Hepatocellular carcinoma and type 2 diabetes mellitus: cytokeratin 8/18 expression in hepatocellular carcinoma and glycogen-storing hepatocytes
Sir,
We have reported two patients with hepatocellular carcinoma (HCC) and type 2 diabetes mellitus (T2DM), who showed abundant glycogen in their liver parenchyma but a marked reduction of glycogen content in HCC.[1] It was suggested that the latter was associated with appearance of a Warburg type glycolysis[1] and discussed in some detail.[2]
Cytokeratins (CKs), the intermediate filament (IF) proteins of epithelia, are sub-divided into type I (CK9-20) and type II (CK1-8) and expressed as type I/II pairs in a cell differentiation manner. In adult liver, hepatocyte IF comprise only CK8/18.[3] CK8/18 expression in normal and diseased liver has been reported, including positive expression in alcoholic steatohepatitis (ASH) and/or non-alcoholic steatohepatitis (NASH) and HCC.[3]
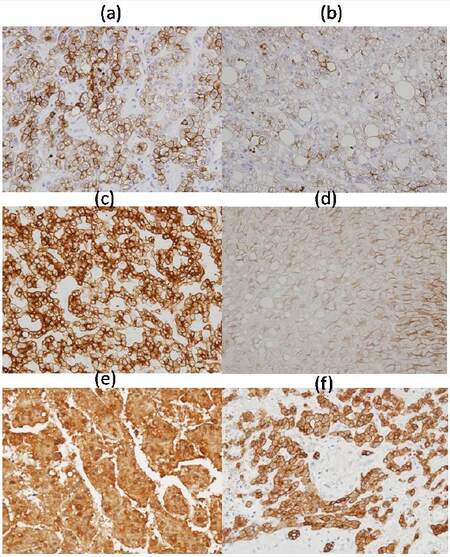
We examined the expression of CK8/18 in the liver to investigate cytoskeletal alterations in hepatocytes, possibly related to changes in hepatocellular glycogen content during hepatocarcinogenesis. Our studies revealed that immunoreactivity for CK8/18 was reduced or frequently even negative in glycogen-rich hepatocytes of background liver [Figure 1b and d], but moderately positive in normal hepatocytes and glycogen-poor cells in HCC [Figure 1a, c, e and f]. Overexpression of CK8/18, as Malory Denk bodies, which are hallmark lesions in ASH and NASH,[3] was not detected [Figure 1b and d]. The results provide evidence for reduced to negative CK8/18 expression in glycogen-rich hepatocytes.
Figure 1. CK8/18 expression in hepatocellular carcinoma (a; case 1, c; case 2, e; control) and background liver (b; case1, d; case 2, f; control), demonstrated with mouse monoclonal antibodies B22.1/B23.1 (Cell Marque, USA) and visualized using the Envision method (Dako) (a-f, ×400). Control (a 79-year-old male, moderately differentiated adenocarcinoma in background of nearly normal liver)
The mechanism of alteration of CK8/18 expression in glycogen-rich hepatocytes has not been elucidated. Su et al.[4] demonstrated that CK8/18 expression was reduced in excessively glycogen-storing (glycogenotic) clear hepatocytes, which also showed a relative reduction of cytoplasmic organelles as demonstrated by electron-microscopic studies. Given simple CK8/18 expression patterns, hepatocytes are sensitive to alterations of cytokeratin architecture.[3] Using hepatic cell culture systems, Mathew et al.[5] reported recently that CK8/18 is involved in the interplay between glucose utilization and insulin signaling. The authors demonstrated that insulin stimulates glucose uptake, glucose-6-phosphatase formation, lactate release, and glycogen formation in hepatocytes via the PI-3 kinase dependent signaling pathway, and that CK8/18 IF loss makes them more efficient glycogen producers.[5] This is in line with the notion that an insulinomimetic effect of oncogenic agents is responsible for the preneoplastic hepatocellular glycogenosis,[2] which is associated with a reduced or negative expression of CK 8/18 in glycogenotic clear cells appearing in chronic human and woodchuck hepadnaviral infection.[4] CK8/18 immunohistochemistry may allow distinct recognition of the glycogen-rich hepatocytes as shown in glycogenotic clear cells under various conditions.[4]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
1. Takegoshi K, Okada E, Nomoto K, Nobata K, Sawasaki T, Terada M, Terakawa H, Kobayashi T, Yabushita K, Sugimoto T, Terahata S. Hepatocellular carcinoma and type 2 diabetes mellitus: two cases highlighting changes in tumor glycogen content. Hepatoma Res 2016;2:26-30.
2. Bannasch P, Klimek F, Mayer D. Early bioenergetic changes in hepatocarcinogenesis: preneoplastic phenotypes mimic responses to insulin and thyroid hormone. J Bioenerg Biomembr 1997;29:303-13.
3. Strnad P, Paschke S, Jang KH, Ku NO. Keratins: markers and modulators of liver disease. Curr Opin Gastroenterol 2012;28:209-16.
4. Su Q, Zerban H, Otto G, Bannasch P. Cytokeratin expression is reduced in glycogenotic clear hepatocytes but increased in ground-glass cells in chronic human and woodchuck hepadnaviral infection. Hepatology 1998;28:347-59.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Takegoshi K, Okada E, Su Q. Hepatocellular carcinoma and type 2 diabetes mellitus: cytokeratin 8/18 expression in hepatocellular carcinoma and glycogen-storing hepatocytes. Hepatoma Res 2016;2:229-30. http://dx.doi.org/10.20517/2394-5079.2016.26
AMA Style
Takegoshi K, Okada E, Su Q. Hepatocellular carcinoma and type 2 diabetes mellitus: cytokeratin 8/18 expression in hepatocellular carcinoma and glycogen-storing hepatocytes. Hepatoma Research. 2016; 2: 229-30. http://dx.doi.org/10.20517/2394-5079.2016.26
Chicago/Turabian Style
Takegoshi, Kunio, Eikichi Okada, Qin Su. 2016. "Hepatocellular carcinoma and type 2 diabetes mellitus: cytokeratin 8/18 expression in hepatocellular carcinoma and glycogen-storing hepatocytes" Hepatoma Research. 2: 229-30. http://dx.doi.org/10.20517/2394-5079.2016.26
ACS Style
Takegoshi, K.; Okada E.; Su Q. Hepatocellular carcinoma and type 2 diabetes mellitus: cytokeratin 8/18 expression in hepatocellular carcinoma and glycogen-storing hepatocytes. Hepatoma. Res. 2016, 2, 229-30. http://dx.doi.org/10.20517/2394-5079.2016.26
About This Article
Copyright
Data & Comments
Data
 Cite This Article 2 clicks
Cite This Article 2 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.