alvaro D
Availability of data and materialsNot applicable.
Financial support and sponsorshipThe study was supported by Consorzio Interuniversitario Trapianti d'Organo, Rome, Italy and by a sponsored research agreement from Vesta Therapeutics (Bethesda, MD).
Conflicts of interestNone.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2018.
REFERENCES
1. Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, Pawlik TM, Gores GJ. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 2014;60:1268-89.
2. Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol 2011;8:512-22.
3. Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, Lind GE, Folseraas T, Forbes SJ, Fouassier L, Geier A, Calvisi DF, Mertens JC, Trauner M, Benedetti A, Maroni L, Vaquero J, Macias RI, Raggi C, Perugorria MJ, Gaudio E, Boberg KM, Marin JJ, Alvaro D. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol 2016;13:261-80.
4. Komuta M, Govaere O, Vandecaveye V, Akiba J, Van Steenbergen W, Verslype C, Laleman W, Pirenne J, Aerts R, Yano H, Nevens F, Topal B, Roskams T. Histological diversity in cholangiocellular carcinoma reflects the different cholangiocyte phenotypes. Hepatology 2012;55:1876-88.
5. Aishima S, Oda Y. Pathogenesis and classification of intrahepatic cholangiocarcinoma: different characters of perihilar large duct type versus peripheral small duct type. J Hepatobiliary Pancreat Sci 2015;22:94-100.
6. Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology 2013;145:1215-29.
7. Andersen JB, Spee B, Blechacz BR, Avital I, Komuta M, Barbour A, Conner EA, Gillen MC, Roskams T, Roberts LR, Factor VM, Thorgeirsson SS. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology 2012;142:1021-31.
8. Nakamura H, Arai Y, Totoki Y, Shirota T, Elzawahry A, Kato M, Hama N, Hosoda F, Urushidate T, Ohashi S, Hiraoka N, Ojima H, Shimada K, Okusaka T, Kosuge T, Miyagawa S, Shibata T. Genomic spectra of biliary tract cancer. Nat Genet 2015;47:1003-10.
9. Nakanuma Y, Sato Y, Harada K, Sasaki M, Xu J, Ikeda H. Pathological classification of intrahepatic cholangiocarcinoma based on a new concept. World J Hepatol 2010;2:419-27.
10. Cardinale V, Carpino G, Reid L, Gaudio E, Alvaro D. Multiple cells of origin in cholangiocarcinoma underlie biological, epidemiological and clinical heterogeneity. World J Gastrointest Oncol 2012;4:94-102.
11. England Rare and less common cancers: Incidence and Mortality in, 2010-2013. A Report from Pubblic Health England, National Cancer Registration and Analysis Service. Available from: http://www.ncin.org.uk/publications/rare_and_less_common_cancers. [Last accessed on 8 Jun].
12. Bertuccio P, Bosetti C, Levi F, Decarli A, Negri E, La Vecchia C. A comparison of trends in mortality from primary liver cancer and intrahepatic cholangiocarcinoma in Europe. Ann Oncol 2013;24:1667-74.
13. Lepage C, Capocaccia R, Hackl M, Lemmens V, Molina E, Pierannunzio D, Sant M, Trama A, Faivre J, EUROCARE-5 Working Group. Survival in patients with primary liver cancer, gallbladder and extrahepatic biliary tract cancer and pancreatic cancer in Europe 1999-2007: results of EUROCARE-5. Eur J Cancer 2015;51:2169-78.
14. Smittenaar CR, Petersen KA, Stewart K, Moitt N. Cancer incidence and mortality projections in the UK until 2035. Br J Cancer 2016;115:1147-55.
15. Llovet JM, Villanueva A, Lachenmayer A, Finn RS. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat Rev Clin Oncol 2015;12:408-24.
17. Alvaro D, Bragazzi MC, Benedetti A, Fabris L, Fava G, Invernizzi P, Marzioni M, Nuzzo G, Strazzabosco M, Stroffolini T, " AISF, Cholangiocarcinoma", committee. Cholangiocarcinoma in Italy: a national survey on clinical characteristics, diagnostic modalities and treatment. Results from the "Cholangiocarcinoma" committee of the Italian Association for the Study of Liver disease. Dig Liver Dis 2011;43:60-5.
18. Farges O, Fuks D, Le Treut YP, Azoulay D, Laurent A, Bachellier P, Nuzzo G, Belghiti J, Pruvot FR, Regimbeau JM. AJCC 7th edition of TNM staging accurately discriminates outcomes of patients with resectableintrahepatic cholangiocarcinoma: by the AFC-IHCC-2009 study group. Cancer 2011;117:2170-7.
19. Liau JY, Tsai JH, Yuan RH, Chang CN, Lee HJ, Jeng YM. Morphological subclassification of intrahepatic cholangiocarcinoma: etiological, clinicopathological, and molecular features. Mod Pathol 2014;27:1163-73.
20. Nakanuma Y, Xu J, Harada K, Sato Y, Sasaki M, Ikeda H, Kim J, Yu E. Pathological spectrum of intrahepatic cholangiocarcinoma arising in non biliary chronic advanced liver diseases. Pathol Int 2011;61:298-305.
21. Komuta M, Spee B, Vander Borght S, De Vos R, Verslype C, Aerts R, Yano H, Suzuki T, Matsuda M, Fujii H, Desmet VJ, Kojiro M, Roskams T. Clinicopathological study on cholangiolocellular carcinomasuggesting hepatic progenitor cellorigin. Hepatology 2008;47:1544-56.
22. Carpino G, Cardinale V, Onori P, Franchitto A, Berloco PB, Rossi M, Wang Y, Semeraro R, Anceschi M, Brunelli R, Alvaro D, Reid LM, Gaudio E. Biliary tree stem/progenitor cells in glands of extrahepatic and intraheptic bile ducts: an anatomical in situ study yielding evidence of maturational lineages. J Anat 2012;220:186-99.
23. Theise ND, Saxena R, Portmann BC, Thung SN, Yee H, Chiriboga L, Kumar A, Crawford JM. The canals of Hering and hepatic stem cells in humans. Hepatology 1999;30:1425-33.
