A potential clinical based score in hepatitis C virus cirrhotic patients to exclude small hepatocellular carcinoma
Abstract
Aim: Hepatitis C virus (HCV) cirrhosis is an important cause of hepatocellular carcinoma (HCC). This study aimed to identify factors of HCC presence among HCV cirrhotic patients with and without small diameter HCC (≤ 3 cm).
Methods: A case control transversal study between 1998 and 2003 including 93 patients: 31 with small diameter HCC and 62 without HCC. Groups were matched by age and gender. Multiple logistic regression analysis using Akaike Information Criteria to estimate the probability of HCC was performed. A model score was generated and bootstrap analysis was performed for internal validation.
Results: Three significant laboratorial variables for HCC presence were found: alanine aminotransferase > 37 U/L [odds ratio (OR): 7.43 (1.61-34.19), P = 0.01], alpha-fetoprotein > 20 ng/mL [OR: 16.2 (4.17-63.01), P < 0.001] and platelet count < 100,000/mm3 [OR: 3.62 (1.43-9.14), P = 0.007]. A model score with an area under curve of 0.79 (95% CI: 0.7-0.89) was built based on these variables. The negative predictive value of those classified as at low risk of HCC was 99.1%.
Conclusion: An easy and practical model score was generated. It may be an auxiliary tool for identification of HCV patients with low probability of small diameter HCC at initial evaluation composed of three serum examinations used in routine outpatient clinical practice.
Keywords
Introduction
Hepatocellular carcinoma (HCC) represents more than 5% of all malignant tumors, and is the fifth most common cancer in men and the eighth in women. The prevalence of this cancer is expected to increase in the coming years[1-3]. HCC incidence varies greatly between geographical regions[4-7]. Hepatitis C virus (HCV) infection is typically prevalent in areas with low incidence (< 3 per 100,000) of HCC, as often found in developed countries. Japan is an exception to this, with 80% of HCC patients infected with HCV[6]. It is generally believed that the presence of cirrhosis and chronic HCV infection contribute to an increased risk of HCC[8]. Other potential underlying risk factors include gender (male), advanced age, hepatitis B virus co-infection, alcohol abuse, a history of blood transfusion, and diabetes[9].
Several cohort studies have shown that early HCC detection increases the potential for application of curative rather than palliative treatment. Screening strategies may allow earlier HCC diagnosis, with a potential positive impact on mortality[10,11]. The European and American guidelines recommend abdominal ultrasonography (US) every 6 months[12,13], but the recently updated Asia-Pacific guidelines, as well as other centers, recommend a combination of US and serum alpha-fetoprotein (AFP) measurement for HCC surveillance[14,15].
In Brazil, HCV is the main etiology of liver cirrhosis[16]. Among a 10-year cohort of 884 Brazilian cirrhotic patients, with almost 60% with HCV etiology, reported an incidence of HCC of 16.9% over 5 years[16]. Improvements in diagnostic imaging and routine surveillance programs have enabled the identification of small liver nodules, meaning that the majority of our HCC cases are now diagnosed in their early stages (80%)[17,18]. As a result, the prognosis for patients with HCC has improved considerably[10,11,19]. However, surveillance adherence rates for HCC are far from ideal in many settings[20]. Moreover, HCC rate detection may be lower outside specialized centers, and the diagnosis of small HCC (≤ 3 cm) can indeed be a challenge in clinical practice. Therefore, it is important to search for reliable markers for early detection or even exclusion of HCC with confidence, to assist in the management of these patients.
The aim of this study was to identify possible factors of HCC presence/absence by analyzing a set of patients with HCV-related cirrhosis, with and without small diameter HCC (≤ 3 cm).
Methods
We performed an observational case-control study in a cohort of HCV-related cirrhosis patients with and without small diameter HCC (≤ 3 cm). The STROBE statement for reporting observational studies was followed[21].
HCC patients
The study included 31 patients (20 male, 11 female) with HCV-related cirrhosis and HCC smaller than 3 cm, who were diagnosed and followed up at a tertiary healthcare center; the Department of Gastroenterology at the University of São Paulo School of Medicine, São Paulo, Brazil between 1998 and 2003. All patients on file eligible for inclusion in the HCC group were included. HCC diagnosis was based on one of the three following criteria: (1) biopsy and histological examination of the nodule; (2) nodules with arterial hyper vascularization and washout in at least two different dynamic imaging methods [abdominal computed tomography (CT) or magnetic resonance imaging (MRI)]; or (3) identification of a suspect growth in at least one dynamic imaging method along with serum AFP > 200 ng/mL.
All biopsies were performed with a 14G Tru-Cut® needle (Medical Technology, Gainsville, FL, USA) with ultrasound-guided puncture performed in the nodule and in the adjacent parenchyma. HCC was diagnosed in 12 (63.1%) of the 19 biopsies performed. The remaining seven cases were included based on the progressive increase of nodule size; with consequent better definition by imaging methods (5 cases) and/or increased AFP level (2 cases).
