Nutrition profile of a liver transplant recipient
Abstract
Malnutrition is almost universally present in patients undergoing liver transplantation. In this report, a male adult patient was followed from his pre-liver transplant phase until chronic post-transplant phase (3 months after the transplant). Improvement in nutrition status, quality of life, and performance status was seen from the pre-transplant to chronic post-transplant phase. Day to day nutrition monitoring and gradual increase in calorie and protein intake was seen in the acute post-transplant phase, but during pre- and chronic post-transplant phase, lack of nutrition support was observed in the patient.
Keywords
Introduction
Liver transplantation (LT) is the only treatment for the end-stage liver disease (ESLD).[1] It is estimated that malnutrition occurs in 65-100% of patients with ESLD.[2,3] Medical nutrition therapy provided by a registered dietician is necessary during all phases of LT for improved surgical outcomes.[4]
Case report
Nutrition therapy for LT is divided into three phases: (1) pre-transplant - provision of adequate nutrients without aggravating ESLD symptoms; (2) acute post-transplant - high protein feeds through various routes to achieve adequate intakes; and (3) chronic post-transplant - aggressive nutrition therapy for improved survival.[4]
Pre-transplant phase
A 54-year-old Indian male patient diagnosed with ethanol and hepatitis C virus-related chronic liver disease underwent living donor LT (Child-Turcotte-Pugh score[5] = 8, Model for ESLD score[6] = 14). Medical history showed the patient suffered from jaundice (for 2 years), ascites (for 3 months) and excessive fatigue (for 15 days). The patient was admitted 12 days before LT. Biochemical parameters before LT depicted deranged results [Table 1].
Biochemical parameters of the patient before the transplant
| Biochemical parameter | Value | Range | Biochemical parameter | Value | Range |
|---|---|---|---|---|---|
| Hb (mg/dL) | 8.5 | 13-17 | Na (mmol/L) | 134 | 137-145 |
| WBC (103/UL) | 8.31 | 4.00-10.00 | K (mmol/L) | 3.7 | 3.5-5.1 |
| Platelets (103/UL) | 100 | 150-410 | Ca (mg/dL) | 8.9 | 8.4-10.2 |
| Alb (g/L) | 3 | 3.5-5.0 | Mg (mg/dL) | 1.5 | 1.6-2.3 |
| Bili (D) (mg/dL) | 0.1 | 0.2-1.3 | P (mg/dL) | 4.3 | 2.5-4.5 |
| Bili (T) (mg/dL) | 1.5 | 0.2-1.3 | Cl (mmol/L) | 106 | 98-107 |
| Total protein (g/L) | 6.4 | 6.3-8.2 | PT | 15.6 | 8.8-12.3 |
| ALT/SGPT (U/L) | 23 | 21-72 | INR | 1.51 | |
| AST/SGOT (U/L) | 34 | 17-51 | CR protein (mg/dL) | 11.6 | 0.0-10.0 |
| γ glutamyl transferase (U/L) | 28 | 15-73 | |||
| Alkaline phosphates (U/L) | 63 | 30-120 | |||
| Urea (mg/dL) | 61 | 10-50 | |||
| Cr (mg/dL) | 1.6 | 0.80-1.50 |
Nutrition status assessment by anthropometry depicted mild malnutrition by mid-arm muscle circumference (MAMC) and severe malnutrition by triceps measurement.[7] Subjective global assessment (SGA) showed moderate malnutrition.[8] Hand grip strength (both hands) showed severe malnutrition.[9]
Body composition analysis depicted standard physique of the patient with normal levels of fat percentage, fat-free mass (FFM), and muscle mass [Table 2].[10]
Nutrition assessment of the patient
| Parameter | Observation | Evaluation |
|---|---|---|
| Anthropometric evaluation | ||
| Weight (kg) | 73.9 | |
| Height (cm) | 176 | |
| Ideal body weight (kg) | 76 | |
| Triceps[7] (cm) | 0.56 | Severe malnutrition |
| MAMC[7] (cm) | 22 | Mild malnutrition |
| SGA[8] | ||
| SGA[8] | 6 | Moderate malnutrition |
| Body composition analysis by bioelectrical impedance analysis[9] | ||
| Weight (kg) | 72.55 | Normal |
| Fat (%) | 22.5 | Normal |
| Fat mass | 16.3 | Normal |
| FFM (kg) | 56.25 | Normal |
| Muscle mass (kg) | 53.35 | Normal |
| BMI | 23.2 | Normal |
Diet history depicted no gastrointestinal (GI) symptoms, dental or oral problem, or food allergies. The simplified nutritional appetite questionnaire (SNAQ) score was 16 hence there was no significant risk of at least 5% weight loss within 6 months.[11] The patient was alcoholic (CAGE score > 2).[12] He was recommended an oral normal diet with supplements providing 2700 kcal, 115 g of proteins with salt (2 g) and fluid restriction (1.5 L/day).[4] Patients’ intake was 1100 kcal and 40 g protein, indicating consumption of 57.6% of the recommended calories.