24. Kuwahara R, Kofman AV, Landis CS, Swenson ES, Barendswaard E, Theise ND. The hepatic stem cell niche: identification by label-retaining cell assay. Hepatology 2008;47:1994-2002.
25. Schmelzer E, Zhang L, Bruce A, Wauthier E, Ludlow J, Yao HL, Moss N, Melhem A, McClelland R, Turner W, Kulik M, Sherwood S, Tallheden T, Cheng N, Furth ME, Reid LM. Human hepatic stem cells from fetal and postnatal donors. J Exp Med 2007;204:1973-87.
26. Cardinale V, Wang Y, Carpino G, Cui CB, Gatto M, Rossi M, Berloco PB, Cantafora A, Wauthier E, Furth ME, Inverardi L, Dominguez-Bendala J, Ricordi C, Gerber D, Gaudio E, Alvaro D, Reid L. Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes, and pancreatic islets. Hepatology 2011;54:2159-72.
27. Turner R, Lozoya O, Wang Y, Cardinale V, Gaudio E, Alpini G, Mendel G, Wauthier E, Barbier C, Alvaro D, Reid LM. Human hepatic stem cell and maturational liver lineage biology. Hepatology 2011;53:1035-45.
28. Farshidfar F, Zheng S, Gingras MC, Newton Y, Shih J, Robertson AG, Hinoue T, Hoadley KA, Gibb EA, Roszik J, Covington KR, Wu CC, Shinbrot E, Stransky N, Hegde A, Yang JD, Reznik E, Sadeghi S, Pedamallu CS, Ojesina AI, Hess JM, Auman JT, Rhie SK, Bowlby R, Borad MJ, Cancer Genome Atlas Network, Zhu AX, Stuart JM, Sander C, Akbani R, Cherniack AD, Deshpande V, Mounajjed T, Foo WC, Torbenson MS, Kleiner DE, Laird PW, Wheeler DA, McRee AJ, Bathe OF, Andersen JB, Bardeesy N, Roberts LR, Kwong LN. Integrative genomic analysis of cholangiocarcinoma identifies distinct IDH-mutant molecular profiles. Cell Rep 2017;18:2780-94.
29. Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, Wolfe C, Hamadeh RR, Moore A, Werdecker A, Gessner BD, Te Ao B, McMahon B, Karimkhani C, Yu C, Cooke GS, Schwebel DC, Carpenter DO, Pereira DM, Nash D, Kazi DS, De Leo D, Plass D, Ukwaja KN, Thurston GD, Yun Jin K, Simard EP, Mills E, Park EK, Catalá-López F, deVeber G, Gotay C, Khan G, Hosgood HD 3rd, Santos IS, Leasher JL, Singh J, Leigh J, Jonas JB, Sanabria J, Beardsley J, Jacobsen KH, Takahashi K, Franklin RC, Ronfani L, Montico M, Naldi L, Tonelli M, Geleijnse J, Petzold M, Shrime MG, Younis M, Yonemoto N, Breitborde N, Yip P, Pourmalek F, Lotufo PA, Esteghamati A, Hankey GJ, Ali R, Lunevicius R, Malekzadeh R, Dellavalle R, Weintraub R, Lucas R, Hay R, Rojas-Rueda D, Westerman R, Sepanlou SG, Nolte S, Patten S, Weichenthal S, Abera SF, Fereshtehnejad SM, Shiue I, Driscoll T, Vasankari T, Alsharif U, Rahimi-Movaghar V, Vlassov VV, Marcenes WS, Mekonnen W, Melaku YA, Yano Y, Artaman A, Campos I, MacLachlan J, Mueller U, Kim D, Trillini M, Eshrati B, Williams HC, Shibuya K, Dandona R, Murthy K, Cowie B, Amare AT, Antonio CA, Casta-eda-Orjuela C, van Gool CH, Violante F, Oh IH, Deribe K, Soreide K, Knibbs L, Kereselidze M, Green M, Cardenas R, Roy N, Tillmann T, Li Y, Krueger H, Monasta L, Dey S, Sheikhbahaei S, Hafezi-Nejad N, Kumar GA, Sreeramareddy CT, Dandona L, Wang H, Vollset SE, Mokdad A, Salomon JA, Lozano R, Vos T, Forouzanfar M, Lopez A, Murray C, Naghavi M; Global Burden of Disease Cancer Collaboration. The global burden of cancer 2013. JAMA Oncol 2015;1:505-27.
30. Poomphakwaen K, Promthet S, Kamsa-Ard S, Vatanasapt P, Chaveepojnkamjorn W, Klaewkla J, Sujirarat D, Pichainarong N. Risk factors for cholangiocarcinoma in Khon Kaen, Thailand: a nested case-control study. Asian Pac Cancer Prev 2009;10:251-8.
31. Shin HR, Oh JK, Masuyer E, Curado MP, Bouvard V, Fang Y, Wiangnon S, Sripa B, Hong ST. Comparison of incidence of intrahepatic and extrahepatic cholangiocarcinoma--focus on East and South-Eastern Asia. Asian Pac J Cancer Prev 2010;11:1159-66.
32. Sripa B, Kaewkes S, Sithithaworn P, Mairiang E, Laha T, Smout M, Pairojkul C, Bhudhisawasdi V, Tesana S, Thinkamrop B, Bethony JM, Loukas A, Brindley PJ. Liver fluke induces cholangiocarcinoma. PLoS Med 2007;4:e201.
33. Lepage C, Capocaccia R, Hackl M, Lemmens V, Molina E, Pierannunzio D, Sant M, Trama A, Faivre J; EUROCARE-5 Working Group. Survival in patients with primary liver cancer, gallbladder and extrahepatic biliary tract cancer and pancreatic cancer in Europe 1999-2007: results of EUROCARE-5. Eur J Cancer 2015;51:2169-78.
34. Donato F, Gelatti U, Tagger A, Favret M, Ribero ML, Callea F, Martelli C, Savio A, Trevisi P, Nardi G. Intrahepatic cholangiocarcinoma and hepatitis C and B virus infection, alcohol intake, and hepatolithiasis: a case-control study in Italy. Cancer Causes Control 2001;12:959-64.