HCC diagnosis was made with imaging in 15 patients (48.3%) and histology in 12 patients (38.8%). A combination of imaging methods and AFP levels was applied in four cases (12.9%). All 31 patients presented up to three liver nodules smaller than 3 cm in total. Some nodules were detected as part of a screening program (55%) involving abdominal US and serum AFP monitoring every 6 months, while some were referrals from other centers with diagnoses of suspected HCC. The mean nodule size was 22 mm. All patients underwent a chest computerized tomography scan and a full-body bone scan to exclude the presence of metastatic HCC.
Control group
Sixty-two patients (40 male, 22 female) with hepatitis C-related cirrhosis, but without HCC were selected from the same tertiary care center. They were paired by age and gender with the HCC group. All patients in the control group were subjected to abdominal US 6 months after data collection, to ensure that HCC had not developed. These patients were systematically screened every 6 months for HCC with US and serum AFP measurements.
The following anthropometric and clinical variables were recorded and used to categorize the control group: age (> 60 years); gender (male/female); treatment with alpha-interferon (yes/no); previous participation in a screening program (yes/no); response to antiviral treatment (yes/no); Child-Pugh score (A/B/C); esophageal varices (yes/no); upper gastrointestinal (GI) bleeding (yes/no); ascites (yes/no); hepatic encephalopathy (yes/no); spontaneous bacterial peritonitis (SBP) (yes/no); weight loss (yes/no); alcohol consumption (yes/no) and abdominal pain (yes/no).
The following serum markers were examined: AFP (≥ 20 ng/mL), total bilirubin (Bil) (> 10 ng/dL), aspartate aminotransferase (AST) (> 41 U/L), alanine aminotransferase (ALT) (> 37 U/L), alkaline phosphatase (AP) (> 129 U/L), gamma-glutamyl transpeptidase (GGT) (> 61 U/L), transferin saturation (> 40%), ferritin (> 150 ng/mL), international normalized ratio (INR) (> 1.20), platelet count (< 100,000/mm3), albumin (< 3.4 g/dL), fibrinogen (< 150 mg/dL), glycemia (> 110 mg/dL). We additionally recorded a descriptive analysis of the HCC histological type as well-, moderately- or poorly-differentiated. Of the 12 histologically confirmed tumors, 11 were moderately-differentiated, and only 1 was well-differentiated, while none were poorly differentiated.
This study was approved by the Institutional Review Board, fulfilling all of the requirements for retrospective studies in human subjects, according to the guidelines of the 1975 Helsinki Declaration.
Statistical analysis
Quantitative variables are presented as median, first quartile and third quartile, and qualitative variables as percentages. Differences between groups (presence/absence of HCC) regarding continuous variables were verified via the Mann-Whitney test and association between categorized variables were checked by Fisher’s test. P-values smaller than 0.05 were considered statistically significant.
Receiver operator curve (ROC) curve was applied to all continuous variables, and cutoff values were selected to maximize the Youden index (MaxSe and MaxSp)[22]. Simple and multivariable logistic regressions were performed to predict HCC presence. Akaike Information Criterion (AIC)[23] was used to select the most informative variables in the backward strategy. Patients with missing data in a specific variable were excluded from the analysis of that variable.
Finally, linear predictors from multiple regressions were resized to a range from 0 to 100, and then a cutoff value was determined by a ROC curve. Performance measures given by sensitivity (Se), specificity (Sp), positive (PPV) and negative (NPV) predicted values were calculated based on a HCC yearly prevalence of 3% (Brazil)[16] and 10% (Japan)[24] and the performance of the model was further analyzed with the bootstrap method[25] with 1000 samples used to estimate the internal validity of performance measures. The R Project for Statistical Computing ver. 3.0.2 (R Core Team, Vienna, Austria, 2014) software package was used for statistical analyses.
Results
We evaluated 93 patients with HCV-related cirrhosis, 31 of which with small HCC and 62 without HCC. Table 1 shows the frequencies and percentages of clinical and laboratory variables of the HCC and control groups. The median age in both groups was 59 years old, the majority were male, and had preserved liver function (Child-Pugh A). No differences between groups could be detected regarding liver related outcomes such as ascites (P = 0.18), spontaneous bacterial peritonitis (P = 1.0), esophageal varices (P = 1.0), variceal bleeding (P = 1.0) or hepatic encephalopathy (P = 0.817).
On the other hand, patients with HCC had higher levels of AFP [10.9 (4.75-45.3) vs. 4.95 (2.92-8.3) ng/mL, P < 0.001], AST [91 (62.5-117) vs. 53.5 (39-84) U/L, P = 0.002], ALT [70 (55.5-110) vs. 47 (30.5-74.5) U/L, P = 0.002], and were less likely to have participated in a screening program (54.84% vs. 95.16%, P < 0.001) than patients in the control group. Furthermore, HCC patients had a lower platelet count than their counterparts in the control group (83.9 vs. 118.5 × 10³ × mm³, P = 0.02), as shown in Table 1.