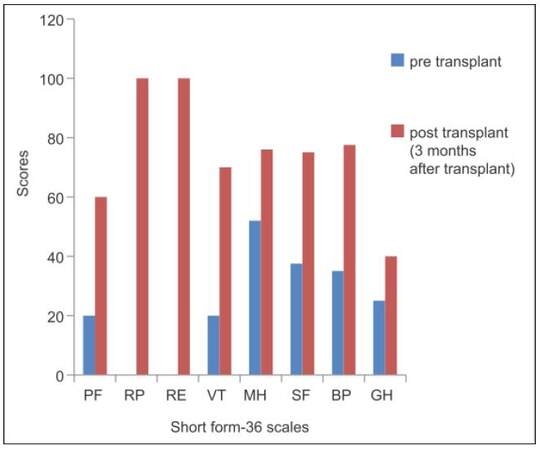
Eastern Cooperative Oncology Group (ECOG) performance status score of 3 indicated that the patient was capable of only limited self-care and unable to carry out any work activities that was ≥ 50% of working hours.[13] Quality of life (QOL) assessment by short form-36 before LT depicted low level in its eight dimensions [Figure 1].[14]
Figure 1. Comparison of quality of life by short form-36 questionnaire pre- and post-transplant. PF: physical functioning; RP: role limitation due to physical health; RE: role limitation due to emotional problem; VT: vitality; MH: mental health; SF: social function; BP: body pain; GH: general health
Acute post-transplant phase
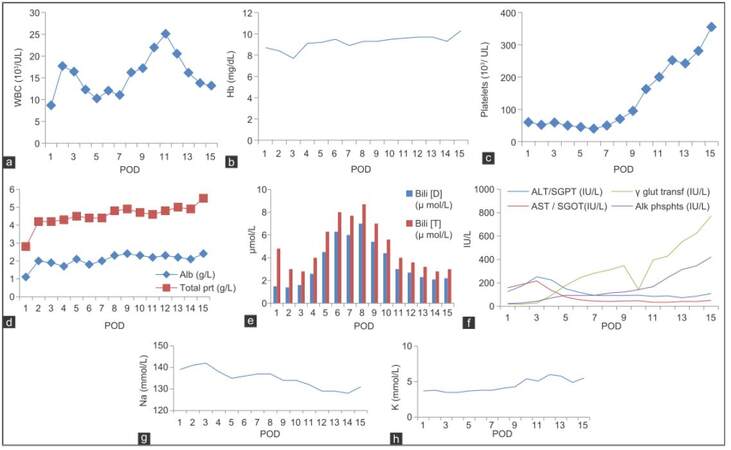
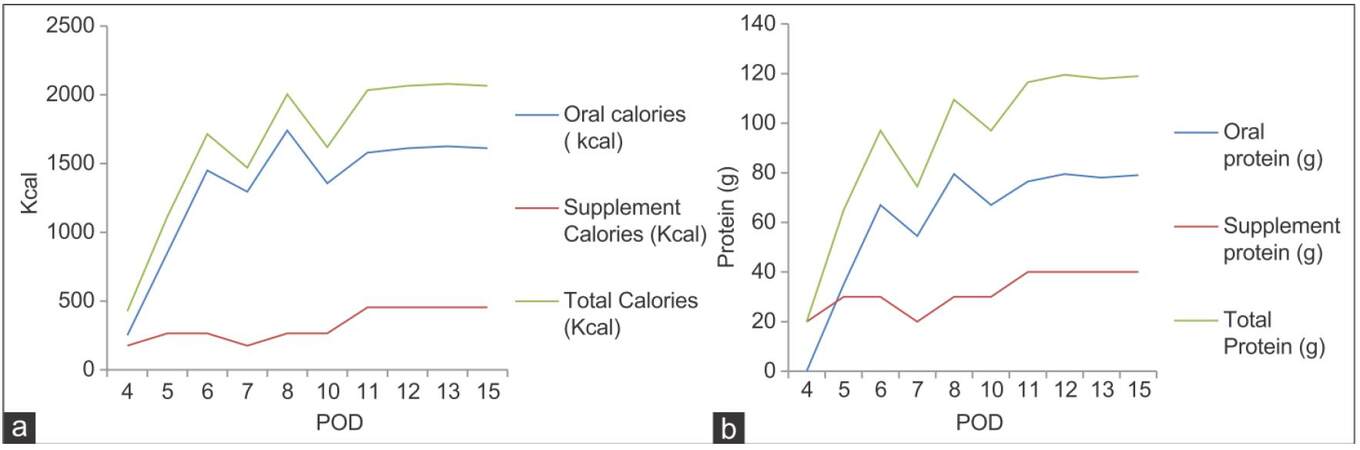
The altered blood parameters are important for implementing the nutrition therapy plan. Deranged biochemical parameters in this phase are presented in Figure 2a-h. The patient had been in intensive care unit for 3 days. At post-operation day (POD), 1 patient was extubated within 24 h and was provided propofol 45 mL (1 kcal/mL) and dextrose normal saline 440 mL (17 kcal/100 mL), KCl 45 mL intravenously. On POD 2 propofol, 120 mL and KCl 120 mL was given. On POD 3 KCl 40 mL along with oral liquids (250 kcal) was given. On POD 4, he was transferred to the LT unit and was given oral high protein normal diet with supplements providing 2,700 kcal and 115 g protein. The patient was not able to complete meals (especially lunch and dinner), because of nausea and lack of appetite. An increasing trend of energy and protein consumption after LT during the hospital stay is indicated in Figure 3. The patient met 76.4% and 103% of the recommended calorie and protein intake, respectively. The patient was discharged on POD 15, on 2,700 kcal and 115 g of proteins (high protein, low potassium normal diet) out of which 375 kcal and 36 g of protein were from low potassium nutrition supplements and about 352 kcal, and 24 g protein was from high calorie-protein biscuits.[4] He was recommended to take multivitamins and potassium binding medications, to monitor glucose regularly, and to avoid the outer source of infection.
Figure 2. Each panel depicts acute post-operative patient profile of WBC (a), hemoglobin (b), platelets (c), albumin and total protein (d), bilirubin (D and T) (e), AST, ALT, γ glutamyl transpeptidase and alkaline phosphates (f), sodium (g), and potassium (h), respectively. Hb: hemoglobin; WBC: white blood cell; Alb: albumin; Bili: bilirubin; AST: aspartate aminotransferase; ALT: alanine aminotransferase; POD: post-operation day