35. Tao LY, He XD, Qu Q, Cai L, Liu W, Zhou L, Zhang SM. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a case-control study in China. Liver Int 2010;30:215-21.
36. Welzel TM, Graubard BI, El-Serag HB, Shaib YH, Hsing AW, Davila JA, McGlynn KA. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study. Clin Gastroenterol Hepatol 2007;5:1221-8.
37. Yamamoto S, Kubo S, Hai S, Uenishi T, Yamamoto T, Shuto T, Takemura S, Tanaka H, Yamazaki O, Hirohashi K, Tanaka T. Hepatitis C virus infection as a likely etiology of intrahepatic cholangiocarcinoma. Cancer Sci 2004;95:592-5.
38. Lee TY, Lee SS, Jung SW, Jeon SH, Yun SC, Oh HC, Kwon S, Lee SK, Seo DW, Kim MH, Suh DJ. Hepatitis B virus infection and intrahepatic cholangiocarcinoma in Korea: a case-control study. Am J Gastroenterol 2008;103:1716-20.
39. Shaib YH, El-Serag HB, Nooka AK, Thomas M, Brown TD, Patt YZ, Hassan MM. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a hospital-based case-control study. Am J Gastroenterol 2007;102:1016-21.
40. Zhou YM, Yin ZF, Yang JM, Li B, Shao WY, Xu F, Wang YL, Li DQ. Risk factors for intrahepatic cholangiocarcinoma: a case-control study in China. World J Gastroenterol 2008;14:632-5.
41. Lee CH, Chang CJ, Lin YJ, Yeh CN, Chen MF, Hsieh SY. Viral hepatitis-associated intrahepatic cholangiocarcinoma shares common disease processes with hepatocellular carcinoma. Br J Cancer 2009;100:1765-70.
42. Choi D, Lim JH, Lee KT, Choi SH, Heo JS, Jang KT, Lee NY, Kim S, Hong ST. Cholangiocarcinoma and Clonorchis sinensis infection: a case-control study in Korea. J Hepatol 2006;44:1066-73.
43. Zhou H, Wang H, Zhou D, Wang H, Wang Q, Zou S, Tu Q, Wu M, Hu H. Hepatitis B virus-associated intrahepatic cholangiocarcinoma and hepatocellular carcinoma may hold common disease process for carcinogenesis. Eur J Cancer 2010;46:1056-61.
44. Welzel TM, Graubard BI, Zeuzem S, El-Serag HB, Davila JA, McGlynn KA. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in SEER-Medicare database. Hepatology 2011;54:463-71.
45. Zhou Y, Zhou Q, Lin Q, Chen R, Gong Y, Liu Y, Yu M, Zeng B, Li K, Chen R, Li Z. Evaluation of risk factors for extrahepatic cholangiocarcinoma: ABO blood group, hepatitis B virus and their synergism. Int J Cancer 2013;15:1867-75.
46. Brandi G, Di Girolamo S, Farioli A, de Rosa F, Curti S, Pinna AD, Ercolani G, Violante FS, Biasco G, Mattioli S. Asbestos: a hidden player behind the cholangiocarcinoma increase? Findings from a case-control analysis. Cancer Causes Control 2013;24:911-8.
47. Patel T. Cholangiocarcinoma—controversieses and challenges. Nat Rev Gastroenterol 2011:8189-200.
48. Broomé U, Olsson R, Lööf L, Bodemar G, Hultcrantz R, Danielsson A, Prytz H, Sandberg-Gertzén H, Wallerstedt S, Lindberg G. Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut 1996;38:610-5.
49. Farrant JM, Hayllar KM, Wilkinson ML, Karani J, Portmann BC, Westaby D, Williams R. Natural history and prognostic variables in primary sclerosing cholangitis. Gastroenterology 1991;100:1710-7.
50. Rosen CB, Nagorney DM, Wiesner RH, Coffey RJ Jr, LaRusso NF. Cholangiocarcinoma complicating primary sclerosing cholangitis. Ann Surg 1991;213:21-5.
51. Erichsen R, Jepsen P, Vilstrup H, Ekbom A, Sørensen HT. Incidence and prognosis of cholangiocarcinoma in Danish patients with and without inflammatory bowel disease: a national cohort study, 1978-2003. Eur J Epidemiol 2009;24:513-20.
52. Chapman MH, Webster GJ, Bannoo S, Johnson GJ, Wittmann J, Pereira SP. Cholangiocarcinoma and dominant strictures in patients with primary sclerosing cholangitis: a 25-year single-centre experience. Eur J Gastroenterol Hepatol 2012;24:1051-8.
53. Bergquist A, Ekbom A, Olsson R, Kornfeldt D, Lööf L, Danielsson A, Hultcrantz R, Lindgren S, Prytz H, Sandberg-Gertzén H, Almer S, Granath F, Broomé U. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol 2002;36:321-7.
54. Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, Gores GJ, American Association for the Study of Liver Diseases. Diagnosis and management of primary sclerosing cholangitis. Hepatology 2010;51:660-78.
55. Claessen MM, Vleggaar FP, Tytgat KM, Siersema PD, van Buuren HR. High lifetime risk of cancer in primary sclerosing cholangitis. J Hepatol 2009;50:158-64.
56. Carpino G, Cardinale V, Renzi A, Hov JR, Berloco PB, Rossi M, Karlsen TH, Alvaro D, Gaudio E. Activation of biliary tree stem cells within peribiliary glands in primary sclerosing cholangitis. J Hepatol 2015;63:1220-8.
57. Huai JP, Ding J, Ye XH, Chen YP. Inflammatory bowel disease and risk of cholangiocarcinoma: evidence from a meta-analysis of population-based studies. Asian Pac J Cancer Prev 2014;15:3477-82.