Descriptive analysis of frequencies and percentages of clinical and laboratory variables of the 93 patients HCV-related cirrhosis patients
| Control (n = 62) | Case (n = 31) | P value | |
|---|---|---|---|
| Gender (male), n (%) | 40 (64.52) | 20 (64.52) | 1 |
| Age (year), median (min-max) | 59 (52.25-66.75) | 59 (52.5-66) | 0.952 |
| AFP (ng/mL) | 4.95 (2.92-8.3) | 10.9 (4.75-45.3) | < 0.001 |
| Bil (mg/dL) | 1.3 (0.82-2.1) | 1.4 (1.05-2) | 0.508 |
| AST (U/L) | 53.5 (39-84) | 91 (62.5-117) | 0.002 |
| ALT (U/L) | 47 (30.5-74.5) | 70 (55.5-110) | 0.002 |
| GGT (U/L) | 50.5 (34-113) | 78 (50.5-188.5) | 0.071 |
| AP (U/L) | 99.5 (80.5-131.75) | 111 (79-136) | 0.496 |
| INR | 1.27 (1.16-1.36) | 1.24 (1.15-1.53) | 0.883 |
| Platelet count (103 × mm3) | 118.5 (68.75-158) | 83.9 (63.75-104.5) | 0.02 |
| Transferin saturation (%) | 44 (28-58) | 44 (30-61.25) | 0.955 |
| Ferritin (ng/mL) | 78.5 (23-258.5) | 325 (140.25-500.5) | 0.199 |
| Albumin (g/dL) | 3.65 (3.37-4) | 3.61 (3.32-3.9) | 0.302 |
| Glucose (mg/dL) | 97 (88-130) | 99 (87.5-108.5) | 0.526 |
| Fibrinogen (mg/dL) | 214 (178-271.5) | 157 (126-190) | 0.04 |
| Screening (%) | 59 (95.16) | 17 (54.84) | < 0.001 |
| Ascites (%) | 22 (35.48) | 16 (51.61) | 0.18 |
| SBP (%) | 1 (1.61) | 1 (3.23) | 1 |
| Variceal bleeding (%) | 7 (11.29) | 4 (12.9) | 1 |
| Esophageal varices (%) | 42 (67.74) | 20 (64.52) | 0.817 |
| Encephalopathy (%) | 7 (11.29) | 4 (12.9) | 1 |
| Abdominal pain (%) | 1 (2.44) | 0 (0) | 1 |
| Weight loss (%) | 5 (12.2) | 1 (3.23) | 0.227 |
| Child-Pugh A/B/C (%) | 44 (70.97) | 17 (54.84) | 0.139 |
| 17 (27.42) | 12 (38.71) | ||
| 1 (1.61) | 2 (6.45) | ||
| Alcohol consumption (%) | 16 (25.81) | 8 (25.81) | 1 |
| Alpha-interferon therapy (%) | 42 (67.74) | 19 (61.29) | 0.644 |
| Treatment response (%) | 10 (23.81) | 0 (0) | 0.056 |
Among HCC patients, 19 (61%) were subjected to antiviral treatment with alpha-interferon, to which none of them responded. However, among the control group, 42 (68%) were subjected to antiviral treatment, and 10 (24%) of these patients achieved sustained virological response (SVR) (P = 0.05).
On multivariate logistic regression [Table 2], higher AFP levels (> 20 ng/mL, P < 0.001), higher ALT levels (> 37 U/L, P = 0.01) and lower platelet count (< 100,000/mm3, P = 0.007) were independent prediction factors of HCC presence, with odds ratios of 16.2 (4.17-63.01), 7.43 (1.61-34.19) and 3.62 (1.43-9.14), respectively.
Odds ratio of risk factors for HCC presence on a multivariate logistic regression analysis
| Group | OR (95% CI) | P value | |
|---|---|---|---|
| Gender | Male | 1 (0.41-2.46) | 1 |
| Age (years) | > 60 | 1.07 (0.44-2.6) | 0.88 |
| AFP (ng/mL) | > 20 | 16.2 (4.17-63.01) | < 0.001 |
| Bil (mg/dL) | > 1.0 | 1.58 (0.61-4.12) | 0.349 |
| AST (U/L) | > 41 | 3.53 (0.95-13.13) | 0.06 |
| ALT (U/L) | > 37 | 7.43 (1.61-34.19) | 0.01 |
| GGT (U/L) | > 61 | 2.65 (0.61-11.43) | 0.192 |
| AP (U/L) | > 129 | - | 0.995 |
| INR | > 1.20 | 0.87 (0.36-2.12) | 0.761 |
| Platelet count (/mm3) | < 100,000 | 3.62 (1.43-9.14) | 0.007 |
| Transferin saturation | > 40% | 0.97 (0.34-2.73) | 0.954 |
| Ferritin (ng/mL) | > 150 | 1.8 (0.3-10.91) | 0.522 |
| Albumin (g/dL) | < 3.4 | 1.46 (0.58-3.67) | 0.425 |
| Glucose (mg/dL) | > 110 | 0.58 (0.2-1.65) | 0.304 |
| Fibrinogen (ng/mL) | < 150 | 1.46 (0.58-3.67) | 0.425 |
| Screening | Yes | 0.06 (0.02-0.24) | < 0.001 |
| Ascites | Yes | 1.94 (0.81-4.66) | 0.138 |
| SBP | Yes | 2.03 (0.12-33.67) | 0.62 |
| Variceal bleeding | Yes | 1.16 (0.31-4.32) | 0.821 |
| Esophageal varices | Yes | 0.87 (0.35-2.15) | 0.756 |
| Encephalopathy | Yes | 1.16 (0.31-4.32) | 0.821 |
| Abdominal pain | Yes | - | 0.992 |
| Weight loss | Yes | 0.24 (0.03-2.17) | 0.204 |