Chronic post-transplant phase
Gradual improvement in all the biochemical parameters was seen after 3 months of LT [Table 3]. The patient regularly visited the hepatologist after the surgery but never visited the dietician. The patient’s intake was 1,983 kcal and 78.9 g protein from the oral diet without any nutritional supplement. The recommended intake amounts to 2,280 kcal and 76 g of protein.[4] Hence, patient met 83.9% of calorie requirements.
Patients' biochemical profile after discharge
| Days after discharge | Hb (mg/dL) | WBC (103/UL) | Platelets (103/UL) | Bil (T) (mg/dL) | Bil (D) (mg/dL) | AST (IU/L) | ALT (IU/L) | Alkaline phosphates | γ glutamyl transferase (IU/L) | Alb (g/dL) | Na (mmol/L) | K (mmol/L) | Cr (mg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 9.5 | 12.02 | 40 | 8 | 6.3 | 54 | 117 | 92 | 245 | 1.8 | 136 | 3.8 | 0.8 |
| 2 | 8.9 | 11.02 | 50 | 7.7 | 6 | 44 | 92 | 94 | 284 | 2 | 137 | 3.8 | |
| 3 | 9.3 | 16.2 | 70 | 8.7 | 7 | 41 | 92 | 113 | 311 | 2.3 | 137 | 4.1 | 0.8 |
| 4 | 9.3 | 17.18 | 95 | 7 | 5.4 | 45 | 92 | 122 | 348 | 2.4 | 134 | 4.3 | 0.8 |
| 5 | 9.5 | 21.93 | 163 | 5.6 | 4.4 | 47 | 95 | 362 | 2.4 | 134 | 5.4 | 0.8 | |
| 6 | 9.6 | 25.6 | 200 | 4 | 3 | 34 | 84 | 167 | 396 | 2.2 | 132 | 5.1 | 0.9 |
| 7 | 9.7 | 20.51 | 252 | 3.6 | 2.7 | 35 | 89 | 245 | 428 | 2.3 | 129 | 6 | 1 |
| 8 | 9.6 | 16.13 | 242 | 3.2 | 2.3 | 41 | 74 | 314 | 552 | 2.2 | 129 | 5.8 | 1 |
| 9 | 9.2 | 8.09 | 185 | 1.5 | 0.9 | 30 | 117 | 82 | 195 | 1.9 | 131 | 4.6 | 0.8 |
| 10 | 10.3 | 10.17 | 355 | 3 | 2.2 | 51 | 109 | 421 | 772 | 2.4 | 131 | 5.5 | 0.9 |
| 12 | 9 | 13.14 | 305 | 2.1 | 1.6 | 52 | 78 | 287 | 733 | 2.2 | 133 | 4.1 | 1 |
| 15 | 9 | 13.19 | 300 | 2.3 | 2 | 105 | 196 | 294 | 737 | 2.3 | 137 | 3.3 | 0.9 |
| 19 | 9.8 | 17.86 | 373 | 2 | 1.7 | 67 | 221 | 325 | 828 | 2.6 | 138 | 3.7 | 0.9 |
| 26 | 11.20 | 15.48 | 301 | 1.0 | 0.8 | 57 | 119 | 213 | 623 | 2.50 | 1.0 | ||
| 33 | 11.30 | 17.37 | 312 | 0.7 | 0.7 | 42 | 86 | 178 | 474 | 2.50 | 4.0 | 0.8 | |
| 34 | 11.70 | 13.27 | 311 | 0.7 | 0.5 | 39 | 83 | 162 | 449 | 2.60 | |||
| 41 | 12.40 | 14.80 | 326 | 0.6 | 44 | 91 | 169 | 382 | 2.90 | 135 | 5.3 | 0.9 | |
| 53 | 11.30 | 13.05 | 328 | 0.3 | 0.2 | 38 | 69 | ||||||
| 54 | 12.20 | 13.22 | 308 | 0.5 | 0.4 | 55 | 102 | 160 | 283 | 2.70 | |||
| 72 | 10.90 | 22.63 | 0.6 | 0.2 | 29 | 42 | 220 | 4.90 | 146 | 4.2 | 1.3 | ||
| 88 | 0.4 | 0.3 | 23 | 32 | 116 | 107 | 3.10 | 140 | 4.8 |