58. Weismüller TJ, Trivedi PJ, Bergquist A, Imam M, Lenzen H, Ponsioen CY, Holm K, Gotthardt D, Färkkilä MA, Marschall HU, Thorburn D, Weersma RK, Fevery J, Mueller T, Chazouillères O, Schulze K, Lazaridis KN, Almer S, Pereira SP, Levy C, Mason A, Naess S, Bowlus CL, Floreani A, Halilbasic E, Yimam KK, Milkiewicz P, Beuers U, Huynh DK, Pares A, Manser CN, Dalekos Eksteen B GN, Invernizzi P, Berg CP, Kirchner GI, Sarrazin C, Zimmer V, Fabris L, Braun F, Marzioni M, Juran BD, Said K, Rupp C, Jokelainen K, Benito de Valle M, Saffioti F, Cheung A, Trauner M, Schramm C, Chapman RW, Karlsen TH, Schrumpf E, Strassburg CP, Manns M, K4 Lindor, G5 Hirschfield, Hansen BE, Boberg KM, International PSC Study Group. Patient age, sex, and inflammatory bowel disease phenotype associate with course of primary sclerosing cholangitis. Gastroenterology 2017;152:1975-84.
60. Tiwari SK, Khan AA, Ibrahim M, Habeeb MA, Habibullah CM. Helicobacter pylori and other Helicobacter species DNA in human bile samples from patients with various hepatobiliary diseases. World J Gastroenterol 2006;12:2181-6.
61. Bulajic M, Maisonneuve P, Schneider-Brachert W, Müller P, Reischl U, Stimec B, Lehn N, Lowenfels AB, Löhr M. Helicobacter pylori and the risk of benign and malignant biliary tract disease. Cancer 2002;95:1946-53.
62. Chang JS, Tsai CR, Chen LT. Medical risk factors associated with cholangiocarcinoma in Taiwan: a population-based case-control study. PLoS One 2013;8:e69981.
63. Pandey M, Shukla M. Helicobacter species are associated with possible increase in risk of hepatobiliary tract cancers. Surg Oncol 2009;18:51-6.
64. Murphy G, Michel A, Taylor PR, Albanes D, Weinstein SJ, Virtamo J, Parisi D, Snyder K, Butt J, McGlynn KA, Koshiol J, Pawlita M, Lai GY, Abnet CC, Dawsey SM, Freedman ND. Association of seropositivity to Helicobacter species and biliary tract cancer in the ATBC study. Hepatology 2014;60:1963-71.
65. Kaewpitoon SJ, Loyd RA, Rujirakul R, Panpimanmas S, Matrakool L, Tongtawee T, Kootanavanichpong N, Pengsaa P, Kompor P, Chavengkun W, Kujapun J, Norkaew J, Ponphimai S, Padchasuwan N, Polsripradist P, Eksanti T, Phatisena T, Kaewpitoon N. Helicobacter species are possible risk factors of cholangiocarcinoma. Asian Pac J Cancer Prev 2016;17:37-44.
66. Leelawat K, Suksumek N, Leelawat S, Lek-Uthai U. Detection of VacA gene specific for Helicobactor pylori in hepatocellular carcinoma and cholangiocarcinoma specimens of Thai patients. Southeast Asian J Trop Med Public Health 2007;38:881-5.
67. Sithithaworn P, Yongvanit P, Duenngai K, Kiatsopit N, Pairojkul C. Roles of liver fluke infection as risk factor for cholangiocarcinoma. J Hepatobiliary Pancreat Sci 2014;21:301-8.
68. Xia J, Jiang SC, Peng HJ. Association between liver fluke infection and hepatobiliary pathological changes: a systematic review and meta-analysis. PLoS One 2015;10:1-19.
69. Li J, Han T, Xu L, Luan X. Diabetes mellitus and the risk of cholangiocarcinoma: an updated meta-analysis. Prz Gastroenterol 2015;10:108-17.
70. Shaib YH, El Serag HB, Davila JA, Morgan R, McGlynn KA. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology 2005;128:620-6.
71. Huang YJ, Wu AT, Chiou YH, Chuang MT, Meng TC, Chien LN, Yen Y. Interactive role of diabetes mellitus and female sex in the risk of cholangiocarcinoma: a population-based nested case–control study. Oncotarget 2017;8:6642-51.
72. Carpino G, Puca R, Cardinale Renzi A V, Scafetta G, Nevi L, Rossi M, Berloco PB, Ginanni Corradini S, Reid LM, Maroder M, Gaudio E, Alvaro D. Peribiliary glands as a niche of extrapancreatic precursors yielding insulin-producing cells in experimental and human diabetes. Stem Cells 2016;34:1332-42.
73. Chaiterakij R, Yang JD, Harmsen WS, Slettedahl SW, Mettler TA, Fredericksen ZS, Kim WR, Gores GJ, Roberts RO, Olson JE, Therneau TM, Roberts LR. Risk factor for intrahepatic cholangiocarcinoma association between metformin use and reduced cancer risk. Hepatology 2013;57:648-55.
74. Ling S, Feng T, Ke Q, Fan N, Li L, Li Z, Dong C, Wang C, Xu F, Li Y, Wang L. Metformin inhibits proliferation and enhances chemosensitivity of intrahepatic cholangiocarcinoma cell lines. Oncol Rep 2014;31:2611-8.
75. Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol 2012;57:69-76.
76. Reddy SK, Hyder O, Marsh JW, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, Pulitano C, Barroso E, Aldrighetti L, Geller DA, Sempoux C, Herlea V, Popescu I, Anders R, Rubbia-Brandt L, Gigot JF, Mentha G, Pawlik TM. Prevalence of nonalcoholic steatohepatitis among patients with resectable intrahepatic cholangiocarcinoma. J Gastrointest Surg 2013;17:748-55.
77. Shin HR, Lee CU, Park HJ, Seol SY, Chung JM, Choi HC, Ahn YO, Shigemastu T. Hepatitis B and C virus, Clonorchis sinensis for the risk of liver cancer: a case-control study in Pusan, Korea. Int J Epidemiol 1996;25:933-40.
78. Li H, Hu B, Zhou ZQ, Guan J, Zhang ZY, Zhou GW. Hepatitis C virus infection and the risk of intrahepatic cholangiocarcinoma and extrahepatic cholangiocarcinoma: evidence from a systematic review and meta-analysis of 16 case-control studies. World J Surg Oncol 2015;13:161-8.