| Child | B | 1.83 (0.72-4.62) | 0.203 |
| C | 5.18 (0.44-60.93) | 0.191 | |
| Alcohol consumption | Yes | 1 (0.37-2.68) | 1 |
| Alpha-Interferon therapy | Yes | 0.75 (0.31-1.85) | 0.538 |
| Treatment response | No | 1.02 (0.3-3.45) | 0.98 |
| Yes | - | 0.993 |
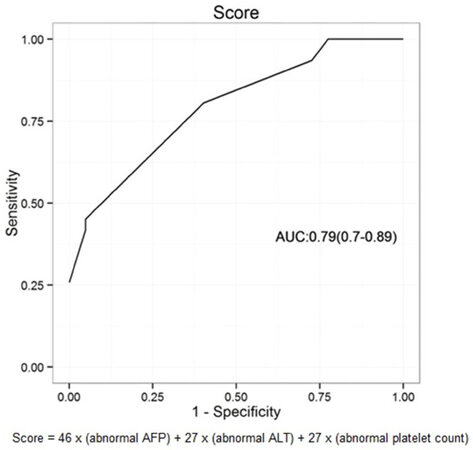
The coefficients of the multivariable model are 3.71 ± 1, 2.96 ± 0.77, 1.72 ± 0.9, 1.7 ± 0.62 for the intercept, AFP > 20, ALT > 37 and platelet count < 100,000. These variables were applied to build a score capable of discriminating higher risk of HCC in HCV cirrhotic patients, with an area under curve (AUC) of 0.79 (95% CI: 0.7-0.89) [Figure 1].
Figure 1. Receiver operator curve analysis of calculated model score for identifying hepatocellular carcinoma. ALT: alanine aminotransferase; AFP: alpha-fetoprotein; AUC: area under curve
Based on the findings, we propose a model score to apply to outpatients with HCV related cirrhosis, but without tumors or nodules on US or CT/MRI images undertaken during routine surveillance:
HCC Risk Score in HCV patients with cirrhosis = 46 × (abnormal AFP) + 27 × (abnormal ALT) + 27 × (abnormal platelet count)
This formula requires the knowledge of the range and limits of the normal values of the aforementioned variables. For example, if AFP > 20 ng/dL, it is considered abnormal, and the score attributable to this variable is 1 (1), but if it ≤ 20 ng/dL its score is 0. Similarly, if the ALT is > 37 U/L, it is considered abnormal, and the score is 1 (1), and finally a platelet count < 100,000/mm3 is considered an abnormal value, and its score is 1 (1).
Three cut-off levels of the model score were considered, and bootstrap analysis was applied to determine the optimal values for sensitivity and specificity [Table 3], since the diagnostic measures were calculated based on internal validation. The cut-off level with the best sensitivity and specificity was 54, with a sensitivity of 81% (64%-94%) and a specificity of 60% (47%-71%). In the scenario of 3% prevalence of HCC risk in HCV cirrhotic patients the best cut off value to exclude HCC is 26 [sensitivity = 100% (89%-100%); specificity = 23% (14%-34%); PPV = 3.7%; NPV = 99.1%], and the best cut off to include HCC is 100 [sensitivity = 26% (14%-43%); specificity = 100% (94%-100%); PPV = 44.6%; NPV = 97.7%]. When we changed the scenario prevalence to 10%, the results show better performances from the positive predictive values, from 5.8% to 18% at a cut off level of 54, from 44.6% to 74.2% at cut off level of 100, and from 3.7% to 12.2% at cut off level of 26.
Discrimination measurements for development of the model score with different prevalent risk HCC scenario (3% and 10%) and results of its internal validation
| Estimate (95% CI) | Optimism | |
|---|---|---|
| General cut off: 54 | ||
| Se | 81% (64%-94%) | −13.1% |
| Sp | 60% (47%-71%) | 6.4% |
| Prevalence scenario | 3% | 10% |
| PPV | 5.8% | 18% |
| NPV | 98.5% | 95% |
| Excluding cut off: 26 | ||
| Se | 100 % (89%-100%) | −7.5% |
| Sp | 23% (14%-34%) | 13% |
| Prevalence scenario | 3% | 10% |
| PPV | 3.7% | 12.2% |
| NPV | 99.1% | 96.9% |
| Including cut off: 100 | ||
| Se | 26% (14%-43%) | −2.6% |
| Sp | 100% (94%-100%) | 0 |
| Prevalence scenario | 3% | 10% |
| PPV | 44.6% | 74.2% |
| NPV | 97.7% | 92.3% |
Discussion
This case control study analyzed clinical and laboratory parameters used in routine daily practice, aiming to identify patients with HCV-related cirrhosis at increased risk of HCC presence. We found that higher serum AFP and ALT levels, and lower platelet count were independent prediction factors of HCC. Such information could be used to develop more cost-effective screening strategies.