The patient was not having any GI problem; he was able to perform daily routine functions. The SNAQ score was 16 which showed no significant risk of at least 5% weight loss within 6 months.[11] QOL assessment depicted improvement of all the eight dimensions 3 months after LT [Figure 1].[14] The performance status assessment by ECOG improved from a score of 3 to 1 which indicated that the patient was restricted in physically strenuous activity but was ambulatory and able to carry out work of a light or sedentary nature.[13] Nutrition status assessment is depicted in Table 4. Anthropometric examination through, MAMC[7] showed similar results as in pre-transplant phase, which is mild malnutrition. Triceps measurement improved from severe malnutrition to normal range.[7] SGA scores improved from moderate malnutrition to normal.[8] Body composition analysis depicted higher levels of fat percentage and FFM after 3 months of LT.[10] Hand grip strength (both hands) showed severe malnutrition similar to pre-transplant phase.[9]
Comparison of nutritional status in pre-transplant and chronic post-transplant phase (3 months after LT)
| Pre-transplant | Post-transplant (3 months after transplant) | |
|---|---|---|
| Anthropometric evaluation | ||
| Weight (kg) | 73.9 | 78.6 |
| Height (cm) | 176 | 176 |
| Triceps[7] (cm) | 0.56 | 1.5 |
| MAMC[7] (cm) | 22 | 21.2 |
| SGA[8] | ||
| SGA[8] (score) | 6 | 2 |
| Body composition analysis by bioelectrical impedance analysis[9] | ||
| Weight (kg) | 72.55 | 76.6 |
| Fat (%) | 22.5 | 28 |
| Fat mass (kg) | 16.3 | 21.45 |
| FFM (kg) | 56.25 | 55.15 |
| Muscle mass (kg) | 53.35 | 52.3 |
| TBW (%) | 53.5 | 47.6 |
| BMI | 23.2 | 24.5 |
| Bone mass (kg) | 2.90 | 2.85 |
Discussion
A high incidence of malnutrition has been seen in LT recipients.[5,14,15] Accurate estimation of the nutritional status of patients with ESLD presents a major challenge due to fluid retention found in patients and the effect of liver function on protein synthesis.[16] Malnutrition has also been associated with poor surgery outcome and increased morbidity and mortality. In India, LT is a relatively new area, and there is a lack of data about the general and nutritional profile of patients undergoing LT. It is essential to identify and correct nutritional deficiencies in LT recipients. Hence, this case report provides information on the day to day nutrition profile and the medical nutrition therapy of a LT recipient with the aim of improving outcomes.