79. Plentz RR, Malek NP. Clinical presentation, risk factors and staging systems of cholangiocarcinoma. Best Pract Res Clin Gastroenterol 2015;29:245-52.
80. Zhou HB, Hu JY, Hu HP. Hepatitis B virus infection and intrahepatic cholangiocarcinoma. World J Gastroenterol 2014;20:5721-9.
81. Zhang H, Biqing Z, He Z, Jianxin L, Wenting Z. HBV infection status and the risk of cholangiocacinoma in Asia: a meta-analysis. Bio Med Research International 2016;2016:3417976.
82. Cardinale V, Bragazzi MC, Alvaro D. New Insights into Epidemiology of Cholangiocarcinoma. In: Brandi G, Ercolani G, editors. Cholangiocarcinoma. Nova Science Pub; 2015. pp. 19-38.
83. Cardinale V, Alvaro D. Molecular Profiling. In: Herman J, Pawlik T, Thomas CR Jr, editors. Biliary Tract and Gallbladder Cancer: A Multidisciplinary Approach, 2nd Edition. Springer Science Pub; 2013. pp. 99-115.
84. Jiao Y, Pawlik TM, Anders RA, Selaru FM, Streppel MM, Lucas DJ, Niknafs N, Guthrie VB, Maitra A, Argani P, Offerhaus GJA, Roa JC, Roberts LR, Gores GJ, Popescu I, Alexandrescu ST, Dima S, Fassan M, Simbolo M, Mafficini A, Capelli P, Lawlor RT, Ruzzenente A, Guglielmi A, Tortora G, de Braud F, Scarpa A, Jarnagin W, Klimstra D, Karchin R, Velculescu VE, Hruban RH, Vogelstein B, Kinzler KW, Papadopoulos N, Wood LD. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat Genet 2013;45:1470-3.
85. Ong CK, Subimerb C, Pairojkul C, Wongkham S, Cutcutache I, Yu W, McPherson JR, Allen GE, Ng CC, Wong BH, Myint SS, Rajasegaran V, Heng HL, Gan A, Zang ZJ, Wu Y, Wu J, Lee MH, Huang D, Ong P, Chan-on W, Cao Y, Qian CN, Lim KH, Ooi A, Dykema K, Furge K, Kukongviriyapan V, Sripa B, Wongkham C, Yongvanit P, Futreal PA, Bhudhisawasdi V, Rozen S, Tan P, Teh BT. Exome sequencing of liver fluke-associated cholangiocarcinoma. Nat Genet 2012;44:690-3.
86. Zou S, Li J, Zhou H, Frech C, Frech C, Jiang X, Chu JS, Zhao X, Li Y, Li Q, Wang H, Hu J, Kong G, Wu M, Ding C, Chen N, Hu H. Mutational landscape of intrahepatic cholangiocarcinoma. Nat Commun 2014;5:5696.
87. Borad MJ, Champion MD, Egan JB, Liang WS, Fonseca R, Bryce AH, McCullough AE, Barrett MT, Hunt K, Patel MD, Young SW, Collins JM, Silva AC, Condjella RM, Block M, McWilliams RR, Lazaridis KN, Klee EW, Bible KC, Harris P, Oliver GR, Bhavsar JD, Nair AA, Middha S, Asmann Y, Kocher JP, Schahl K, Kipp BR, Barr Fritcher EG, Baker A, J4 Aldrich, Kurdoglu A, Izatt T, Christoforides A, Cherni I, Nasser S, Reiman R, Phillips L, McDonald J, Adkins J, Mastrian SD, Placek P, Watanabe AT, Lobello J, Han H, Von Hoff D, Craig DW, Stewart AK, Carpten JD. Integrated genomic characterization reveals novel, therapeutically relevant drug targets in FGFR and EGFR pathways in sporadic intrahepatic cholangiocarcinoma. PLoS Genet 2014;10:e1004135.
88. Fujimoto A, Furuta M, Shiraishi Y, Gotoh K, Kawakami Y, Arihiro K, Nakamura T, Ueno M, Ariizumi S, Nguyen HH, Shigemizu D, Abe T, Boroevich KA, Nakano K, Sasaki A, Kitada R, Maejima K, Yamamoto Y, Tanaka H, Shibuya T, Shibata T, Ojima H, Shimada K, Hayami S, Shigekawa Y, Aikata H, Ohdan H, Marubashi S, Yamada T, Kubo Hirano S M, Ishikawa O, Yamamoto M, Yamaue H, Chayama K, Miyano S, Tsunoda T, Nakagawa H. Whole-genome mutational landscape of liver cancers displaying biliary phenotype reveals hepatitis impact and molecular diversity. Nat Commun 2015;6:6120.
89. Gao Q, Zhao YJ, Wang XY, Guo WJ, Gao S, Wei L, Shi JY, Shi GM, Wang ZC, Zhang YN, Shi YH, Ding J, Ding ZB, Ke AW, Dai Z, Wu FZ, Wang H, Qiu ZP, Chen ZA, Zhang ZF, Qiu SJ, Zhou J, He XH, Fan J. Activating mutations in PTPN3 promote cholangiocarcinoma cell proliferation and migration and are associated with tumor recurrence in patients. Gastroenterology 2014;146:1397-407.
90. Boulter L, Guest RV, Kendall TJ, Wilson DH, Wojtacha D, Robson AJ, Ridgway RA, Samuel K, Van Rooijen N, Barry ST, Wigmore SJ, Sansom OJ, Forbes SJ. WNT signaling drives cholangiocarcinoma growth and can be pharmacologically inhibited. J Clin Invest 2015;125:1269-85.
91. Chan-On W, Nairismägi ML, Ong CK, Lim WK, Dima S, Pairojkul C, Lim KH, McPherson JR, Cutcutache I, Heng HL, Ooi L, Chung A, Chow P, Cheow PC, Lee SY, Choo SP, Tan IBH, Duda D, Nastase A, Myint SS, Wong BH, Gan A, Rajasegaran V, Young Ng CC, Nagarajan S, Jusakul A, Zhang S, Vohra P, Yu W, Huang DC, Sithithaworn P, Yongvanit P, Wongkham S, Khuntikeo N, Bhudhisawasdi V, Popescu I, Rozen SG, Tan P, Teh BT. Exome sequencing identifies distinct mutational patterns in liver fluke-related and non-infection-related bile duct cancers. Nat Genet 2013;45:1474-8.