The median age in both groups was 59 years old. Velázquez et al.[26] demonstrated that an age of ≥ 55 years is an independent risk factor for HCC among patients with cirrhosis and HCV. Other published data suggest a higher incidence of HCC from the age of 60[6]. Lok et al.[27] also found that older age is a predictive factor for HCC development. The HCC group (31 patients) had male:female ratio of 1.8:1; this finding is consistent with data from the literature showing that the prevalence of HCC is 2 to 4 times higher in male patients[24].
We found no differences in liver related outcomes, such as ascites, spontaneous bacterial peritonitis, esophageal varices, variceal bleeding or hepatic encephalopathy between groups. This suggests that HCC does not alter the pathogenesis of the early clinical stages of HCV-related cirrhosis in more advanced stages. A previous study showed that hepatic encephalopathy and ascites were not related to the development of HCC, although esophageal varices were[28]. The latter was also observed by Lok et al.[27]. Bolondi et al.[29] assessed the cost-effectiveness of HCC screening by comparing 313 patients with cirrhosis and 104 patients with cirrhosis and HCC, and identified the functional classes Child-Pugh B and C as independent risk factors for HCC. Our results are different, possibly due to the small number of patients and also because most of them had preserved liver function (Child-Pugh A). However they do point to the need for identifying multiple risk factors, beyond the clinical stage of cirrhosis to allow earlier identification of risk. This is of great importance in improving the management and prognosis of patients with HCC.
Sustained virological response (SVR) occurred in 24% of the control group, while no patients in the HCC group exhibited SVR. Several studies have demonstrated the beneficial impact of HCV clearance with interferon in reducing HCC occurrence[30]. In a multiple logistic regression analysis, AFP, ALT and platelet count were related to higher risk of HCC. In our previous cohort study of patients with cirrhosis, we found the following risk factors for HCC; AFP > 20 ng/mL, albumin < 3.4 g/dL and patients of East Asian ethnicity as the best of seven possible models applied to predict HCC risk[16]. In the present study AFP > 20 ng/mL was confirmed as a predictive risk factor for the presence of HCC. The diagnostic importance of AFP has been the subject of much scientific debate in recent years. In some studies, a high base value of AFP has been considered a risk factor for HCC, with a cut-off level of 20 ng/mL for determining groups of high and low risk[29]. AFP levels above 400 ng/mL in the presence of a hepatic nodule in imaging finding, is a conclusive HCC diagnosis[28]. However, small HCC tumors (< 2 cm) involve low-level secretion of AFP and thus, in most cases the patients cannot be diagnosed using this test alone[31]. In a prospective study, Tong et al.[32] analyzed 31 patients with cirrhosis and hepatitis B virus or HCV who had developed HCC; they found AFP values above 400 ng/mL in only 4(13%). It is important to note that the AFP levels may be higher in individuals with chronic viral hepatitis (B or C), but without HCC compared with similar patients with other etiologies of cirrhosis. This is caused by the inflammatory activity and hepatocyte regeneration in the most severe cases of viral hepatitis. Gupta et al.[31] conducted a systematic review evaluating AFP as an instrument for the detection of HCC in patients with hepatitis C; they concluded that AFP has limited utility in this setting. Most authors have found that an isolated measurement of serum AFP levels had limited success for early HCC screening[14,33], but even small changes in AFP levels may be a predictor for HCC[34,35]. In fact, dynamic AFP measurement could identify patients at higher risk of HCC occurrence, as recently shown by Bird et al.[36].
Early HCC detection remains challenging, but novel serum biomarkers are under evaluation, such as microRNAs (miRNAs)[37,38], creatine/betaine ratio[39], the combination of chaperonin containing TCP1 complex (CCT) and IQ-motif-containing GTPase-activating protein-3 (IQGAP3)[40] and circulating c-Myc and p53 proteins[41].
The lower blood platelet count in HCC patients can be explained by a longer evolution of chronic liver disease with subsequent advanced portal hypertension and hypersplenism. Velázquez et al.[26] showed that platelet count < 75,000/mm3 was an independent positive predictive value for HCC development. In this analysis, the cut-off level for platelet count was 100,000/mm3 according to previously defined levels[42,43]. Lok et al.[27] also demonstrated the association of HCC risk with low platelet count through the HALT-C study cohort. In a recent prospective study of the ANRS CO12 CirVir cohort including 1323 patients with HCV cirrhosis, Ganne-Carrié et al.[44] found five variables independently associated with HCC development at 1, 3, and 5 years: age > 50 years, past excessive alcohol intake, GGT above the upper limit of normal, absence of SVR during follow-up and platelets < 100,000/mm3. The latter was also evidenced in our work and in the retrospective study by Noh et al.[45] as a predictor of HCC.