A gradual improvement in the nutrition, biochemical, and functional parameters was seen after 3 months of transplant. Nutrition assessment by SGA, triceps, and body composition analysis showed better nutrition status 3 months after LT. During the acute post-transplant phase, continuous observation by medical and nutrition experts helped to fulfill nutritional needs through various feeding routes. However, the difference in calorie and protein intake in chronic post-transplant phase is due to lack of counseling from nutrition experts. Hence, proper nutrition monitoring is required during all phases of transplant to maintain the overall health of the patient.
Acknowledgments
Dr. A. S. Soin: Chief Hepatobiliary and Liver Transplant Surgeon and Chairman of Medanta Institute of Liver Transplantation and Regenerative Medicine, Medanta, The Medicity, Gurgaon, India, for permitting the author to collect information regarding liver transplant patients from their institute.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
1. O'Leary JG, Lepe R, Davis GL. Indications for liver transplantation. Gastroenterology 2008;134:1764-76.
2. Stephenson GR, Moretti EW, El-Moalem H, Clavien PA, Tuttle-Newhall JE. Malnutrition in liver transplant patients: preoperative subjective global assessment is predictive of outcome after liver transplantation. Transplantation 2001;72:666-70.
3. Lautz HU, Selberg O, Körber J, Bürger M, Müller MJ. Protein-calorie malnutrition in liver cirrhosis. Clin Investig 1992;70:478-86.
4. Plauth M, Cabré E, Riggio O, Assis-Camilo M, Pirlich M, Kondrup J, DGEM (German Society for Nutritional Medicine), Ferenci P, Holm E, Vom Dahl S, Müller MJ, Nolte W, ESPEN (European Society for Parenteral and Enteral Nutrition). ESPEN guidelines on Enteral Nutrition: Liver disease. Clin Nutr 2006;25:285-94.
5. Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60:646-9.
6. Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, Kremers W, Lake J, Howard T, Merion RM, Wolfe RA, Krom R; United Network for Organ Sharing Liver Disease Severity Score Committee. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology 2003;124:91-6.
7. Jelliffe DB, Jelliffe EF, Zerfas A, Neumann CG. Community Nutritional Assessment with Special Reference to Less Technically Less Developed Countries. Oxford: Oxford University Press; 1989.
8. Detsky AS, McLaughlin JR JR, Baker JP, Johnston N, Whittaker S, Mendelson RA, Jeejeebhoy KN. What is subjective global assessment of nutritional status? JPEN J Parenter Enteral Nutr 1987;11:8-13.
9. TANITA. Multi-Frequency Body Composition Analyzer MC-180MA Instruction Manual. 2005. pp. 1-61.
10. Wilson MM, Thomas DR, Rubenstein LZ, Chibnall JT, Anderson S, Baxi A, Diebold MR, Morley JE. Appetite assessment: simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents. Am J Clin Nutr 2005;82:1074-81.
12. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649-55.
13. Ware JE Jr, Gandek B. Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) project. J Clin Epidemiol 1998;51:903-12.
14. Hasse JM. Nutritional implications of liver transplantation. Henry Ford Hosp Med J 1990;38:235-40.
15. Sanchez AJ, Aranda-Michel J. Nutrition for liver transplant patients. Liver Transpl 2006;12:1310-6.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Bakshi N, Singh K. Nutrition profile of a liver transplant recipient. Hepatoma Res 2016;2:98-102. http://dx.doi.org/10.4103/2394-5079.168958
AMA Style
Bakshi N, Singh K. Nutrition profile of a liver transplant recipient. Hepatoma Research. 2016; 2: 98-102. http://dx.doi.org/10.4103/2394-5079.168958
Chicago/Turabian Style
Bakshi, Neha, Kalyani Singh. 2016. "Nutrition profile of a liver transplant recipient" Hepatoma Research. 2: 98-102. http://dx.doi.org/10.4103/2394-5079.168958
ACS Style
Bakshi, N.; Singh K. Nutrition profile of a liver transplant recipient. Hepatoma. Res. 2016, 2, 98-102. http://dx.doi.org/10.4103/2394-5079.168958
About This Article
Copyright
Data & Comments
Data

 Cite This Article 5 clicks
Cite This Article 5 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.