92. Sia D, Losic B, Moeini A, Cabellos L, Hao K, Revill K, Bonal D, Miltiadous O, Zhang Z, Hoshida Y, Cornella H, Castillo-Martin M, Pinyol R, Kasai Y, Roayaie S, Thung SN, Fuster J, Schwartz ME, Waxman S, Cordon-Cardo C, Schadt E, Mazzaferro V, Llovet JM. Massive parallel sequencing uncovers actionable FGFR2-PPHLN1 fusion and ARAF mutations in intrahepatic cholangiocarcinoma. Nat Commun 2015;6:6087.
93. Nault JC, Zucman-Rossi J. Genetics of hepatobiliary carcinogenesis. Semin Liver Dis 2011;31:173-87.
94. Chen CP, Haas-Kogan D. Neoplasms of the hepatobiliary system: clinical presentation, molecular pathways and diagnostics. Expert Rev Mol Diagn 2010;10:883-95.
95. Kumar M, Zhao X, Wang XW. Molecular carcinogenesis of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: one step closer to personalized medicine? Cell Biosci 2011;1:5.
96. Yoon JH, Higuchi H, Werneburg NW, Kaufmann SH, Gores GJ. Bile acids induce cyclooxygenase-2 expression via the epidermal growth factor receptor in a human cholangiocarcinoma cell line. Gastroenterology 2002;122:985-93.
97. Yoon JH, Canbay AE, Werneburg NW, Lee SP, Gores GJ. Oxysterols induce cyclooxygenase-2 expression in cholangiocytes: implications for biliary tract carcinogenesis. Hepatology 2004;39:732-8.
98. Jaiswal M, LaRusso NF, Burgart LJ, Gores GJ. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res 2000;60:184-90.
99. Jaiswal M, LaRusso NF, Shapiro RA, Billiar TR, Gores GJ. Nitric oxide-mediated inhibition of DNA repair potentiates oxidative DNA damage in cholangiocytes. Gastroenterology 2001;120:190-9.
100. Ishimura N, Bronk SF, Gores GJ. Inducible nitric oxide synthase upregulates cyclooxygenase-2 in mouse cholangiocytes promoting cell growth. Am J Physiol Gastrointest Liver Physiol 2004;287:G88-95.
101. Seol MA, Chu IS, Lee MJ, Yu GR, Cui XD, Cho BH, Ahn EK, Leem SH, Kim IH, Kim DG. Genome-wide expression patterns associated with oncogenesis and sarcomatous transdifferentation of cholangiocarcinoma. BMC Cancer 2011;11:78.
102. Jinawath N, Chamgramol Y, Furukawa Y, Obama K, Tsunoda T, Sripa B, Pairojkul C, Nakamura Y. Comparison of gene expression profiles between Opisthorchis viverrini and non-Opisthorchis viverrini associated human intrahepatic cholangiocarcinoma. Hepatology 2006;44:1025-38.
103. Wang X, Chen W, Huang Y, Sun J, Men J, Liu H, Luo F, Guo L, Lv X, Deng C, Zhou C, Fan Y, Li X, Huang L, Hu Y, Liang C, Hu X, Xu J, Yu X. The draft genome of the carcinogenic human liver fluke Clonorchis sinensis. Genome Biol 2011;12:R107.
104. Young ND, Campbell BE, Hall RS, Jex AR, Cantacessi C, Laha T, Sohn WM, Sripa B, Loukas A, Brindley PJ, Gasser RB. Unlocking the transcriptomes of two carcinogenic parasites, Clonorchis sinensis and Opisthorchis viverrini. PLoS Negl Trop Dis 2010;4:e719.
105. Sriraksa R, Zeller C, El-Bahrawy MA, Dai W, Daduang J, Jearanaikoon P, Chau-In S, Brown R, Limpaiboon T. CpG-island methylation study of liver fluke-related cholangiocarcinoma. Br J Cancer 2011;104:1313-8.
106. Sakoda LC, Gao YT, Chen BE, Chen J, Rosenberg PS, Rashid A, Deng J, Shen MC, Wang BS, Han TQ, Zhang BH, Cohen-Webb H, Yeager M, Welch R, Chanock S, Fraumeni JF Jr, Hsing AW. Prostaglandin-endoperoxide synthase 2 (PTGS2) gene polymorphisms and risk of biliary tract cancer and gallstones: a population-based study in Shanghai, China. Carcinogenesis 2006;27:1251-6.
107. Kipp BR, Fritcher EG, Clayton AC, Gores GJ, Roberts LR, Zhang J, Levy MJ, Halling KC. Comparison of KRAS mutation analysis and FISH for detecting pancreatobiliary tract cancer in cytology specimens collected during endoscopic retrograde cholangiopancreatography. J Mol Diagn 2010;12:780-6.
108. Huang L, Frampton G, Liang LJ, Demorrow S. Aberrant DNA methylation profile in cholangiocarcinoma. World J Gastrointest Pathophysiol 2010;1:23-9.
109. Huang L, Frampton G, Rao A, Zhang KS, Chen W, Lai JM, Yin XY, Walker K, Culbreath B, Leyva-Illades D, Quinn M, McMillin M, Bradley M, Liang LJ, DeMorrow S. Monoamine oxidase A expression is suppressed in human cholangiocarcinoma via coordinated epigenetic and IL-6-driven events. Lab Invest 2012;92:1451-60.
110. Melum E, Karlsen TH, Schrumpf E, Bergquist A, Thorsby E, Boberg KM, Lie BA. Cholangiocarcinoma in primary sclerosing cholangitis is associated with NKG2D polymorphisms. Hepatology 2008;47:90-6.