This study found that serum levels of ALT, AFP and platelet count could be used to determine the risk of small HCC with a sensitivity of 81% and specificity of 60%. The major strength of this formula is the tests are easy to apply, and the score is simple to calculate. Therefore, this model is an auxiliary tool for identification of patients with HCV at elevated risk of HCC by applying a formula with three serum exams used in routine outpatient clinical practice throughout the world. An even better application of the aforementioned model would be to rule out the presence of small HCC in the initial evaluation of the patient, since the negative predictive value was 99.1% for those stratified as low risk (a score of 26). For example, in a patient with HCV and cirrhosis, the presence of two abnormal variables, imply a higher risk of HCC with a score of 54. In another hypothetical scenario with a patient score of 26, due to no abnormal variables, the patient could be excluded from the high risk group. For maximization of the specificity of the model score, the cut-off of 100 reflects, for instance, the three abnormal variables. We tested the score performance based on a HCC prevalence of 3% (Brazil) and in another scenario with an HCC prevalence of 10% (Japan), showing that the higher the HCC prevalence, better the score performs in identifying individuals with HCC. Recently, El-Serag et al.[34] proposed models to predict HCC risk with the same variables we found (AFP > 20 ng/mL, platelets < 100,000/mm3 and higher ALT) from the analysis of the change in AFP values according to HCC development. Flemming et al.[46] evaluated a risk model using six baseline clinical variables, including age, diabetes, gender, ethnicity, etiology of cirrhosis, and severity of liver dysfunction independently associated with HCC occurrence. The authors showed C-indices of 0.704 and 0.691 in the derivation and internal validation cohorts, respectively[46]. By comparison, the score proposed in this paper achieved a C-index of 0.79 (0.7-0.89). Attallah et al.[47] reported the simplified HCC-ART score for HCC detection in chronic hepatitis C patients from Egypt based on age, AFP, AST/ALT ratio, albumin and alkaline phosphatase. The AUROC curve for discriminating patients with HCC (n = 227) from those with liver cirrhosis (n = 341) was 0.95. Like our work, they used easily obtainable laboratory tests.
Our study is somewhat limited by the fact that the model score was developed only on a Brazilian HCV population between ages of 38 and 77 years, and still requires external validation with other etiologies, but a bootstrap internal validation was applied and we accessed the optimal diagnostic measures such that our model score is still useful, practical, readily available and easy to apply in primary or tertiary health centers in developing countries.
In conclusion, a score model was created from the results of the case control study based on serum levels of ALT, AFP and platelet count. This score facilitates the identification of patients with small diameter HCC (≤ 3 cm), and mainly those at lowest risk of its presence in the absence of ALT, AFP and platelet count alterations in the thresholds defined in this study. The score is not intended to predict HCC development. Instead, its strength is to rule out small HCC in HCV cirrhotic patients, considering that the negative predictive value of those classified as low risk of HCC presence was 99.1%. This information may assist screening strategies in the population of patients with HCV-related cirrhosis. Further studies in other populations, including non-HCV related cirrhosis are needed to address its role in HCC detection.
Declarations
Authors’ contributionsDesigned and performed the research: Paranaguá-Vezozzo DC, Matielo CEL
Analyzed data and wrote the article: Paranaguá-Vezozzo DC, de Campos Mazo DF
Revised the article critically: Nacif LS, Pessoa MG, Pereira GLR, de Lima RGR, Zitelli PMY, Ono SK, Carrilho FJ
Approved the final version of the manuscript: all authors
Data source and availabilityThe relevant raw data from this study can be available upon request for non-commercial purpose to the corresponding author.
Financial support and sponsorshipThis study was in part supported by Alves de Queiroz Family Fund for Research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interestThe authors declare no conflicts of interest in this work.
Patient consentInformed consent was obtained.
Ethics approvalThis study was approved by the Institutional Review Board fulfilling all of the requirements for retrospective studies in humans, according to the guidelines of the 1975 Helsinki Declaration.
Copyright© The Author(s) 2018.
REFERENCES
2. Yang JD, Roberts LR. Hepatocellular carcinoma: a global view. Nat Rev Gastroenterol Hepatol 2010;7:448-58.
3. Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist 2010;15:5-13.
4. Ahmed F, Perz JF, Kwong S, Jamison PM, Friedman C, Bell BP. National trends and disparities in the incidence of hepatocellular carcinoma, 1998-2003. Prev Chronic Dis 2008;5:A74.
5. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108.
6. Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology 2004;127:S5-16.
7. El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007;132:2557-76.
8. Fattovich G, Giustina G, Degos F, Tremolada F, Diodati G, Almasio P, Nevens F, Solinas A, Mura D, Brouwer JT, Thomas H, Njapoum C, Casarin C, Bonetti P, Fuschi P, Basho J, Tocco A, Bhalla A, Galassini R, Noventa F, Schalm SW, Realdi G. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology 1997;112:463-72.
9. Liang TJ, Heller T. Pathogenesis of hepatitis C-associated hepatocellular carcinoma. Gastroenterology 2004;127:S62-71.
10. El-Serag HB, Kramer JR, Chen GJ, Duan Z, Richardson PA, Davila J. Effectiveness of AFP and ultrasound tests on hepatocellular carcinoma mortality in HCV-infected patients in the USA. Gut 2011;60:992-7.
11. Mittal S, Kanwal F, Ying J, Chung R, Sada YH, Temple S, Davila JA, El-Serag HB. Effectiveness of surveillance for hepatocellular carcinoma in clinical practice: a United States cohort. J Hepatol 2016;65:1148-54.