111. Karamitopoulou E, Tornillo L, Zlobec I, Cioccari L, Carafa V, Borner M, Schaffner T, Brunner T, Diamantis I, Zimmermann A, Terracciano L. Clinical significance of cell cycle- and apoptosis-related markers in biliary tract cancer: a tissue microarray-based approach revealing a distinctive immunophenotype for intrahepatic and extrahepatic cholangiocarcinomas. Am J Clin Pathol 2008;130:780-6.
112. Miller G, Socci ND, Dhall D, D'Angelica M, DeMatteo RP, Allen PJ, Singh B, Fong Y, Blumgart LH, Klimstra DS, Jarnagin WR. Genome wide analysis and clinical correlation of chromosomal and transcriptional mutations in cancers of the biliary tract. J Exp Clin Cancer Res 2009;28:62.
113. Borger DR, Tanabe KK, Fan KC, Lopez HU, Fantin VR, Straley KS, Schenkein DP, Hezel AF, Ancukiewicz M, Liebman HM, Kwak EL, Clark JW, Ryan DP, Deshpande V, Dias-Santagata D, Ellisen LW, Zhu AX, Iafrate AJ. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist 2012;17:72-97.
114. Yang B, House MG, Guo M, Herman JG, Clark DP. Promoter methylation profiles of tumor suppressor genes in intrahepatic and extrahepatic cholangiocarcinoma. Mod Pathol 2005;18:412-20.
115. Oliveira DV, Zhang S, Chen X, Calvisi DF, Andersen JB. Molecular profiling of intrahepatic cholangiocarcinoma: the search for new therapeutic targets. Expert Rev Gastroenterol Hepatol 2017;11:349-56.
116. Rhee H, Ko JE, Chung T, Jee BA, Kwon SM, Nahm JH, Seok JY, Yoo JE, Choi JS, Thorgeirsson SS, Andersen JB, Lee HS, Woo HG, Park YN. Transcriptomic and histopathological analysis of cholangiolocellular differentiation trait in intrahepatic cholangiocarcinoma. Liver Int 2018;38:113-24.
117. Rizvi S, Gores GJ. Emerging molecular therapeutic targets for cholangiocarcinoma. J Hepatol 2017;67:632-44.
118. NakanumaY, SatoY. Cystic and papillary neoplasm involving peribiliary glands: a biliary counterpart of branch-type intraductal papillary mucinous [corrected] neoplasm? Hepatology 2012;55:2040-1.
119. Cardinale V, Wang Y, Carpino G, Mendel G, Alpini G, Gaudio E, Reid LM, Alvaro D. The biliary tree--a reservoir of multipotent stem cells. Nat Rev Gastroenterol Hepatol 2012;9:231-40.
120. Cardinale V, Renzi A, Carpino G, Torrice A, Bragazzi MC, Giuliante F, DeRose AM, Fraveto A, Onori P, Napoletano C, Franchitto A, Cantafora A, Grazi G, Caporaso N, D'Argenio G, Alpini G, Reid LM, Gaudio E, Alvaro D. Profiles of cancer stem cell subpopulations in cholangiocarcinomas. Am J Pathol 2015;185:1724-39.
121. Gandou C, Harada K, Sato Y, Igarashi S, Sasaki M, Ikeda H, Nakanuma Y. Hilar cholangiocarcinoma and pancreatic ductal adenocarcinoma share similar histopathologies, immunophenotypes, and development-related molecules. Hum Pathol 2013;44:811-21.
122. Nakanuma Y, Harada K, Sasaki M, Sato Y. Proposal of a new disease concept "biliary diseases with pancreatic counterparts." Anatomical and pathological bases. Histol Histopathol 2014;29:1-10.
123. Cardinale V, Gatto M, Alvaro D. Clinical features of intrahepatic cholangiocarcinoma. In: Brandi G, Ercolani G, editors. Cholangiocarcinoma. Nova Science Pub; 2015. pp. 125-34.
124. Rimola J, Forner A, Reig M, Vilana R, de Lope CR, Ayuso C, Bruix J. Cholangiocarcinoma in cirrhosis: absence of contrast washout in delayed phases by magnetic resonance imaging avoids misdiagnosis of hepatocellular carcinoma. Hepatology 2009;50:791-8.
125. Iavarone M, Piscaglia F, Vavassori S, Galassi M, Sangiovanni A, Venerandi L, Forzenigo LV, Golfieri R, Bolondi L, Colombo M. Contrast enhanced CT-scan to diagnose intrahepatic cholangiocarcinoma in patients with cirrhosis. J Hepatol 2013;58:1188-93.
126. Kim SJ, Lee JM, Han JK, Kim KH, Lee JY, Choi BI. Peripheral mass-forming cholangiocarcinoma in cirrhotic liver. AJR Am J Roentgenol 2007;189:1428-34.
127. Weber SM, Ribero D, O'Reilly EM, Kokudo N, Miyazaki M, Pawlik TM. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford) 2015;17:669-80.
128. Chen J, He J, Deng M, Wu HY, Shi J, Mao L, Sun Q, Tang M, Fan XS, Qiu YD, Huang Q. Clinicopathologic, radiologic, and molecular study of 23 combined hepatocellular-cholangiocarcinomas with stem cell features, cholangiolocellular type. Hum Pathol 2017;64:118-27.
129. Khan SA, Davidson BR, Goldin RD, Heaton N, Karani J, Pereira SP, Rosenberg WM, Tait P, Taylor-Robinson SD, Thillainayagam AV, Thomas HC, Wasan H, Gastroenterology British Society of. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut 2012;61:1657-69.
130. Bledsoe JR, Shinagare SA, Deshpande V. Difficult diagnostic problems in pancreatobiliary neoplasia. Arch Pathol Lab Med 2015;139:848-57.
131. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol 2009;51:237-67.
132. Lindor KD, Kowdley KV, Harrison ME, American College of Gastroenterology. ACG clinical guideline: primary sclerosing cholangitis. Am J Gastroenterol 2015;110:646-59; quiz 660.
133. Navaneethan U, Njei B, Lourdusamy V, Konjeti R, Vargo JJ, Parsi MA. Comparative effectiveness of biliary brush cytology and intraductal biopsy for detection of malignant biliary strictures: a systematic review and meta-analysis. Gastrointest Endosc 2015;81:168-76.
134. Arbelaiz A, Azkargorta M, Krawczyk M, Santos-Laso A, Lapitz A, Perugorria MJ, Erice O, Gonzalez E, Jimenez-Agüero R, Lacasta A, Ibarra C, Sanchez-Campos A, Jimeno JP, Lammert F, Milkiewicz P, Marzioni M, Macias RIR, Marin JJG, Patel T, Gores GJ, Martinez I, Elortza F, Falcon-Perez JM, Bujanda L, Banales JM. Serum extracellular vesicles contain protein biomarkers for primary sclerosing cholangitis and cholangiocarcinoma. Hepatology 2017;66:1125-43.
135. Alvaro D. The challenge of cholangiocarcinoma diagnosis: the turning point is in extracellular vesicles? Hepatology 2017;66:1029-31.
136. Primrose JN, Fox R, Palmer DH, Prasad R, Mirza D, Anthoney DA, Corrie P, Falk S, Wasan HS, Ross PJ, Wall LR, Wadsley J, Evans TRJ, Stocken D, Praseedom R, Cunningham D, Garden OJ, Stubbs C, Valle JW, Bridgewater JA. Adjuvant capecitabine for biliary tract cancer: the BILCAP randomized study. J Clin Oncol 2017;35 Suppl 15:abstr4006.
137. Javle M, Lowery M, Shroff RT, Weiss KH, Springfeld C, Borad MJ, Ramanathan RK, Goyal L, Sadeghi S, Macarulla T, El-Khoueiry A, Kelley RK, Borbath I, Choo SP, Oh DY, Philip PA, Chen LT, Reungwetwattana T, Van Cutsem E, Yeh KH, Ciombor K, Finn RS, Patel A, Sen S, Porter D, Isaacs R, Zhu AX, Abou-Alfa GK, Bekaii-Saab T. Phase II study of BGJ398 in patients with FGFR-altered advanced cholangiocarcinoma. J Clin Oncol 2018;36:276-82.
138. Bragazzi MC, Ridola L, Safarikia S, Matteo SD, Costantini D, Nevi L, Cardinale V. New insights into cholangiocarcinoma: multiple stems and related cell lineages of origin. Ann Gastroenterol 2018;31:42-55.
139. Rycaj K, Tang DG. Cell-of-origin of cancer versus cancer stem cells: assays and interpretations. Cancer Res 2015;75:4003-11.
141. Cardinale V, Wang Y, Carpino G, Reid LM, Gaudio E, Alvaro D. Mucin producing cholangiocarcinoma might derive from biliarytree stem/progenitor cells located in peribiliary glands. Hepatology 2012;55:2041-2.
142. Yamashita T, Wang XW. Cancer stem cells in the development of liver cancer. J Clin Invest 2013;123:1911-8.
143. Raggi C, Invernizzi P, Andersen JB. Impact of microenvironment and stem-like plasticity in cholangiocarcinoma: molecular networks and biological concepts. J Hepatol 2015;62:198-207.
144. Chen WT, Tseng CC, Pfaffenbach K, Kanel G, Luo B, Stiles BL, Lee AS. Liver-specific knockout of GRP94 in mice disrupts cell adhesion, activates liver progenitor cells, and accelerates liver tumorigenesis. Hepatology 2014;59:947-57.
145. Vander Borght S, Komuta M, Libbrecht L, Katoonizadeh A, Aerts R, Dymarkowski S, Verslype C, Nevens F, Roskams T. Expression of multidrug resistance-associated protein 1 in hepatocellular carcinoma is associated with a more aggressive tumour phenotype and may reflect a progenitor cell origin. Liver Int 2008;28:1370-80.
146. Govaere O, Komuta M, Berkers J, Spee B, Janssen C, de Luca F, Katoonizadeh A, Wouters J, van Kempen LC, Durnez A, Verslype C, De Kock J, Rogiers V, van Grunsven LA, Topal B, Pirenne J, Vankelecom H, Nevens F, van den Oord J, Pinzani M, Roskams T. Keratin 19: a key role player in the invasion of human hepatocellular carcinomas. Gut 2014;63:674-85.
147. Magee JA, Piskounova E, Morrison SJ. Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell 2012;21:283-96.
148. Moeini A, Sia D, Zhang Z, Camprecios G, Stueck A, Dong H, Montal R, Torrens L, Martinez-Quetglas I, Fiel MI, Hao K, Villanueva A, Thung SN, Schwartz ME, Llovet JM. Mixed hepatocellular cholangiocarcinoma tumors: Cholangiolocellular carcinoma is a distinct molecular entity. J Hepatol 2017;66:952-61.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Cardinale V, Bragazzi MC, Carpino G, Matteo SD, Overi D, Nevi L, Gaudio E, Alvaro D. Intrahepatic cholangiocarcinoma: review and update. Hepatoma Res 2018;4:20. http://dx.doi.org/10.20517/2394-5079.2018.46
AMA Style
Cardinale V, Bragazzi MC, Carpino G, Matteo SD, Overi D, Nevi L, Gaudio E, Alvaro D. Intrahepatic cholangiocarcinoma: review and update. Hepatoma Research. 2018; 4: 20. http://dx.doi.org/10.20517/2394-5079.2018.46
Chicago/Turabian Style
Cardinale, Vincenzo, Maria Consiglia Bragazzi, Guido Carpino, Sabina Di Matteo, Diletta Overi, Lorenzo Nevi, Eugenio Gaudio, Domenico Alvaro. 2018. "Intrahepatic cholangiocarcinoma: review and update" Hepatoma Research. 4: 20. http://dx.doi.org/10.20517/2394-5079.2018.46
ACS Style
Cardinale, V.; Bragazzi MC.; Carpino G.; Matteo SD.; Overi D.; Nevi L.; Gaudio E.; Alvaro D. Intrahepatic cholangiocarcinoma: review and update. Hepatoma. Res. 2018, 4, 20. http://dx.doi.org/10.20517/2394-5079.2018.46
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 39 clicks
Cite This Article 39 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.