12. European Organisation for Research and Treatment of Cancer; European Association for the Study of the Liver. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-43.
13. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358-80.
14. Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 2017;11:317-70.
15. Singal A, Volk ML, Waljee A, Salgia R, Higgins P, Rogers MA, Marrero JA. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther 2009;30:37-47.
16. Carrilho FJ, Kikuchi L, Branco F, Goncalves CS, Mattos AA; Brazilian HCC Study Group. Clinical and epidemiological aspects of hepatocellular carcinoma in Brazil. Clinics (Sao Paulo) 2010;65:1285-90.
17. Paranaguá-Vezozzo DC, Ono SK, Alvarado-Mora MV, Farias AQ, Cunha-Silva M, França JI, Alves VA, Sherman M, Carrilho FJ. Epidemiology of HCC in Brazil: incidence and risk factors in a ten-year cohort. Ann Hepatol 2014;13:386-93.
18. Paranagua-Vezozzo DC, Matielo CE, Chagas AL, Kikuchi LO, Mello ES, Alves VA, Ono-Nita SK, Carrilho FJ. Incidence of hepatocellular carcinoma in cirrhotic patients in Sao Paulo, Brazil. Hepatology 2006;44:A504-5.
19. van Meer S, de Man RA, Coenraad MJ, Sprengers D, van Nieuwkerk KM, Klümpen HJ, Jansen PL, IJzermans JN, van Oijen MG, Siersema PD, van Erpecum KJ. Surveillance for hepatocellular carcinoma is associated with increased survival: results from a large cohort in the Netherlands. J Hepatol 2015;63:1156-63.
20. Goldberg DS, Taddei TH, Serper M, Mehta R, Dieperink E, Aytaman A, Baytarian M, Fox R, Hunt K, Pedrosa M, Pocha C, Valderrama A, Kaplan DE. Identifying barriers to hepatocellular carcinoma surveillance in a national sample of patients with cirrhosis. Hepatology 2017;65:864-74.
21. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453-7.
23. Burnham KP, Anderson DR. Model selection and multi-model inference: a practical information-theoretic approach. New York: Springer-Verlag; 2002.
24. Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 2004;127:S35-50.
25. Efron B. Estimating the error rate of a prediction rule: improvement on cross-validation. J Am Statist Assoc 1983;78:316-31.
26. Velázquez RF, Rodríguez M, Navascués CA, Linares A, Pérez R, Sotorríos NG, Martínez I, Rodrigo L. Prospective analysis of risk factors for hepatocellular carcinoma in patients with liver cirrhosis. Hepatology 2003;37:520-7.
27. Lok AS, Seeff LB, Morgan TR, di Bisceglie AM, Sterling RK, Curto TM, Everson GT, Lindsay KL, Lee WM, Bonkovsky HL, Dienstag JL, Ghany MG, Morishima C, Goodman ZD; HALT-C Trial Group. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology 2009;136:138-48.
29. Bolondi L, Sofia S, Siringo S, Gaiani S, Casali A, Zironi G, Piscaglia F, Gramantieri L, Zanetti M, Sherman M. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost effectiveness analysis. Gut 2001;48:251-9.
30. Messori A, Badiani B, Trippoli S. Achieving sustained virological response in hepatitis C reduces the long-term risk of hepatocellular carcinoma: an updated meta-analysis employing relative and absolute outcome measures. Clin Drug Investig 2015;35:843-50.
31. Gupta S, Bent S, Kohlwes J. Test characteristics of alpha-fetoprotein for detecting hepatocellular carcinoma in patients with hepatitis C. A systematic review and critical analysis. Ann Intern Med 2003;139:46-50.
32. Tong MJ, Blatt LM, Kao VW. Surveillance for hepatocellular carcinoma in patients with chronic viral hepatitis in the United States of America. J Gastroenterol Hepatol 2001;16:553-9.
33. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2.
34. El-Serag HB, Kanwal F, Davila JA, Kramer J, Richardson P. A new laboratory-based algorithm to predict development of hepatocellular carcinoma in patients with hepatitis C and cirrhosis. Gastroenterology 2014;146:1249-55.
35. Richardson P, Duan Z, Kramer J, Davila JA, Tyson GL, El-Serag HB. Determinants of serum alpha-fetoprotein levels in hepatitis C-infected patients. Clin Gastroenterol Hepatol 2012;10:428-33.
36. Bird TG, Dimitropoulou P, Turner RM, Jenks SJ, Cusack P, Hey S, Blunsum A, Kelly S, Sturgeon C, Hayes PC, Bird SM. Alpha-fetoprotein detection of hepatocellular carcinoma leads to a standardized analysis of dynamic AFP to improve screening based detection. PLoS One 2016;11:e0156801.
37. Lu CY, Lin KY, Tien MT, Wu CT, Uen YH, Tseng TL. Frequent DNA methylation of MiR-129-2 and its potential clinical implication in hepatocellular carcinoma. Genes Chromosomes Cancer 2013;52:636-43.
38. Amr KS, Ezzat WM, Elhosary YA, Hegazy AE, Fahim HH, Kamel RR. The potential role of miRNAs 21 and 199-a in early diagnosis of hepatocellular carcinoma. Gene 2016;575:66-70.
39. Zeng J, Huang X, Zhou L, Tan Y, Hu C, Wang X, Niu J, Wang H, Lin X, Yin P. Metabolomics identifies biomarker pattern for early diagnosis of hepatocellular carcinoma: from diethylnitrosamine treated rats to patients. Sci Rep 2015;5:16101.
40. Qian EN, Han SY, Ding SZ, Lv X. Expression and diagnostic value of CCT3 and IQGAP3 in hepatocellular carcinoma. Cancer Cell Int 2016;16:55.
41. Attallah AM, El-Far M, Abdelrazek MA, Omran MM, Attallah AA, Elkhouly AA, Elkenawy HM, Farid K. Combined use of nuclear phosphoprotein c-Myc and cellular phosphoprotein p53 for hepatocellular carcinoma detection in high-risk chronic hepatitis C patients. Br J Biomed Sci 2017;74:170-5.
42. Sato T, Tateishi R, Yoshida H, Ohki T, Masuzaki R, Imamura J, Goto T, Kanai F, Obi S, Kato N, Shiina S, Kawabe T, Omata M. Ultrasound surveillance for early detection of hepatocellular carcinoma among patients with chronic hepatitis C. Hepatol Int 2009;3:544-50.
43. Masuzaki R, Tateishi R, Yoshida H, Goto E, Sato T, Ohki T, Imamura J, Goto T, Kanai F, Kato N, Ikeda H, Shiina S, Kawabe T, Omata M. Prospective risk assessment for hepatocellular carcinoma development in patients with chronic hepatitis C by transient elastography. Hepatology 2009;49:1954-61.
44. Ganne-Carrié N, Layese R, Bourcier V, Cagnot C, Marcellin P, Guyader D, Pol S, Larrey D, de Lédinghen V, Ouzan D, Zoulim F, Roulot D, Tran A, Bronowicki JP, Zarski JP, Riachi G, Calès P, Péron JM, Alric L, Bourlière M, Mathurin P, Blanc JF, Abergel A, Serfaty L, Mallat A, Grangé JD, Attali P, Bacq Y, Wartelle C, Dao T, Benhamou Y, Pilette C, Silvain C, Christidis C, Capron D, Bernard-Chabert B, Zucman D, Di Martino V, Trinchet JC, Nahon P, Roudot-Thoraval F; ANRS CO12 CirVir Study Group. Nomogram for individualized prediction of hepatocellular carcinoma occurrence in hepatitis C virus cirrhosis (ANRS CO12 CirVir). Hepatology 2016;64:1136-47.
45. Noh R, Lee DH, Kwon BW, Kim YH, Kim SB, Song IH. Clinical impact of viral load on the development of hepatocellular carcinoma and liver-related mortality in patients with hepatitis C virus infection. Gastroenterol Res Pract 2016;2016:7476231.
46. Flemming JA, Yang JD, Vittinghoff E, Kim WR, Terrault NA. Risk prediction of hepatocellular carcinoma in patients with cirrhosis: the ADRESS-HCC risk model. Cancer 2014;120:3485-93.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Paranaguá-Vezozzo DC, Matielo CEL, Mazo DFC, Nacif LS, Pessoa MG, Pereira GLR, Lima RGR, Zitelli PMY, Ono SK, Carrilho FJ. A potential clinical based score in hepatitis C virus cirrhotic patients to exclude small hepatocellular carcinoma. Hepatoma Res 2018;4:11. http://dx.doi.org/10.20517/2394-5079.2018.17
AMA Style
Paranaguá-Vezozzo DC, Matielo CEL, Mazo DFC, Nacif LS, Pessoa MG, Pereira GLR, Lima RGR, Zitelli PMY, Ono SK, Carrilho FJ. A potential clinical based score in hepatitis C virus cirrhotic patients to exclude small hepatocellular carcinoma. Hepatoma Research. 2018; 4: 11. http://dx.doi.org/10.20517/2394-5079.2018.17
Chicago/Turabian Style
Paranaguá-Vezozzo, Denise Cerqueira, Celso Eduardo Lourenço Matielo, Daniel Ferraz de Campos Mazo, Lucas Souto Nacif, Mario Guimaraes Pessoa, Gleicy Luz Reinoso Pereira, Roque Gabriel Rezende de Lima, Patricia Momoyo Yoshimura Zitelli, Suzane Kioko Ono, Flair José Carrilho. 2018. "A potential clinical based score in hepatitis C virus cirrhotic patients to exclude small hepatocellular carcinoma" Hepatoma Research. 4: 11. http://dx.doi.org/10.20517/2394-5079.2018.17
ACS Style
Paranaguá-Vezozzo, DC.; Matielo CEL.; Mazo DFC.; Nacif LS.; Pessoa MG.; Pereira GLR.; Lima RGR.; Zitelli PMY.; Ono SK.; Carrilho FJ. A potential clinical based score in hepatitis C virus cirrhotic patients to exclude small hepatocellular carcinoma. Hepatoma. Res. 2018, 4, 11. http://dx.doi.org/10.20517/2394-5079.2018.17
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 2 clicks
Cite This Article 2 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.